Summary
Glioblastoma (GBM) stem cells (GSCs) contribute to poor prognosis in patients with GBM. Identifying molecular markers is crucial for developing targeted therapies. Here, we identify cluster of differentiation 97 (CD97) as an optimal GSC surface antigen for potential targeting by chimeric antigen receptor (CAR) T cell therapy through in vitro antibody screening. CD97 is consistently expressed in all validated patient-derived GSCs and positively correlated with known intracellular GSC markers. Silencing CD97 reduces GSC tumorigenicity-related activities, including self-renewal, proliferation, and tumor progression. Transcriptome analysis reveals that CD97 activates mTORC2, leading to AKT S473 phosphorylation and enhanced expression of the downstream genes ARHGAP1, BZW1, and BZW2. Inhibiting mTORC2 with JR-AB2-011 suppresses GSC tumorigenicity and downstream gene expression. We develop CD97-CAR T helper (Th) 9 cells, which exhibit potent cytotoxic effects in vitro and extend survival in mice. These findings suggest that CD97 is a promising GSC-enriched antigen and that targeting it with CAR Th9 cells offers a potential therapeutic strategy for GBM.
Keywords: glioblastoma, glioblastoma stem cell, tumorigenicity, self-renewal, CD97, mTORC2, CAR T, Th9 cell
Graphical abstract

Highlights
-
•
In vitro antibody screening identified CD97 as an optimal GSC surface antigen
-
•
CD97 strongly correlates with intracellular GSC markers
-
•
CD97 regulates ARHGAP1, BZW1, and BZW2 expression via mTORC2-AKT signaling
-
•
CD97-targeting CAR Th9 cells show a potent antitumor response
Zhou et al. identify CD97 as a regulator of GSC maintenance via mTORC2 signaling activation. Targeting CD97 with CAR Th9 cells exhibits potent cytotoxic effects and prolongs survival in mice, highlighting CD97 as a promising antigen for GBM-targeted therapy.
Introduction
Glioblastoma (GBM) is the most common malignant primary brain tumor with a median overall survival of 14–16 months despite intensive treatment.1,2 One of the leading causes of GBM aggressiveness, high recurrence, and resistance to therapy is cancer heterogeneity, especially GBM stem cells (GSCs).3 GSCs are subpopulations of cells capable of initiating tumor progression to organize proliferation and produce pathognomonic heterogeneity and more differentiated cancer cells.3 Identification and validation of a molecular marker of GSCs are important to classify tumor cell features and translate them into GSC-targeted therapies. However, potential GSC-targeted therapies, especially chimeric antigen receptor (CAR) T cell therapy, are not yet well established due to the current lack of reliable tumor antigens for targeting.
The success of Food and Drug Administration approval for CD19-CAR T cells as a treatment for B cell malignancies4 has greatly encouraged researchers to develop CAR T therapy for patients with GBM. However, early clinical outcomes with CAR T cells targeting IL-13 receptor α chain 2,5 epidermal growth factor receptor variant III,6 and human epidermal growth factor receptor 27 were limited. Therefore, studies are urgently needed to discover reliable GSC-specific surface antigens and reveal the functions of recognized antigens to develop GSC-targeted CAR T cells. Although several surface markers have been identified for GSCs, such as CD133,8,9,10 CD44,11,12 CD15,13,14 CD9,15 L1CAM,16 CD321,17 and CD49f,18 their potency in CAR development is limited due to their expression in normal neural stem cells or astrocytes. A recent study suggested the rational development of CD133-targeting immunotherapies, including CD133-targeting CAR T cells for GBM and brain metastasis,19,20 but the use of CD133 as a specific surface antigen of GSCs is controversial.19 Following in-depth research, a large number of studies demonstrated that CD133-negative cells also form brain tumors.21 Moreover, the cell surface antigens having a biological function in regulating GSC maintenance and tumorigenesis are restricted, and the potency for developing antitumor CAR T cells is challenged.
Here, we used a human surface protein antibody array and found that all validated patient-derived GSCs uniformly and specifically express the surface antigen cluster of differentiation 97 (CD97). CD97 showed a remarkable presence on GSCs and was associated with adverse outcomes in patients with GBM. It showed a strong positive association with intracellular GSC markers, such as Nestin, Oct3/4, and Nanog, suggesting its suitability as an exclusive surface marker for GSCs. Functionally, CD97 activates mTORC2-AKT signaling to maintain the tumorigenicity of GSCs. Furthermore, CD97high GSCs promote aggressive tumor growth in vitro and in vivo and confer a poor prognosis to patients with GBM. Finally, we developed CD97-targeting CAR T helper (Th) 9 cells and showed a potential antitumor response against GBM. Collectively, these findings suggest that CD97 is a functional GSC surface antigen, and CD97-targeting CAR Th9 cell therapy may be a potent strategy for treatment-refractory GBM.
Results
Identification of CD97 as a potential surface antigen for GSCs
To identify a generally optimal enriched surface antigen of GSCs, we first screened GSCs (83 and X01), serum-induced differentiated GSCs (X01-Diff), and normal astrocytes (Ast) using commercially available cell surface protein antibody panels (Figure 1A). The antibody array generated data for 242 human cell surface proteins and 9 isotype controls. The percentage of positive cells was determined for each cell surface protein using a high-throughput confocal imaging system, and values from replicates were averaged. We identified potential GSC surface antigens based on the following three criteria: (1) the candidate was not or minimally expressed in Ast, (2) the candidate was expressed with high levels in 83 and X01 GSCs, and (3) the candidate showed compromised expression levels in differentiated GSCs. Thus, we identified CD97 as our top candidate (Figure 1B). The characterization of CD97 as a potential surface antigen of GSCs is shown in the representative confocal immunofluorescence (IF) micrographs and the accompanying quantitative evaluation (Figures 1C and 1D).
Figure 1.
Identification of surface antigens for GSCs
(A) Schematic illustrating the experimental design. Human astrocytes and GSCs (83 and X01) were profiled using multiple antibody libraries.
(B) Heatmap showing the top 32 genes of human cell surface antigens, ranked based on the fold change values of fluorescence intensity compared to normal astrocytes or differentiated GSCs.
(C and D) High-throughput confocal immunofluorescence (IF) staining images showing staining with the anti-CD97 antibody (C) and quantification of fluorescence in the antibody array (values normalized to those of astrocytes) (D). Scale bar, 50 μm. All error bars represent the mean ± standard error of the mean (SEM; n = 3 independent experiments).
(E) Quantitative reverse-transcription PCR (RT-qPCR) and immunoblot (IB) analyses of CD97 expression in astrocytes, GSCs, and serum-differentiated GSCs (83-Diff and X01-Diff). β-actin was used as a loading control. Error bars represent the mean ± standard deviation (SD; n = 3 independent experiments).
(F) Flow cytometry analysis of astrocytes, GSCs, and serum-differentiated GSCs (83-Diff and X01-Diff) using a phycoerythrin (PE)-conjugated anti-CD97 antibody.
(G) Flow cytometry analysis of CD97 expression on patient-derived GSCs, stained with a PE-conjugated anti-CD97 antibody.
(H and I) CD97 protein expression in NT and GBM tissues (H) and the expression in each GBM subtype (I) according to the CPTAC database. NT, nontumor; CL, classical; MES, mesenchymal; NL, neural; PN, proneural.
(J) Kaplan-Meier overall survival curves for patients with GBM presenting high and low CD97 protein expression according to the CPTAC database. Groups were divided by optimal cutpoints using “survminer” R package.
The p value was determined using the log rank (Mantel-Cox) test. ∗∗∗∗p < 0.0001, ∗∗∗p < 0.001, ∗p < 0.05, t test in (C), (D), (E), and (H). See also Figure S1.
CD97, which is encoded by the gene ADGRE5, is a member of the epidermal growth factor subfamily of the adhesion G protein-coupled receptor class of cell surface proteins.22 CD97 was first reported to be involved in cell proliferation, adhesion, and migration in different immunophenotypes of diseases.22 Recent studies have implicated abundant CD97 expression detected in acute myeloid leukemia,23 thyroid,24 hepatocellular,25 colorectal,26 and pancreatic cancers,27 as well as GBM.28,29,30,31,32,33,34,35,36,37 Functionally, CD97 has been shown not only to confer cell adhesion through interaction with other extracellular matrix proteins but also to stimulate angiogenesis and induce cancer invasion. However, its association with GBM, particularly in GSC biology, has yet to be thoroughly investigated. Consistent with the expression profiles obtained from antibody screening, the CD97 mRNA and protein were expressed at high levels in GSCs, and CD97 expression was noticeably decreased after GSC differentiation (Figure 1E). CD97 was further validated as a hit using flow cytometry, and a high expression level of CD97 was detected in 83 and X01, and its expression was markedly decreased in GSCs under serum-induced differentiation conditions (Figure 1F). We evaluated eight additional patient-derived GSCs (131, 528, 448, GSC772, GSC211, GSC924, GSC028, and GSC428) using flow cytometry to further confirm these findings in a wider range of GSCs, and CD97 was ubiquitously overexpressed in all tested GSCs (Figure 1G). Collectively, these data suggest that CD97 could be an ideal GSC surface antigen.
CD97 is correlated with a poor prognosis for patients with GBM
Subsequently, to investigate the clinical relevance of CD97 in patients with GBM, we performed an in silico analysis utilizing publicly available glioma datasets. Higher levels of CD97 protein abundance were observed in patients with GBM than in nontumor tissues from the Clinical Proteomic Tumor Analysis Consortium cohort (CPTAC)38 (Figure 1H). Additionally, CD97 was expressed at high levels in all three GBM subtypes, which were classified based on different molecular signatures (Figure 1I).39 Notably, upregulated CD97 protein expression was also associated with a poor prognosis for those patients (Figure 1J). These findings were consistent with the results using mRNA glioma datasets from The Cancer Genome Atlas (TCGA), the Chinese Glioma Genome Atlas (CGGA), and the Repository of Molecular Brain Neoplasia Data (REMBRANDT) (Figure S1). These results strongly indicate that high expression of CD97, either in protein or mRNA level, is remarkably correlated with worse outcomes for patients with GBM.
CD97 identifies the GSC population and maintains GSC tumorigenicity
To determine the role of CD97 as an optimal surface antigen in GSC identification, we performed a bioinformatics analysis. First, we interrogated single-cell RNA sequencing datasets using Restall40 and Richards41 cohorts and found evidence that ADGRE5/CD97 is highly expressed in GSCs (Figure 2A). Next, we calculated the Pearson correlation coefficient (R) between individual genes associated with conventional, well-known GSC markers (Nestin,9,42 Sox2,43 Nanog,44 Oct3/4,45 PROM1/CD133,10 CD44,11 FUT4/CD15,13 and ITGA6/CD49f18) and ADGRE5/CD97 using public databases, including TCGA, CCGA, REMBRANDT, and CPTAC (protein) (Figures 2B, 2C, and S2). Notably, Nestin exhibited a consistently high positive correlation coefficient with CD97 in both mRNA (Figure 2B) and protein (Figure 2C) levels of all public databases (R > 0.2). While CD44, CD15, and CD49f showed positive correlations in all databases, the R-values for these correlations were not consistently high across the databases (some were below 0.1) (Figure S2).
Figure 2.
Validation of CD97 as an optimal GSC marker
(A) Bubble plot comparing expression of ADGRE5/CD97 and stem cell-related genes on GSCs and GBM tumors using single-cell RNA sequencing datasets from the study by Restall and Richards.
(B) Scatterplots to compare the RNA expression level of ADGRE5 and NES according to patients with GBM in the TCGA, CCGA, and REMBRANDT databases. CD97 is encoded by ADGRE5 gene. Nestin is encoded by NES gene.
(C) Scatterplots to compare the protein expression level of CD97 and Nestin according to patients with GBM in the CPTAC database.
(D) Flow cytometry plots in which CD97-PE was co-stained along with other GSC markers, including Nestin, Oct 3/4, Nanog, CD133 or CD49f, CD15, or CD44 in 83, X01, and GSC772. Each gate on the x and y axis separates positive from negative cells. The numbers in each quadrant indicate the percentages of cells.
(E) Limiting dilution assay (LDA) using CD97high CD49fhigh, CD97high CD49f−/low, CD97−/low CD49fhigh, and CD97−/low CD49f−/low GSC 83 cells sorted through flow cytometry.
(F–H) Confocal images of CD97 (green) and Nestin (red) co-immunofluorescence staining in brain tissues of the X01-xenograft (F) and human patients with GBM who have NestinLow (G) and NestinHigh (H). Scale bar, 50 μm. Quantification analysis of the percentage of the cell number CD97+/Nestin+ and CD97−/Nestin+ cells.
All error bars represent mean ± SD (n = 3 independent experiments) in (E)–(H). ∗∗∗∗p < 0.0001, ∗∗∗p < 0.001, t test. n.s, non-significant. See also Figures S2–S5.
To provide more direct evidence of the positive correlation between CD97 and GSC markers, we compared CD97 expression with aforementioned GSC markers by flow cytometry analysis (Figure 2D). We found that almost all intracellular GSC markers, such as Nestin, Oct3/4, and Nanog, showed high co-staining with CD97 in 83 and X01 cells ranging from 75.6% to 98.7%, and GSC772 cells with moderate CD97 expression showed over half co-staining (Figure 2D). However, some of the intracellular markers, in contrast to CD97, also showed overexpression in the general GBM cell line (U87 and U251; Figure S3). In addition, 83 and X01 cells did not contain any cells with detectable CD133 expression, consistent with the findings from other reports.46 CD44, another putative GSC surface marker, was detected with high expression in 83 cells (Figure 2D), as well as in a general glioma cell line, U87 (Figure S3A). Thus, CD44 is disqualified as a specific surface antigen for GSCs. Another GSC-specific surface antigen CD49f was detected in all GSCs (Figures 2D and S3), but did not exhibit a high positive correlation coefficient with CD97 expression in public databases (Figure S2). CD15 has also been reported as a GSC-enriched surface marker, but it showed no detectable expression in our tested GSCs (Figure 2D). Moreover, the immunoblotting analysis showed that CD97 expression was associated with a downregulation of typical intracellular marker expression under serum-induced differentiation conditions of GSCs (Figure S4). To further confirm the self-renewal ability of CD97+ GSCs compared with other GSC makers, we performed flow cytometry to categorize GSCs (83, X01, and 528) into four distinct groups with CD49f: CD97high CD49fhigh, CD97high CD49f−/low, CD97−/low CD49fhigh, and CD97−/low CD49f−/low. We then evaluated the self-renewal capacity of these GSCs through limiting dilution assay (LDA). Supportively, the results demonstrated a gradient decrease in sphere formation abilities across these groups, with CD97high CD49fhigh, CD97high CD49f−/low, CD97−/low CD49fhigh, and CD97−/low CD49f−/low. This indicates that CD97 expression is significantly positively correlated with sphere formation ability in GSCs (Figures 2E and S5). Taken together, unlike intracellular markers that can unambiguously identify GSCs, currently used surface antigens, except for CD97, do not clearly define a comprehensive GSC population.
To further validate CD97 as a GSC-specific surface antigen, we conducted IF staining using X01 GSC xenograft GBM tissues (Figure 2F). The results showed that most of the Nestin-positive cells, known as GSCs, were co-stained with CD97, supporting the highly positive correlation between Nestin and CD97. In addition, the Nestin-positive GBM cells showed high co-localization with CD97 in NestinLow and NestinHigh patient GBM tissues (Figures 2G and 2H), further supporting that CD97 is an optimal GSC surface marker. These findings collectively support the potential utility of CD97 as a surface antigen for GSCs.
To verify CD97 as a GSC surface antigen and its function in GBM initiation, we examined the characteristics of CD97-enriched GSCs. GSC772 was grouped into two subpopulations based on CD97 expression profiles; the top 38.5% was defined as CD97high, and the bottom 18.8% was defined as CD97−/low using flow cytometry (Figure 3A). The isolated CD97high population exhibited high expression of the stemness-related markers CD133, Nestin, and CD44 at both the mRNA and protein levels compared to the CD97−/low population (Figures 3B and 3C). As expected, sorted CD97high cells showed significantly increased proliferation (Figure 3D) and self-renewal (Figure 3E) compared to CD97−/low cells. Additionally, we replicated these findings by sorting GSC924 based on CD97 expression and confirming the enrichment of stem cell markers and the promotion of cell growth and self-renewal in CD97high cells (Figures 3F–3J).
Figure 3.
Characterization of CD97high cells in vitro and in vivo
(A) GSC772 cells were stained with an anti-CD97 antibody and analyzed using flow cytometry. The Neg.Ctrl group is shown in gray and the Anti-CD97 group is shown in red.
(B–E) RT-qPCR (B) and IB analysis (C) of stemness-related genes, proliferation assays (D), and LDAs (E) in the CD97high and CD97−/low subpopulations of GSC772. β-actin was used as a loading control.
(F) GSC924 cells were stained with an anti-CD97 antibody and analyzed using flow cytometry as in (A).
(G–J) RT-qPCR (G) and IB analysis (H) of stemness-related genes, proliferation assays (I), and LDAs (J) in the CD97high and CD97−/low subpopulations of GSC924. GAPDH was used as a loading control.
(K) Kaplan-Meier survival curves of mice orthotopically implanted with CD97high or CD97−/low GSC772 cells (n = 8, 2.5 × 105 cells/mouse). MST, median survival time. Log rank (Mantel-Cox) test.
(L and M) Representative axial MRI (L) and the quantified tumor size (M) of mice bearing orthotopic xenografts of CD97high or CD97−/low GSC772 cells. Regions of interest (ROIs) within the tumor are indicated by white polygons.
All error bars represent mean ± SD (n = 3 independent experiments). ∗∗∗∗p < 0.0001, ∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.05, t test in (B)–(J), (L), and (M).
Next, to identify the in vivo tumorigenic potential of CD97-enriched GSCs, we established orthotopic transplantation models using CD97high and CD97−/low GSC772. The overall survival of tumor-bearing animals was significantly shorter in the CD97high GSC772-injected group (86.5 days) than in the CD97−/low GSC772-injected group (101.5 days) (Figure 3K). Importantly, the onset of tumors was significantly faster, and magnetic resonance imaging (MRI) at 70 days after implantation showed that the tumors derived from CD97high GSC772 were significantly larger than those derived from CD97−/low GSC772 (Figures 3L and 3M). Overall, these data strongly indicate that CD97high cells have the characteristics of GSCs in vitro and high tumorigenicity in vivo.
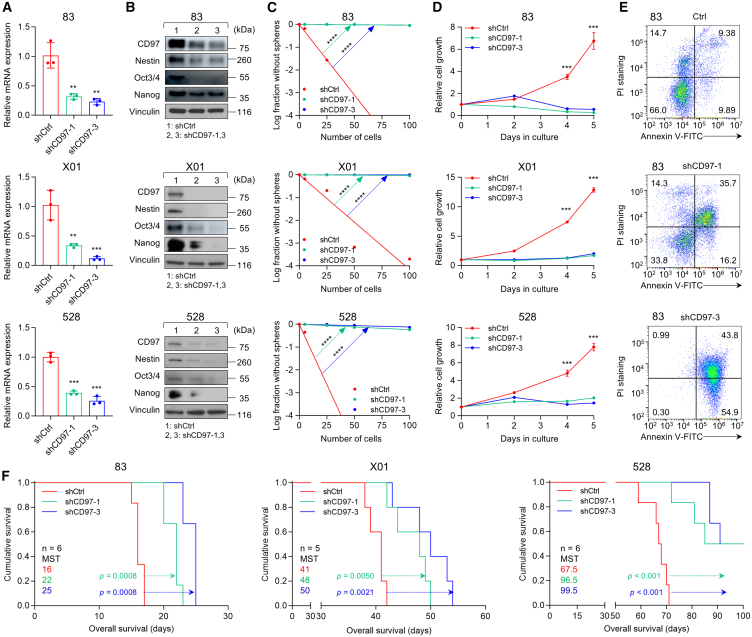
The presence of GSCs implies the ability to proliferate indefinitely and exhibits a high degree of plasticity, contributing to the intratumor heterogeneity observed in GBM.47 To explore the role of CD97 in maintaining GSC tumorigenicity, we manipulated CD97 expression in three types of GSCs (83, X01, and 528) with the highest CD97 expression among all tested GSCs, performed an in vitro LDA and proliferation assay, and generated an orthotopic mouse model. Silencing CD97 with two independent short hairpin RNA (shRNA)-expressing lentiviruses significantly reduced CD97 mRNA and protein expression (Figures 4A and 4B). Additionally, CD97 knockdown in GSCs resulted in the downregulation of intracellular GSC markers (Nestin, Oct3/4, and Nanog), as confirmed by immunoblotting (Figure 4B) and flow cytometry analysis (Figure S6). Consequently, CD97 knockdown significantly led to decreased sphere-forming ability of GSCs, as measured using LDA, a renowned method to determine the GSC self-renewal capacity (Figure 4C). CD97 knockdown significantly decreased GSC proliferation and increased cell death (Figures 4D, 4E, and S7). CD97 knockout further confirmed the downregulation of GSC markers, along with reduced sphere-forming ability and cell growth in GSCs (Figure S8). However, CD97 knockdown in CD97−/low GSCs and non-GSCs did not show much effect on cell growth or expression of stemness markers (Figure S9). Furthermore, we observed the restoration of self-renewal capacity and cell proliferation, underscoring the role of CD97 in these processes, in rescue experiments where CD97 was re-expressed in the CD97-knockdown cells (Figure S10). Then, we investigated the role of CD97 in the tumor progression of GBM by intracranially injecting mice with GSCs transduced with shCtrl or shCD97s. Knockdown of CD97 expression in 83, X01, and 528 GSCs remarkably prolonged the survival of the mice (Figure 4F). Collectively, these results highlight that CD97 expression is crucial for maintaining GSC tumorigenicity processes, including self-renewal, proliferation, and tumor progression.
Figure 4.
CD97 regulates GSC tumorigenicity
(A–D) RT-qPCR (A) of CD97 expression, IB analysis (B) of GSC intracellular markers, LDAs (C), and cell proliferation assays (D) in three different GSCs (83, X01, and 528 cells) infected with shCtrl, shCD97-1, or shCD97-3 lentivirus. β-actin, GAPDH, and vinculin were used as loading controls. All error bars represent mean ± SD (n = 3 independent experiments). ∗∗∗∗p < 0.0001, ∗∗∗p < 0.001, ∗∗p < 0.01, t test.
(E) Flow cytometry analysis of Annexin V and PI staining in GSCs 83 infected with shCtrl, shCD97-1, or shCD97-3 lentivirus.
(F) Kaplan-Meier survival curves of mice bearing orthotopic xenografts of three GSCs infected with shRNA lentivirus (n = 5 or 6, 1 × 104 for 83 and X01, or 1 × 105 for 528 cells/mouse). MST, median survival time. Log rank (Mantel-Cox) test.
See also Figures S6–S10.
Screening downstream targets of CD97 in GSCs
We used an integrated approach to comprehensively determine the biological functions mediated by CD97 expression and to understand the regulatory role of CD97. We performed transcriptome profiling using RNA sequencing (RNA-seq) analysis with 83, X01, and 528 GSCs after the knockdown of CD97 and found that 131 genes were significantly downregulated by silencing CD97 in all of those GSCs (Figure 5A). Gene Ontology Biological Process analysis showed enrichment in the regulation of stem cell population maintenance in the shCtrl group, indicating a potential role of CD97 in GSC maintenance (Figure 5B). Each gene identified as being downregulated upon CD97 knockdown was carefully examined for its significant functions in biological processes. Thus, the biological process category of Gene Ontology terms was analyzed for enrichment of these commonly downregulated genes using the Database for Annotation, Visualization, and Integrated Discovery. The results showed that cell-cell adhesion, nucleobase-containing compound metabolic processes, and positive regulation of hormone biosynthetic processes were the only three significantly enriched signaling pathways in GSCs with CD97 knockdown (Figure 5C). Among them, cell-cell adhesion showed the highest gene ratio, which included poly(rC)-binding protein 1 (PCBP1), basigin (BSG), Rho GTPase-activating protein 1 (ARHGAP1), basic leucine zipper and W2 domain-containing protein 1 (BZW1), basic leucine zipper and W2 domain-containing protein 2 (BZW2), and drebrin (DBN1) (Figure 5C). Indeed, the downregulation of the mRNA expression of these cell-cell adhesion-associated genes was confirmed in GSCs with CD97 knockdown (Figure 5D). To determine the possible relationship between the expression of CD97 and these related genes, we analyzed the clinical prognosis using publicly available GBM datasets and found that patients expressing high levels of both CD97 and ARHGAP1, BZW1, or BZW2 experienced worse survival outcomes than patients with low levels in TCGA (Figure 5E), CGGA (Figure S11A), and REMBRANDT databases (Figure S11B), while coexpression of CD97 and BSG, DBN1, or PCBP1 did not consistently show significant correlations with the survival probability of patients in either database (Figures S11C–S11E). Furthermore, we assessed the regulators of the cell-cell adhesion-associated genes by analyzing the 50 hallmark signaling pathways along with the transcriptome results using gene set enrichment analysis. Among the significant hallmark signaling pathways enriched in the shCtrl group, we observed that mTOR signaling pathways (including PI3K/AKT/mTOR, also known as mTORC1 and mTORC2) were enriched in the shCtrl group (Figures 5F and 5G). Therefore, we hypothesize that among the candidate genes, CD97 regulates the expression of ARHGAP1, BZW1, and BZW2 through mTOR signaling.
Figure 5.
Screening of downstream targets of CD97 in GSCs
(A) Schematic representation of the experimental strategy for the transcriptome analysis. Heatmap of the hierarchical clustering analysis showing the expression of 131 common downregulated genes in CD97-knockdown GSCs (right).
(B) Gene set enrichment analysis (GSEA) plot for the regulation of stem cell population maintenance signature from the Gene Ontology Biological Process (GOBP) category comparing the shCtrl and shCD97 groups. NES, normalized enrichment score.
(C) Bubble chart showing the top 10 biological processes of Gene Ontology terms for genes downregulated in CD97-knockdown GSCs. Size, gene number; color, −log10 (p value).
(D) RT-qPCR of PCBP1, BSG, ARHGAP1, BZW1, BZW2, and DBN1 expression in three different GSCs (83, X01, and 528 cells) infected with shCtrl or shCD97 lentivirus. β-actin was used as a loading control. All error bars represent mean ± SD (n = 3 independent experiments). ∗∗∗∗p < 0.0001, ∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.05, t test.
(E) Kaplan-Meier overall survival curves for patients with GBM presenting the high and low expression of the CD97/ARHGAP1 (left), CD97/BZW1 (middle), and CD97/BZW2 (right) mRNAs according to TCGA GBM database. Log rank (Mantel-Cox) test.
(F) Bubble chart showing the top 10 hallmark genes enriched in shCtrl GSCs. Size, gene number; color, −log2 (p value).
(G) GSEA plot for the PI3K/AKT/mTOR signaling signature from the Hallmark category comparing the shCtrl and shCD97 groups.
See also Figure S11.
CD97 regulates downstream gene expression through mTORC2 signaling
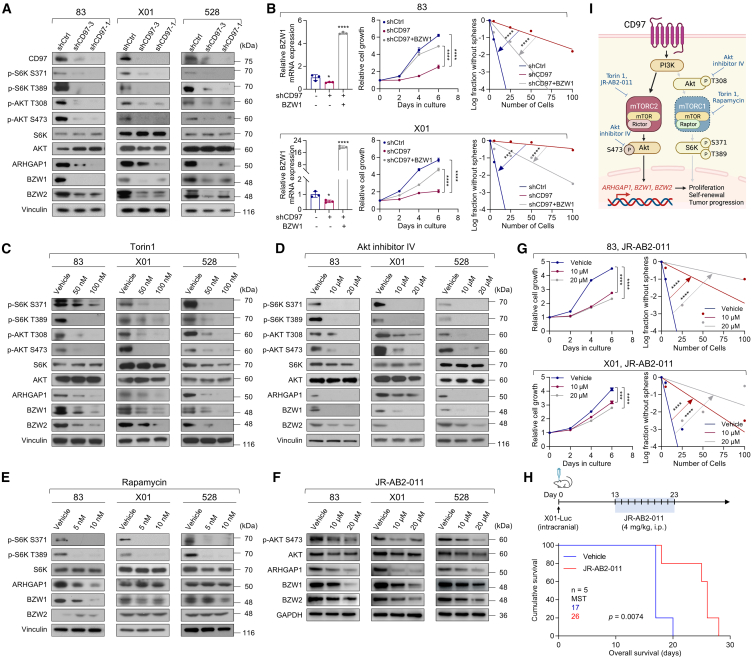
mTOR is an intracellular serine/threonine conserved protein kinase in two distinct complexes, mTORC1 and mTORC2. mTORC1, which is rapamycin sensitive, controls cell growth at least partially through its ability to phosphorylate S6K and activates downstream PI3K/AKT signaling.48,49 On the other hand, by phosphorylating AKT on Ser473, mTORC2, which is rapamycin insensitive, regulates cell proliferation, survival, migration, and cytoskeletal remodeling.50,51,52 As an extension of the aforementioned results, we rigorously tested the functions of the downstream signaling pathway for CD97. The isolated CD97high population regulated AKT phosphorylation (S473) related to mTORC2 signaling and exhibited increased expression of ARHGAP1, BZW1, and BZW2 (Figure S12A). Supportively, distinct staining with anti-ARHGAP1, anti-BZW1, and anti-BZW2 antibodies was detected in CD97high GSC772 tumors (Figure S12B). Additionally, silencing CD97 using shRNAs substantially attenuated S6K (S371 and T389) and AKT (T308 and S473) phosphorylation in three different GSCs (Figure 6A). Accordingly, the expression levels of ARHGAP1, BZW1, and BZW2 were significantly reduced in CD97-knockdown GSCs (Figure 6A). Next, we conducted rescue experiments with the three genes, BZW1, BZW2, and ARHGAP1, in CD97-knockdown GSCs. All of three genes overexpressed in CD97-knockdown GSCs partially rescued shCD97-mediated repression of GSC cell growth and self-renewal in three different GSCs (Figures 6B and S13). These results show that these three genes, associated with cell-cell adhesion, are key molecules in CD97-mediated mTOR signaling for the maintenance of GSC proliferation and self-renewal and prompted us to investigate the potential involvement of mTOR downstream of CD97.
Figure 6.
mTORC2 inhibition prevents proliferation and self-renewal in GSCs
(A) IB analysis of CD97, p-S6K, p-AKT, S6K, AKT, ARHGAP1, BZW1, and BZW2 levels in three different GSCs (83, X01, and 528 cells) infected with shCtrl or shCD97 lentivirus.
(B) RT-qPCR of BZW1 expression (left), cell proliferation assay (middle), and LDAs (right) in 83 and X01 GSCs infected with shCtrl and shCD97 lentivirus, followed by BZW1 lentivirus infection.
(C–F) IB analysis of p-S6K, p-AKT, S6K, AKT, ARHGAP1, BZW1, and BZW2 levels in three different GSCs (83, X01, and 528 cells) treated with Torin1 48 h (C), AKT inhibitor IV 24 h (D), 24 h rapamycin (E), and JR-AB2-011 24 h (F).
(G) Cell proliferation assays (left) and LDAs (right) were performed on 83 and X01 GSCs after treatment with JR-AB2-011.
(H) Kaplan-Meier survival curves of mice orthotopically implanted with X01-Luc cells (n = 5, 1 × 105 cells/mouse) and intraperitoneally (i.p.) treated with JR-AB2-011 (4 mg/kg) or vehicle. MST, median survival time. Log rank (Mantel-Cox) test.
(I) Schematic representation of the CD97-related signaling pathway regulating the proliferation, self-renewal, and tumor progression of GSCs. Vinculin and GAPDH, and β-actin were used as loading controls in IB, β-actin was used as a loading control in RT-PCR.
All error bars represent mean ± SD (n = 3 independent experiments) in (B) and (G). ∗∗∗∗p < 0.0001, ∗∗∗p < 0.001, ∗p < 0.05, t test. See also Figures S12–S15.
Given the potential involvement of mTOR downstream of CD97, we subsequently aimed to identify mTORC1 or mTORC2 signaling involved with ARHGAP1, BZW1, and BZW2 gene expression by administering small-molecular inhibitors. Torin1 (an inhibitor of mTORC1 and mTORC2) and AKT inhibitor IV induced decreases in not only the phosphorylation of S6K (S371 and T389) and AKT (T308 and S473) but also the expression of three target molecules (Figures 6C and 6D). Subsequently, we blocked each signaling pathway with two different classes of mTOR-specific inhibitors (rapamycin, a mTORC1 inhibitor, and JR-AB2-011, a mTORC2 inhibitor) and analyzed the functional state of each pathway in GSCs to understand the mechanism by which signaling triggered by CD97 contributes to the expression of its target genes. Rapamycin treatment reduced the phosphorylation of S6K (S371 and T389) but did not consistently alter the expression levels of the three target proteins (Figure 6E). On the other hand, treatment with JR-AB2-011 altered the phosphorylation of AKT (S473), which is important for mTORC2 activation, and, interestingly, downregulated the expression of three target proteins (Figure 6F). Based on the cell proliferation assay and LDA, all inhibitors that selectively inhibited mTOR/AKT signaling therapeutically blocked the growth and self-renewal of GSCs since our results suggested that CD97 activates mTORC1 and mTORC2 signaling, but only mTORC2 is involved in downstream gene expression (Figures 6G and S14). In animal models, JR-AB2-011 treatment significantly decreased tumor size and prolonged the survival of mice bearing X01-Luc (X01 GSC transduced with a luciferase-expressing vector) orthotopic xenograft tumors (Figures 6H and S15A); also, reduced mTOR2 involved downstream gene expression (Figure S15B). Collectively, these data strongly suggest that CD97 regulates ARHGAP1, BZW1, and BZW2 expression through the mTORC2/AKT pathway, which is involved in the tumorigenesis of GSCs (Figure 6I).
Development of CD97-targeting CAR Th9 cells and their antitumor activity
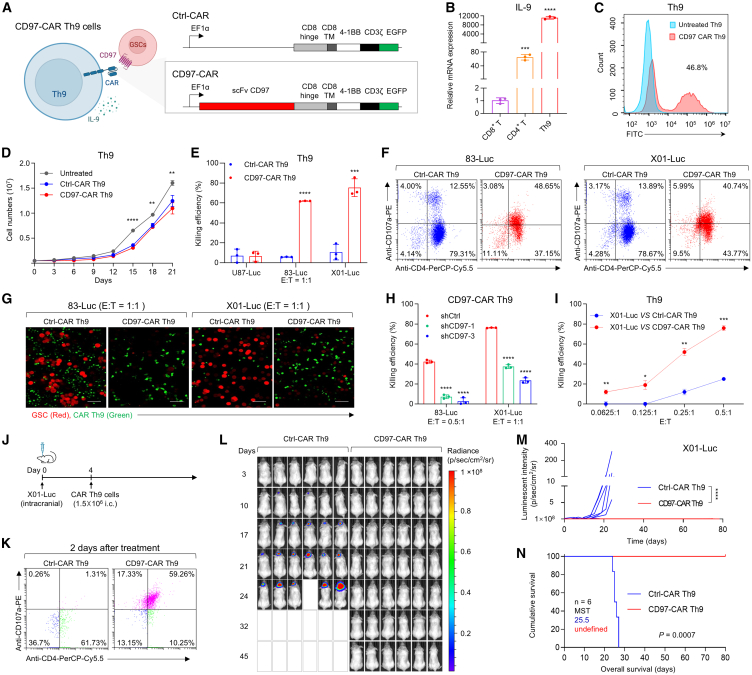
Cell surface antigens might be good targets for one of the most promising anticancer therapies, known as CAR T cells, which have developed artificial molecules capable of recognizing and subsequently destroying cancer cells that specifically express antigens on their surface.53,54,55 The biologically relevant properties of the CD97 antigen suggest that it may be an attractive candidate therapeutic target for GBM CAR T cell therapy. In this study, our approach focuses on the development of CD97-targeting CAR Th9 cells, a subset of CD4+ T cells characterized by their production of interleukin 9 (IL-9).56,57 Recent studies have indicated that Th9 cells possess the capability to exert potent cytolytic activity against tumor cells, exhibit reduced exhaustion, and maintain long-term persistence.56,58 First, we generated an anti-CD97 human antibody in a hybridoma and characterized the extracellular single-chain variable fragment (scFv) sequence of CD97 (Figure S16A). Next, we designed and created a second-generation lentiviral CD97-CAR construct with the aforementioned validated CD97-specific scFv fused with intracellular signaling domains, including 4-1BB and CD3ζ (Figure 7A). The CD97-CAR lacking the scFv sequences was used as a control (control CAR, Ctrl-CAR). Then, CD97-CAR was successfully transduced into Th9 cells with high IL-9 expression (Figure 7B) through the transduction efficiency detected by the GFP signal using flow cytometry and representative fluorescence imaging analyses (Figures 7C and S16B), and the CD97-CAR Th9 cells sustained expansion for 3 weeks (Figure 7D). The CD97-CAR Th9 cells exhibited significantly greater expansion capacity and cytotoxic activity compared to both CD97-CAR peripheral blood mononuclear cell and CD97-CAR CD8+ T cells (Figures S16C and S16D). Following transduction and expansion, we co-cultured CD97-CAR Th9 cells or Ctrl-CAR Th9 cells with GSCs (83-Luc and X01-Luc cells) and non-GSC U87-Luc cells at an effector-to-target (E:T) ratios of 1:1 to assess the functionality of CD97-CAR Th9 cells in vitro. CD97-CAR Th9 cells demonstrated robust cytotoxic activity against 83-Luc and X01-Luc GSCs, both of which exhibit high levels of CD97 expression (Figure 7E). Additionally, CD97-CAR Th9 cells or Ctrl-CAR Th9 cells were co-cultured with 83-Luc GSC at 2:1 and 5:1 E:T ratios to further evaluate the specific tumor-killing ability of CD97-CAR Th9 cells (Figure S17A). Since CD107a, a marker for degranulation of cytotoxic lymphocytes, is utilized to investigate CAR T cell activation and its triggering of cytotoxic effects,59 we examined surface CD107a expression on CAR Th9 cells co-cultured with GSCs via multi-parameter flow cytometry. The percentages of CD4+ CD107a+ T cells were increased to more than 40% in CD97-CAR Th9 cells compared to Ctrl-CAR Th9 cells with around 13%, demonstrating cytotoxicity and the degranulation capacity in vitro (Figure 7F). To further confirm the cytotoxicity and degranulation capacity of CD97-CAR Th9 cells, we assessed the expression of IFN-γ, a key marker of cytotoxic lymphocyte degranulation. The percentage of CD4+ IFN-γ+ Th9 cells was higher in CD97-CAR Th9 cells compared to Ctrl-CAR Th9 cells when cultured with 83-Luc and X01-Luc GSCs (Figure S17C). Moreover, when CD97-CAR Th9 cells or Ctrl-CAR Th9 cells were co-cultured with RFP lentivirus-transfected GSCs (83-Luc and X01-Luc GSCs) at an E:T ratios of 1:1 for 24 h, subsequent confocal analysis revealed that CD97-CAR Th9 cells exhibited a stronger tumor-killing ability compared to Ctrl-CAR Th9 cells (Figures 7G and S17B). Furthermore, the co-culture of 83-Luc and X01-Luc cells with CD97 knockdown mediated by shRNAs with CD97-CAR Th9 cells decreased CAR Th9 cell cytotoxic efficacy (Figure 7H). We further analyzed the CD97-CAR Th9 cytotoxic effect at different lower co-culture E:T ratios (0.0625:1, 0.125:1, 0.25:1, and 0.5:1); X01-Luc cells were selected as a high performer in terms of CD97-CAR antitumor activity (Figure 7I). Together, these data reveal robust, antigen-specific CD97-CAR Th9 cell responses upon contact with CD97+ GSCs.
Figure 7.
Antitumor activity of CD97-targeting CAR Th9 cells against GBM
(A) Schematic showing the structure of the second-generation lentiviral vectors encoding CD97-CAR, including the 4-1BB and CD3ζ costimulatory domains. TM, transmembrane domain.
(B) RT-qPCR analysis of IL-9 expression in CD8+ T cells, CD4+ T cells, and Th9 cells.
(C) Flow cytometry analysis of CAR expression detected by the fusion protein EGFP.
(D) Expansion kinetics of untreated, Ctrl-CAR, and CD97-CAR T cells in vitro (n = 3 independent experiments).
(E) Antigen-specific in vitro cytotoxicity of CD97-CAR Th9 cells or Ctrl-CAR Th9 cells toward U87-Luc, 83-Luc, or X01-Luc cells, evaluated using a luciferase activity assay.
(F) Flow cytometry analysis of CD4 and CD107a expression on CD97-CAR Th9 cells following incubation with 83-Luc and X01-Luc cells.
(G) Representative confocal microscopy images showing CD97-CAR Th9 cells or Ctrl-CAR Th9 cells interacting with 83-Luc and X01-Luc cells at an E:T ratio of 1:1 for 24 h. Scale bar, 50 μm.
(H and I) Antigen-specific in vitro cytotoxicity of CD97-CAR Th9 cells toward 83-Luc or X01-Luc cells infected with shCD97 or shCtrl lentivirus (H) and against X01-Luc cells at different E:T ratios (I).
(J) Schematic experimental design to evaluate the antitumor effects of CD97-targeting CAR Th9 cells. Mice were implanted with X01-Luc cells (n = 6, 1× 105 cells/mouse) and intracranially injected with CD97-CAR or Ctrl-CAR Th9 cells (1.5 × 106 cells/mouse) on day 4.
(K) Flow cytometry analysis showing the expression of CD4 and CD107a within the tumor two days post-treatment of CD97-CAR Th9 or Ctrl-CAR Th9 cells.
(L) Bioluminescence images of mice as in (J).
(M) Quantification of bioluminescence intensity of photons emitted from each tumor in the images in (L). Data are presented as mean ± SD, two-way ANOVA at day 24, ∗∗∗∗p < 0.0001.
(N) Kaplan-Meier survival curve of mice as in (J). MST, median survival time. Log rank (Mantel-Cox) test. GAPDH was used as a loading control.
All error bars represent mean ± SD (n = 3 independent experiments) in (B), (D), (E), (H), and (I). ∗∗∗∗p < 0.0001, ∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.05, t test. See also Figures S16–S18.
To further validate the CD97-targeting therapeutic strategy, we first assessed the efficacy of in vivo tumor-killing activity of CD97-CAR Th9 cells by intravenous injections using an orthotopic model of X01-Luc cells (Figures S17D–S17H). Compared to mice treated with untreated T cells, CD97-CAR T cells significantly increased the CD4⁺ CD107a⁺ T cell population at 2 and 7 days after injection, reduced tumor growth, reversed body weight loss, and improved the survival rate of the mice (Figures S17E–S17H). These findings highlight the potent in vivo tumor-killing efficacy of CD97-targeting therapy. Given the clinical studies have demonstrated that locally targeted CAR T cell therapy for GBM, when administered directly into the brain, effectively overcomes inter- and intra-patient heterogeneity and circumvents the blood-brain barrier, this method has proven to be safe and clinically effective in select patients.60,61 In light of these findings, we next investigated the in vivo tumor-killing activity of CD97-CAR Th9 cells through intracranial injections to further validate the efficacy of the CD97-targeting CAR Th9 cell therapeutic strategy. Mice orthotopically injected with X01-Luc were given intracranial injections with 1.5 × 106 CD97-CAR Th9 cells or Ctrl-CAR Th9 cells on day 4 (Figure 7J). We first examined surface CD107a expression on CD97-CAR Th9 cells in the tumor. The percentage of CD4+ CD107a+ T cells was increased to 59.26% after 2 days of intracranial injections of CD97-CAR Th9 cells (compared with 1.31% in Ctrl-CAR Th9 cell-injected group, Figure 7K) and remained at 48.36% until 7 days (Figure S17I), demonstrating cytotoxicity and the degranulation capacity in vivo. Continuing with the investigation, the CD97-CAR Th9 cells remarkably reduced tumor growth without losing body weight, as evidenced by bioluminescence measurements (Figures 7L, 7M, and S17J). Moreover, the CD97-CAR Th9 cells led to a substantial and significantly improved survival rate compared to that achieved with Ctrl-CAR Th9 cells (Figure 7N). To further validate the antitumor efficacy and potential toxicity of CD97-CAR T cells, we developed a murine version of the CD97 CAR. This version utilizes the same human CD97 scFv while substituting the human signaling molecules with murine CD28 and CD3ζ (mCD97-CAR), in accordance with the methodologies described in the referenced literature.62,63 Activated murine T cells were subsequently transduced with this vector to enable CAR expression. Although the transfection efficiency was only 11% (Figure S18A), the cytotoxic killing ability of mCD97-CAR T cells increased gradually as the E:T ratios in the co-culture with CD97-overexpressing murine syngeneic GBM cells (GL261-Luc-CD97) were raised (1:1, 5:1, and 10:1) (Figures S18A and S18B). Next, mice orthotopically injected with GL261-Luc-CD97 received intracranial injections with 1 × 106 mCD97-CAR T cells or untreated T cells on day 4 (Figure S18C). The results showed that treatment with mCD97-CAR T cells significantly reduced tumor growth, reversed body weight loss, and markedly improved the survival rate of mice compared to those treated with untreated T cells (Figures S18C–S18F). Collectively, our results highlight that CD97-specific targeting of CAR Th9 cells exerts potent antitumor effects on the GBM xenograft model and potentially represents a new therapeutic option for GBM.
Discussion
In the current study, we identified CD97 as an optimal GSC-enriched antigen that promotes GSC tumorigenicity via the mTORC2/AKT signaling axis, which increases ARHGAP1, BZW1, and BZW2 gene expression. Moreover, we developed CD97-targeting CAR Th9 cells that exerted a potent cytolytic effect and significantly prolonged the survival rate of a mouse xenograft model. Given the dire prognosis of GBM and the absence of effective therapies to treat GBM, CD97-CAR Th9 cell therapy may represent a promising option for patients with GBM.
GSC identification has been dependent on intracellular neural stem cell-associated proteins64 or the utilization of diverse surface antigens, employing combinations of double or multidimensional expression with varying levels of effectiveness.11,12,13,14,15,16,17,18,19,20,21 Intracellular markers of the GSC, including Nestin, Oct3/4, and Nanog, show high expression in neurosphere cultures and are required for the maintenance of GSC identity.42,64 However, intracellular neural stem cell-associated proteins were also expressed in non-GSC cells, suggesting that GSC-specific expression of these intracellular markers is not necessarily defined as GSC markers.65,66 We also analyzed the expression levels of the representative GSC surface antigens, including CD133, CD44, CD15, and CD49f. Here, our results revealed that CD133 is high (GSC772 cells, over 90%) or rarely expressed (83 and X01 cells, less than 1%) in GSCs, and also expressed in GBM cell lines (19%–24%). While CD44 is a mesenchymal-like GSC antigen with high specificity (83 cells, 99.7%), CD44 also has strong positivity in U87 cell lines (100%), making it difficult to categorize as a GSC surface antigen. The CD15-positive population was rare and represented less than 5.5% of our tested GSCs and GBM cell lines. Another stemness marker, CD49f, closely aligns with CD97. The population of CD97high CD49fhigh and CD97high CD49f−/low GSCs significantly induced the sphere formation ability compared to CD97−/low CD49fhigh and CD97−/low CD49f−/low GSCs. Notably, CD97high CD49fhigh GSCs exhibited greater self-renewal capacity than both CD97high CD49f−/low and CD97−/low CD49fhigh GSCs, suggesting that CD97 could be a more reliable stemness marker for GSCs than CD49f. Moreover, CD97 is expressed in 23%–100% of the GSCs we analyzed, and GBM cell lines U87 and U251 show almost no or lower expression. Overall, our study highlights the limitations of using conventional surface antigens in identifying GSCs and emphasizes the potential utility of CD97 as an enriched surface antigen in GSCs.
While abundant CD97 expression has been reported in GBM, including those associated with GSC,17,30,33,34,36,37 it remains largely unverified and limited mainly to in vitro studies.28,29,30,31,32,33,34,35,36,37 The GSCs they used have some limitations: CD133-positive glioma-initiating cells in screening procedure30 or adherent brain tumor-initiating cells for validation.33 Additionally, recent studies have demonstrated the different mechanisms of CD97 involved in gliomagenesis. One study has demonstrated that the interaction of CD97 and β-arrestin promoted glycolytic metabolism by the mitogen-activated protein kinase pathway and developed an anti-CD97 antibody-drug conjugate that exhibited only an in vitro killing effect36; this group advocated CD97 as a regulator of tumor metabolism in GBM. Although the role of two functional CD97 fragments in GBM proliferation and invasion via Gα12/13-mediated signaling, such as Rho pathway, are considered, the details remain unclear.37 In the current study, we discovered the CD97 mechanism in the maintenance and tumorigenesis of GSCs, offering an entirely different perspective. Our comparative transcriptome analysis also reveals that CD97 controlled GSCs features by activating mTORC2-AKT (S473) signaling. Another recent study showed that upregulated CD97 in GBM promotes GBM cell invasion through PI3-AKT signaling,35 but they validated using only a single type of GBM cell line (U251) in vitro and specifically the phosphorylated site (T308) implicated in mTORC1. Indeed, CD97 participates in both mTORC1 and mTORC2 signaling pathways, but cell-cell adhesion-related genes, such as ARHGAP1, BZW1, and BZW2, are regulated through the mTORC2 signaling pathway. The mTORC2 phosphorylates AGC kinases such as AKT, SGK, and PKC family members, each with known oncogenic roles in tumor cell survival50,51,52 and metastasis.66 Additionally, our findings suggest that downstream mTORC2-AKT signaling is involved in the maintenance of GSCs. Pharmacological inhibition of mTORC2 signaling with JR-AB2-011 significantly decreased GSC proliferation and self-renewal capacity and improved in vivo survival rate. Although our preclinical findings supported an important role in the CD97-dependent mTORC2 signaling pathway, it is still necessary to comprehensively analyze the safety profile of this inhibitor.
CD4+ Th9 cells appear to function in a broad range of autoimmune disorders, allergic inflammation, and cancer and have recently been in the spotlight.57,67 Several investigations have identified that CD4+ T cells also have direct antitumor activity, even though their role is broadly presumed to be as helper cells providing proinflammatory cytokines and chemokines.57,58,68,69,70 CD4+ T cells directly interacted with microglia, promoting IFN-γ-dependent microglia activation and phagocytosis in GBM,69 and orchestrated with tumor-associated myeloid cells, triggering remote inflammatory cell death that indirectly eradicates IFN-unresponsive and major histocompatibility complex-deficient tumors.70 Additionally, a recent study showed that the phenotype, proliferative capacity, and requirement for CD4+ T cells during CAR T cell production in vitro are important to increase persistence and mediate better tumor control in vivo.71 These more favorable outcomes with enhanced therapeutic efficacy are associated with increased persistence of CAR T cells and an increased memory T cell pool, resulting in increased proliferative capacity and long-term persistence in vivo. Unlike other T helper subsets, Th9 cells have unique properties because they can recruit monocytes and effectively lead to the clearance of tumor antigen loss variants through the induction of host immunity.58 Therefore, it could be proposed as a solution to overcome the challenges faced by CAR utilizing CD8+ cytotoxic effector T cells, including difficulties in impaired T cell trafficking and the immunosuppressive tumor microenvironment, leading to T cell exhaustion and poor expansion.72 These findings prompted us to develop CD97-targeting CAR Th9 cells and subsequently observe their antitumor effects.
As the precise contribution of CD97-targeting CAR Th9 cell-mediated cytotoxicity activity, we investigated CD107a, known as lysosomal-associated membrane protein-1, which has been described as a marker of immune cell degranulation.59 Degranulation serves as an essential prerequisite for perforin-granzyme-mediated killing and is a crucial component of the immediate lytic function exhibited by antigen-specific immune cells, including natural killer, CD8+, and CD4+ T cells.59,73 CD97-targeting CAR Th9 cells induced the increase and maintenance of CD4+CD107a+ cells 7 days after injection into the tumor. However, additional research is necessary to fully understand the underlying molecular mechanisms. These CAR Th9 cells might be delivered in multiple doses over time to titrate the desired effect with fewer side effects. The dose of cytotoxic CAR Th9 cells might also be titrated based on need, and further studies are needed.74,75
Indeed, the potential of CD97-CAR T cells to target cancer stem cells (CSCs) in a variety of tumor types is a promising area of research that merits exploration. CD97 is not only overexpressed in GBM but also in several other malignancies, including, but not limited to, breast,76 pancreas,24 and prostate cancers77 where CSCs play a pivotal role in tumor progression, metastasis, and therapy resistance. The utility of CD97-CAR T therapy in targeting CSCs across these diverse tumor types could revolutionize the approach to cancer treatment, offering a more promising and potentially curative strategy. This would be particularly relevant for aggressive and recurrent tumors, where conventional therapies fail to eliminate CSCs, leading to relapse and poor prognosis. Exploring the efficacy and specificity of CD97-CAR T cells in various cancer models could shed light on their potential as a versatile and powerful therapeutic tool against a broad spectrum of tumors.
Overall, this study suggests that CD97 serves as a functional surface antigen for GSCs. An important implication of these findings is that studies aimed at discovering therapeutic targets for GBM should not rely solely on identifying GSCs, and the targets must have functions that significantly contribute to self-renewal and tumorigenesis via CD97-related mTORC2 signaling. Moreover, CD97high GSCs are a conserved requirement for aggressive tumorigenic properties, thus underscoring the importance of CD97 as a potential therapeutic target. This study directly informs a clinical development strategy for CD97-targeting CAR Th9 cell therapy and reveals the strengths of targeting CD97. Thus, our study illustrates how CD97 might influence the biology of GSCs and offers a potential approach to targeted therapy for GBM.
Limitations of the study
While our study identifies CD97 as a promising target for GBM therapy, several limitations should be considered. First, the therapeutic efficacy and safety of CD97-CAR Th9 cells were demonstrated in vitro and in mouse models; thus, their effectiveness in humans remains uncertain. Second, although CD97 expression was consistent across the patient-derived GSCs we tested, GBM is a highly heterogeneous disease, and CD97 expression may vary among different patients. Third, the long-term impact of mTORC2 inhibition on GSCs and normal cells was not explored, nor were possible resistance mechanisms that could arise with treatment. Future studies should address these limitations by evaluating the clinical applicability and long-term effects of CD97-targeted therapies in diverse patient populations.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Jinlong Yin (jlyin@henu.edu.cn).
Materials availability
All materials and reagents used in this study are available from the lead contact upon request.
Data and code availability
-
•
RNA-seq data have been deposited at Gene Expression Omnibus and are publicly available as of the date of publication. Accession numbers are listed in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.
Acknowledgments
The authors would like to extend special thanks to Xun Jin (Tianjin Medical University Cancer Institute and Hospital) for providing GSC428 and to Rongjun Qian (Henan Provincial People’s Hospital) for supplying GBM patient samples. This work was supported by grants from the National Natural Science Foundation of China (82173228) and Project of Basic Research Fund of Henan Institute of Medical and Pharmacological Sciences (2024BP0206). Cartoons in Figures 1A, 5A, 6I, and 7A were created with BioRender.com.
Author contributions
J.Y. conceived the study and supervised the project with contributions from B.S. S.Z., W.L., R.N., Z.Y., Q.Z., M.G., S.S.K., Y.D., M.L., and S.I.C. performed the experiments and analyzed the results. S.Z., W.L., X.J., S.I.C., and J.Y. wrote the manuscript. X.J. performed the bioinformatics analysis. T.C. performed mCD97 CAR cloning. S.Z., W.L., X.J., J.B.P., S.I.C., B.S., and J.Y. discussed the data and reviewed the manuscript.
Declaration of interests
The authors declare no competing interests.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-CD97 antibody [EPR4427] ab108368 | Abcam | Cat# ab108368; RRID: AB_10865208 |
| Nestin | BD Biosciences | Cat# 611658; RRID: AB_399176 |
| OCT4/POU5F1 Polyclonal antibody | Proteintech | Cat# 11263-1-AP; RRID: AB_2167545 |
| Nanog | Sangon Biotech | Cat# D155241; RRID: AB_3661675 |
| Phospho-p70 S6 Kinase (Ser371) Antibody | Cell Signaling Technology | Cat# 9208; RRID: AB_330990 |
| Phospho-p70 S6 Kinase (Thr389) (108D2) Rabbit mAb | Cell Signaling Technology | Cat# 9234; RRID: AB_2269803 |
| p70 S6 Kinase (49D7) Rabbit mAb | Cell Signaling Technology | Cat# 2708; RRID: AB_390722 |
| Akt Antibody | Cell Signaling Technology | Cat# 9272; RRID: AB_329827 |
| p-Akt S473 | Cell Signaling Technology | Cat# 9271; RRID: AB_329825 |
| Phospho-Akt (Thr308) (C31E5E) Rabbit mAb | Cell Signaling Technology | Cat# 2965; RRID: AB_2255933 |
| Anti-ARHGAP1 Antibody (C-10) | Santa Cruz Biotechnology | Cat# sc-398671; RRID: AB_2924417 |
| CD133 antibody | Proteintech | Cat# 18470-1-AP; RRID: AB_2172859 |
| Human CD44s Pan Specific MAb (Clone 2C5) | R&D Systems | Cat# BBA10; RRID: AB_356933 |
| Human/Mouse/Rat SOX2 Antibody | R&D Systems | Cat# AF2018; RRID: AB_355110 |
| Anti-Vinculin antibody produced in rabbit | Sigma-Aldrich | Cat# V4139; RRID: AB_262053 |
| β-Actin (I102) polyclonal antibody | Bioworld Technology | Cat# AP0060; RRID: AB_2797445 |
| Anti-GAPDH rabbit polyclonal antibody | Sangon Biotech | Cat# D110016; RRID:AB_2904600 |
| FACS: PE anti-human CD97 | BioLegend | Cat# 336307; RRID: AB_1227612 |
| FACS: Nestin Monoclonal Antibody (196908), APC | Thermo Fisher Scientific | Cat# MA5-23650; RRID: AB_2608686 |
| FACS: Alexa Fluor(R) 647 anti-OCT4 (OCT3) | BioLegend | Cat# 653710; RRID: AB_2566689 |
| FACS: Alexa Fluor(R) 647 anti-Nanog | BioLegend | Cat# 674010; RRID: AB_2632605 |
| FACS: APC anti-human CD133 | BioLegend | Cat# 397905; RRID: AB_2876721 |
| FACS: Alexa Fluor(R) 488 anti-human CD44 | BioLegend | Cat# 397507; RRID: AB_2876719 |
| FACS: Alexa Fluor(R) 488 anti-human CD15 (SSEA-1) | BioLegend | Cat# 301910; RRID: AB_493257 |
| FACS: APC anti-human/mouse CD49f | BioLegend | Cat# 313615; RRID: AB_1575047 |
| FACS: BD Pharmingen™ APC Mouse Anti-Human IFN-γ | BD Biosciences | Cat# 554702; RRID: AB_398580 |
| FACS: Mouse Anti-Human CD3 Monoclonal Antibody | BD Biosciences | Cat# 560176; RRID: AB_1645475 |
| FACS: PerCP/Cyanine5.5 anti-human CD4 | BioLegend | Cat# 317427; RRID: AB_1186124 |
| FACS: PE anti-human CD107a (LAMP-1) | BioLegend | Cat# 328607; RRID: AB_1186062 |
| BZW1 antibody | GeneTex | Cat# GTX120426; RRID: AB_2885492 |
| BZW2 antibody | Bethyl Laboratoriess | Cat# A304-608A-T; RRID: AB_3661728 |
| Alexa Fluor® 488-anti-rabbit IgG | Thermo Fisher Scientific | Cat# A-11001; RRID: AB_2534069 |
| Alexa Fluor® 568 anti-mouse IgG | Thermo Fisher Scientific | Cat# A10042; RRID: AB_2534017 |
| Bacterial and virus strains | ||
| Trelief ® 5 Chemically Competent Cell | Tsingke Biotech | Cat# TSC-C01 |
| pLKO.1-puro | Bob Weinberg Lab Materials | Addgene plasmid #8453 |
| LentiCRISPRv2GFP | Walter et al.78 | Addgene plasmid #82416 |
| Biological samples | ||
| Human peripheral blood mononuclear cells (PBMCs) | Healthy donors | N/A |
| GBM specimens | Henan Provincial People’s Hospital (Henan, China, #2020-107) | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| B27 supplement | Invitrogen | Cat# 17504044 |
| Recombinant Human EGF Protein | R&D Systems | Cat# 236-EG |
| Recombinant Human FGF basic/FGF2/bFGF | R&D Systems | Cat# 4114-TC |
| containing Torin1 | SelleckChem | Cat# S2827 |
| Akt inhibitor IV | CruzCredit | Cat# sc-394003 |
| Rapamycin | SelleckChem | Cat# S1039 |
| JR-AB2-011 | Targetmol | Cat# T11728 |
| Purified NA/LE Mouse Anti-Human CD3 | BD Biosciences | Cat# 555336; RRID: AB_395742 |
| Purified NA/LE Mouse Anti-Human CD28 | BD Biosciences | Cat# 555725; RRID: AB_396068 |
| InVivoMAb anti-human-IFN- γ | Bio X Cell | Cat# BE0235; RRID: AB_2687717 |
| Purified NA/LE Hamster Anti-Mouse CD3e Clone 145-2C11 | BD Biosciences | Cat# 553057; RRID: AB_394590 |
| Purified anti-mouse CD28 | BioLegend | Cat# 102102; RRID: AB_312867 |
| Recombinant Human IL-4 | Novoprotein | Cat# CX03 |
| Recombinant Human TGF-beta 1 | Novoprotein | Cat# CA59 |
| Recombinant Human IL-2 | Novoprotein | Cat# C013 |
| Human IL-2 Recombinant Protein | Peprotech | Cat# 200-02 |
| 4′,6-diamidino-2-phenylindole (DAPI, Thermo-Fisher Scientific) | Solarbio | Cat# C0065 |
| Accutase | MPBio | Cat# 091000449 |
| Trypsin-EDTA (0.25%) | Gibco | Cat# A11105-01 |
| Penicilin-Streptomycin Solutions (100X) | WELGENE | Cat# LS202-02 |
| Lipofectamine 2000 | Invitrogene | Cat# 11668-019 |
| Polybrene | Sigma | Cat# TR-1003 |
| Paraformaldehyde (PFA) | Biosesang | Cat# PC2031 |
| Triton X-100 | Bio Basic | Cat# BT0198 |
| RIPA buffer | Solarbio | Cat# R0020 |
| skim milk | Epizyme | Cat# PS112L |
| PEG300 | Targetmol | Cat# T7022 |
| Dimethyl sulfoxide DMSO (cell culture grade) | Biosharp | Cat# BL165B |
| Tween-80 | Sangon Biotech | Cat# A100442-0500 |
| DMEM/F-12 | WELGENE | Cat# LM-002-04 |
| RPMI-1640 Medium | ATCC | Cat# 30-2001 |
| Penicillin-streptomycin (10,000 U/mL) | Gibco | Cat# 15140122 |
| NEAA Cell Culture Additive (100×) | Oricellbio | Cat# NEAA-10201-100 |
| High Glucose DMEM | Hyclone | Cat# SH30243.01 |
| FBS | Hyclone | Cat# SH30243.03 |
| Ficoll-Paque plus | GE Healthcare | Cat# 17144003 |
| Celltracker CM-DiI dye | Yeasen | Cat# 40718ES50 |
| Critical commercial assays | ||
| EasySep™ Human CD4+ T cell Isolation Kit | STEMCELL | Cat# 17952 |
| EasySep™ Mouse T cell Isolation Kit | STEMCELL | Cat# 19851 |
| Fluorokinase Reporter Gene Assay Kit | Beyotime | Cat# RG005 |
| Lenti-X concentrator | Clontech (TaKaRa) | Cat# 631232 |
| Rneasy Mini Kit | QIAGENE | Cat# 74104 |
| AmpiGene cDNA Synthesis Kit | Enzo | Cat# ENZ-KIT 106 |
| AmpiGene qPCR Green Mix Hi-ROX | Enzo | Cat# ENZ-NUC104-1000 |
| Annexin V-FITC Apoptosis Assay Kit | Beyotime | Cat# C1062S |
| CCK-8 Cell Proliferation and Cytotoxicity Assay Kit | Solarbio | Cat# CA1210 |
| Deposited data | ||
| Bulk RNA-Seq data in this paper | Gene Expression Omnibus | GSE201960 |
| Experimental models: cell lines | ||
| Human: 83 GSC | Weiwei et al.79 | N/A |
| Human: X01 GSC | Weiwei et al.79 | N/A |
| Human: 528 GSC | Jinlong et al.80 | N/A |
| Human: 131 GSC | Weiwei et al.79 | N/A |
| Human: 448 GSC | Weiwei et al.79 | N/A |
| Human: GSC211 | This paper | N/A |
| Human: GSC924 | This paper | N/A |
| Human: GSC028 | This paper | N/A |
| Human: GSC428 | This paper | N/A |
| Human: GSC772 | Weiwei et al.79 | N/A |
| Human: HA1800 | ScienCell Research Laboratories | Cat#1800 |
| Human: U87 | ATCC | Cat# HTB-14™ |
| Human: U251 | Cellosaurus | Cat# CVCL_0021 |
| Human: 293T | ATCC | Cat# CRL-3216™ |
| Mouse: GL261 | Neuros Creative Biolabs | CAT#: NCL-2108P28 |
| Human: Th9 cells | This paper | N/A |
| Mouse: CD3+T cells | This paper | N/A |
| Experimental models: organisms/strains | ||
| BALB/c nude mice | SPF (Beijing) biotechnology Co. Ltd. | N/A |
| NTG mice | SPF (Beijing) biotechnology Co. Ltd. | N/A |
| C57BL/6J mice | SPF (Beijing) biotechnology Co. Ltd. | N/A |
| Oligonucleotides | ||
| shRNA targeting sequence: shCtrl, CCTAAGGTTAAGTCGCCCTCG | Merck | N/A |
| shRNA targeting sequence: shCD97-1, GCCGAACTGGAGGAGATATAT | Merck | TRC Clone ID: TRCN0000356764 |
| shRNA targeting sequence: shCD97-3, GCGATCCTTATGGCTCATTAT | Merck | TRC Clone ID: TRCN0000008235 |
| PCR amplified oligomers: BZW1, sense ATAGAAGATTCTAGAGCTAGCATGAATAA TCAAAAGCAGCAAAAGC |
Tsingke | N/A |
| PCR amplified oligomers: BZW2, sense CGGCTAGCCGATGGATCCGCTCTCAGAGCT | Tsingke | N/A |
| PCR amplified oligomers: ARHGAP1, sense GGCTAGCCATGAATAAGCATCAGAAGCC | Tsingke | N/A |
| PCR amplified oligomers: CD97, sense GCTAGCCATGGGAGGCCGCGTCTTTCT | Tsingke | N/A |
| sgRNA targeting sequence: sgCtrl, CGAATATTATTTCTATCGGG | Tsingke | N/A |
| sgRNA targeting sequence: sgCD97-1, CCTTACCTGGATGGTGACCT | Tsingke | N/A |
| sgRNA targeting sequence: sgCD97-2, CTCCAAGACAAGCTCAGCCG | Tsingke | N/A |
| Primers for CD97, PCBP1, BSG, ARHGAP1, BZW1, BZW2, DBN1, β-actin, Nestin, CD133, GAPDH, and CD44 see Table S1 | Tsingke | N/A |
| Recombinant DNA | ||
| pLKO-shCtrl | This paper | N/A |
| pLKO-shCD97-1 | This paper | N/A |
| pLKO-shCD97-3 | This paper | N/A |
| pCDH-EF1-copGFP-T2A-Puro | Addgene Kazuhiro Oka Lab Materials | Addgene plasmid #72263 |
| pCDH-GFP-CD97 | This paper | N/A |
| pCDH-GFP-BZW1 | This paper | N/A |
| pCDH-GFP-BZW2 | This paper | N/A |
| pCDH-GFP-ARHGAP1 | This paper | N/A |
| pCDH-GFP-hCD97 CAR | This paper | N/A |
| pCDH-GFP-mCD97 CAR | This paper | N/A |
| LentiCRISPRv2GFP-sgCtrl | This paper | N/A |
| LentiCRISPRv2GFP-SgCD97-1 | This paper | N/A |
| LentiCRISPRv2GFP-SgCD97-2 | This paper | N/A |
| Software and algorithms | ||
| Harmony™ software | PerkinElmer Inc. | https://www.perkinelmer.com.cn/ |
| LightCycler 480 software | Roche | https://lifescience.roche.com/ |
| FlowJo software v10.4 | FlowJo | https://www.flowjo.com |
| Microsoft Excel | Microsoft | https://www.microsoft.com/zhcn/microsoft-365/microsoft-office |
| GraphPad PRISM v8 software | Graphpad | https://www.graphpad.com/ |
| Extreme Limiting Dilution Analysis (ELDA) software | WEHI | http://bioinf.wehi.edu.au/software/elda/ |
| Para Vision v5.1 software | Bruker | https://www.bruker.com/zh.html |
| Living Image software | Caliper Life Sciences | www.caliperls.com |
| FastQC (v0.11.7) | Babraham Bioinformatics | https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ |
| Bowtie2 aligner (v2.3.4.1) | Bowtie 2 | https://bowtie-bio.sourceforge.net/bowtie2/index.shtml |
| StringTie (v2.1.3b) | Center For Computational Biology | https://ccb.jhu.edu/software/stringtie/ |
| LPEseq package (v0.99.5) | BioInformatics and BioStatistics | http://statgen.snu.ac.kr/2024/03/06/lpeseq/ |
| DAVID (v6.8) | National Institutes of Health | https://david.ncifcrf.gov/tools.jsp |
| GenePattern | GenePattern | https://www.genepattern.org/ |
| ZEN Microscopy Software | Zeiss | https://www.zeiss.com.cn/ |
| Adobe Photoshop | Adobe | https://www.adobe.com/cn/products/photoshop.html |
| Social Sciences software (v16) | IBM SPSS Statistics | https://www.ibm.com/products/spss-statistics |
| CytExpert software v1.0 | Beckman | https://www.beckman.com/flow-cytometry/research-flow-cytometers/cytoflex/software |
| Other | ||
| PVDF membranes | Millipore | Cat# IPVH00010 |
| Amersham ECL Prime western blotting detection reagent | Cytiva Lifescience | Cat# RPN2232 |
| 96-well plates | Falcon® | Cat# 3300 |
| 96-well black plates | LABSELECT | Cat# 31112 |
| BD LypoplateTM Screening Panels | BD Biosciences | Cat# 560747 |
Experimental model and subject details
Cell lines and cell culture
83, X01, 528, 131, 448, and GSC772 GSCs were obtained as described in our previous reports.79,80 GSC428 was generously provided by Professor Xun Jin of the Tianjin Medical University Cancer Institute and Hospital. GSC211, GSC924, and GSC028 GSCs were newly isolated using Patel’s method.81 GBM specimens were collected from patients after obtaining informed written consent and approval from the Ethics Committees at Henan Provincial People’s Hospital (Henan, China, #2020-107). All GSCs were authenticated by STR testing and maintained in DMEM/F-12 (Gibco) supplemented with B27 (Invitrogen), EGF (10 ng/mL, R&D Systems), and bFGF (5 ng/mL for X01, 448, 131, and 83; 10 ng/mL for GSC772). Human HA1800 astrocytes were purchased from ScienCell Research Laboratory. U87 and 293T cells were purchased from the American Type Culture Collection (ATCC). U251 cells were purchased from Cellosaurus and GL261 cells were obtained from Neuros Creative Biolabs. The cells were maintained in high-glucose DMEM (HyClone) supplemented with 10% fetal bovine serum (FBS; HyClone), 1% Penicillin-Streptomycin (P/S; Gibco), and 1% NEAA Cell Culture Additive (Oricellbio). X01-Luc, 83-Luc, GL261-Luc, and U87-Luc cells were transduced with a luciferase-expressing vector for in vivo monitoring and coculture experiments with CAR T cells. All cell cultures were incubated at 37°C in a humidified incubator with 5% CO2 and repeatedly screened for mycoplasma.
In vivo animal model
All animal experiments were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee of the National Cancer Center, Republic of Korea, and the Animal Care and Use Committee of Laboratory Animal Center, Henan University (HUSOM2020-018). BALB/c nude mice were purchased from Orient Bio (Korea) and SiPeiFu (SPF) (Beijing) Biotechnology Co., Ltd. (China)], and NTG mice were purchased from SiPeiFu (SPF) (Beijing) Biotechnology Co., Ltd. The animals were group-housed in ventilated cages under controlled temperature and humidity with a 12 h light-dark cycle. Every animal was randomized by body weight before the experiments. For the orthotopic mouse model,79,80 GSCs were first resuspended in DMEM/F-12 supplemented with B27, EGF (10 ng/mL), and bFGF (5 ng/mL), and then transplanted into the left striatum of BALB/c nude mice by stereotactic injection. The injection coordinates were 2.2 mm to the left of the midline and 0.2 mm posterior to the bregma at a depth of 3.5 mm. The brain of each mouse was harvested and fixed with 4% PFA. All mice used in this study were 5-week-old female mice, assigned to groups randomly. Female animals were involved, and sex was not considered as a biological variable.
Human GBM tissue and healthy blood samples
Peripheral blood mononuclear cells (PBMCs) were isolated from fresh blood samples of healthy donors (n = 3), and GBM specimens for GSCs isolation were collected from patients after obtaining informed written consent and approval from the Ethics Committees at Henan Provincial People’s Hospital (Henan, China, #2020-107).
Method details
Screening of cell-surface markers
Cell surface markers on GSCs were screened using a panel of monoclonal antibodies against human cell surface markers (BD Lypoplate Screening Panels, BD Biosciences). Astrocytes, 83, and X01 cells (5 × 103 cells) were plated in Falcon 96-well imaging plates (GSCs were seeded into wells that had been precoated with laminin overnight). The medium was changed the next day, as indicated in the cell culture method (X01-Diff was changed with 10% FBS containing GSC media), followed by 3 days of culture. The cells were then incubated with primary and secondary antibodies with intermediate washing steps according to the manufacturer’s instructions. Fluorescence images were obtained and analyzed using an Operetta CLS microscope (PerkinElmer Inc.) and Harmony software (PerkinElmer Inc.).
Lentivirus production and infection
All shRNA vectors to deplete endogenous human CD97 were purchased from Merck. To generate the lentiviral shRNA construct against human CD97, the following sequences were cloned into the pLKO-lentiviral vectors (shCtrl, 5′-CCTAAGGTTAAGTCGCCCTCG-3’; shCD97-1, 5′-GCCGAACTGGAGGAGATATAT-3’; shCD97-3, 5′-GCGATCCTTATGGCTCATTAT-3′). The lentiviral sgRNA construct against human CD97, the following sequences were cloned into the LentiCRISPRv2GFP78 vectors (sgCtrl, 5′-CGAATATTATTTCTATCGGG-3’; sgCD97-1, 5′-CCTTACCTGGATGGTGACCT-3’; sgCD97-2, 5′-CTCCAAGACAAGCTCAGCCG-3). For the generation of the pCDH-GFP-BZW1 and pCDH-GFP-CD97 constructs for the lentiviral transduction, pCDH-GFP (MiaolingBio) was used, and PCR was performed with the following oligomers: PCR amplified oligomers were as follows: BZW1, sense 5′- ATAGAAGATTCTAGAGCTAGCATGAATAATCAAAAGCAGCAAAAGC-3′ and antisense 5′-GATCGCAGATCCTTCGCGGCCGCTCAGTCACCTTCTTCAGCTTCAGA-3′, BZW2, sense 5′-CGGCTAGCCGATGGATCCGCTCTCAGAGCT-3′ and antisense 5′- GCGGCCGCAGGTTCAGAGCCCGCT-3′, ARHGAP1, sense 5′-GGCTAGCCATGAATAAGCATCAGAAGCC-3′ and antisense 5′- GCGGCCGCTTAATTTTCCTCACCTTCCG-3′, CD97, sense 5′-GCTAGCCATGGGAGGCCGCGTCTTTCT-3′ and antisense 5′- TTGCGGCCGCAAAGCGTAATCTGGAACATCGTATGGGTATCATATGCCGGACTCTGATG-3’. All oligomers were purchased from Tsingke. All constructs were verified by DNA sequencing (Tsingke). Lentiviruses were produced as previously reported.80,82 Briefly, 3.5 × 106 293T cells were plated on 100 mm culture dishes and incubated for 24 h before transfection. Then, 4.5 μg of lentiviral constructs (pLKO-shCtrl, pLKO-shCD97-1, pLKO-shCD97-3, pCDH-GFP-BZW1, and pCDH-GFP-CD97), 3 μg of psPAX2 (Addgene), and 1.5 μg of pMD2.G (Addgene) were cotransfected with 27 μL of Lipofectamine 2000 (Invitrogen). The medium was changed 6 h after transfection, and the medium containing lentivirus was harvested at 48 h after transfection. The viral particles were concentrated and purified using a Lenti-X concentrator (Clontech Laboratory). Cells were infected with the lentivirus in the presence of 6 μg/mL polybrene.
Quantitative reverse transcription-polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using a RNeasy Mini Kit (QIAGEN, MD, USA) according to the manufacturer’s instructions. Total RNA (1 μg) was used as a template to synthesize cDNAs using an Ampigene cDNA Synthesis Kit (Enzo Biochem). RT-qPCR analysis was performed with a Roche Light Cycler 480 Instrument using Ampigene qPCR Green Mix Hi-ROX (Enzo Biochem). The results were analyzed with LightCycler 480 software (Roche). The expression levels of the target genes were normalized to that of β-actin or Vinculin. The following PCR primers were used: CD97 (sense 5′-AGAGCGAGAACACCTGTCAA-3′ and antisense 5′-TCCACATCTGTGCAGACCTT-3′), PCBP1 (sense 5′-CGGCTTCTTATGCACGGAAA-3′ and antisense 5′- TTCCTCCAGCTTGTCGATGA-3′), BSG (sense 5′-AGTACTCCTGCGTCTTCCTC-3′ and antisense 5′-GACGACTTCACAGCCTTCAC-3′), ARHGAP1 (sense 5′-TACCAGGTGCTTCGTTTCCT-3′ and antisense 5′-TGGTGAAGGTGTTGATGGGA-3′), BZW1 (sense 5′-AACAGATGTCCCGTGGTGAT-3′ and antisense 5′-TTGCTCTGCTACAAGCTCCT-3′), BZW2 (sense 5′-TGCCAACTCTGTTACCTCGT-3′ and antisense 5′-AAGCTCCTTAAGACCTGCGT-3′), DBN1 (sense 5′-ATGAGAGGCTCAGGTTCGAG-3′ and antisense 5′-TCATCCCGATGGTCACCAAA-3′), β-actin (sense 5′-GAGGCACTCTTCCAGCCTTC-3′ and antisense 5′-GGATGTCCACGTCACACTTC -3′), GAPDH (sense 5′-GGAGTCCACTGGCGTCTTCAC-3′ and antisense 5′-GAGGCATTGCTGATGATCTTGAGG-3′), Nestin (sense 5′-AAGTCTGCGGGACAAGAGAA-3′ and antisense 5′-TCCACAGACTCCAGTGGTTC-3′), CD133 (sense 5′-GAAAGTGGCATCGTGCAAAC-3′ and antisense 5′-GTACACGTCCTCCGAATCCA-3′), and CD44 (sense 5′-AGCACCATTTCAACCACACC-3′ and antisense 5′-CGGATTTGAATGGCTTGGGT-3′).
Immunoblot analysis (IB)
Proteins were extracted with RIPA buffer supplemented with complete protease inhibitors (Solarbio), separated by electrophoresis, transferred to PVDF membranes (Bio-Rad), and blocked with 5% skim milk (Epizyme Biomedical Technology Co., Ltd.). The membranes were incubated with primary antibodies against CD97 (Abcam), Nestin (BD Transduction Laboratories), Oct3/4 (Proteintech), Nanog (Sangon biotech), p-S6K S371 (Cell Signaling Technology), p-S6K T389 (Cell Signaling Technology), S6K (Cell Signaling Technology), p-AKT T308 (Cell Signaling Technology), p-AKT S473 (Cell Signaling Technology), AKT (Cell Signaling Technology), ARHGAP1 (Santa Cruz), BZW1 (GeneTex), BZW2 (Bethyl Laboratories), CD133 (Proteintech), CD44 (R&D Systems), SOX2 (R&D Systems), Vinculin (Sigma-Aldrich), and β-actin (Bioworld Technology) overnight at 4°C. The immunoreactive bands were visualized using peroxidase-labeled affinity purified secondary antibodies (KPL) and the Amersham ECL Prime western blotting detection reagent (Cytiva Lifescience).
Flow cytometry
Cells were isolated with Accutase (for GSCs, Sigma-Aldrich) or 0.25% trypsin-EDTA (for adherent cells, Gibco) and washed once with PBS. The cells were stained with anti-human CD97 (PE, BioLegend), anti-human Nestin (APC, Thermofisher), anti-human Oct3/4 (Alexa Fluor 647, BioLegend), anti-human Nanog (Alexa Fluor 647, BioLegend), anti-human CD133 (APC, BioLegend), anti-human CD44 (Alexa Fluor 488, BioLegend), and anti-human CD15 (Alexa Fluor 488, BioLegend) antibodies at 4°C for 20 min. Anti-human/mouse CD49f (APC, BioLegend) and anti-human CD97 (PE, BioLegend) antibodies were added before CD97high GSC sorting. For each cell line, a corresponding negative control was utilized at the time of sample collection. For experiments to detect cytotoxicity, anti-human CD3 (APC, BD Biosciences), anti-human CD4 (PerCP/Cyanine5.5, BioLegend), INF-ϒ (APC, BD Biosciences) or anti-human CD107a (PE, BioLegend) antibodies at 4°C for 20 min. Data were acquired using a BD FACSCalibur and BD LSRFortessa. The cells were sorted using a BD FACSAria III SORP cell sorter. The data were analyzed using FlowJo software v10.4.
PI staining and annexin V staining
Cellular apoptosis was assessed using propidium iodide (PI, BD) staining and Annexin V (Themo Fisher) staining. The cultured GSCs were washed with phosphate-buffered saline (PBS), and resuspended in binding buffer. Subsequently, cells were stained with Annexin V-FITC and PI according to the manufacturer’s protocol. The stained cells were then analyzed using flow cytometry.
Cell proliferation assays
GSCs were plated at a density of 1 × 103 cells/well in 96-well plates containing DMEM/F-12 supplemented with B27, EGF (10 ng/mL), and bFGF (5 ng/mL) for in vitro proliferation assays. The luminescence of viable cells was detected using a CCK-8 Cell Proliferation and Cytotoxicity Assay Kit according to the manufacturer’s protocol. The CCK-8 Cell Proliferation and Cytotoxicity Assay is a homogeneous method for determining the number of viable cells in culture based on the quantitation of the amount of dehydrogenase in the mitochondria of viable cells present, which indicates the presence of metabolically active cells. The luminescence signal was detected using a SpectraMax Microplate Reader (Molecular Device) according to the manufacturer’s protocol. The results were analyzed and visualized using Microsoft Excel 2016 and GraphPad PRISM v8 software.
LDAs
For in vitro LDAs, GSCs were plated at decreasing densities (100, 50, 25, and 5 cells/well) in 96-well plates containing DMEM/F-12 supplemented with B27, EGF (10 ng/mL), and bFGF (5 ng/mL). The LDAs results were processed after 7 to 10 days using Extreme Limiting Dilution Analysis (ELDA) software, which is available at http://bioinf.wehi.edu.au/software/elda/.
Inhibitor treatment
GSCs were plated at a density of 6 × 105 cells/well densities in 60 mm culture dishes and incubated with DMEM/F-12 supplemented with B27, EGF (10 ng/mL), and bFGF (5 ng/mL) containing Torin1 (50 and 100 nM, for 48 h; SelleckChem, Houston, TX, USA), AKT inhibitor IV (10 and 20 μM, for 24 h; Cruz Credit, Santa Cruz, CA, USA), Rapamycin (5 and 10 nM, for 24 h; SelleckChem), and JR-AB2-011 (10 and 20 μM, for 48 h; MedChemExpress, Monmouth Junction, NJ, USA). GSCs were collected, and IB was performed as described above.
GSCs were plated in 96-well plates at 103 cells/well densities for in vitro proliferation assays and at decreasing densities (100, 50, 25, and 5 cells/well) for LDAs. The GSCs were incubated with Torin1 (50 and 100 nM, for 4 days), AKT inhibitor IV (10 and 20 μM, for 3 days), Rapamycin (5 and 10 nM, for 4 days), and JR-AB2-011 (10 and 20 μM, for 6 days) and analyzed as described above.
Mice models
To observe the characteristics of CD97 in vivo, sorted CD97high and CD97-/low GSC772 cells (1 × 105) were orthotopically injected into the brains of BALB/c nude mice, as mentioned above. Animals were monitored by using magnetic resonance imaging (MRI). All MRI examinations were performed using a high-field 7.0 Tesla Bruker BioSpec 70/20 USR (Bruker) and analyzed using Para Vision v5.1 software (Bruker) as described in a previous report.79
To examine the pharmacological effects of JR-AB2-011 in vivo, X01-Luc cells (1 × 105) were orthotopically injected into the brain of BALB/c nude mice, as mentioned above. Thirteen days after intracranial tumor injection, the mice were daily intraperitoneally (i.p.) injected with JR-AB2-011 (4 mg/kg) or vehicle (45% PEG-300, TOP SCIENCE; 5% DMSO, Sigma-Aldrich; and 5% Tween-80, Sangon Biotech in PBS) for 10 days.
To assess CD97-CAR Th9 cell antitumor activity in vivo, X01-Luc cells (1 × 105) were orthotopically injected into the brains of NTG mice, as mentioned above. On day 4 after the intracranial tumor injection, CD97-CAR Th9 or Ctrl-CAR Th9 cells (1.5 × 106) in 8 μL total volume were stereotactically injected into the brain of mice using the following co-ordinates: the injection coordinates were 2.2 mm to the left of the midline and 0.2 mm posterior to the bregma at a depth of 3.5 mm. In addition, treatment with tail intravenous administration of CD97-CAR Th9 or Ctrl-CAR Th9 cells was administered intravenously on days 4 and 17 after intracranial tumor injection (1× 107).
The mice were monitored by detecting bioluminescence using the IVIS Lumina XRMS Series III system (PerkinElmer). The photons emitted from the luciferase-expressing tumor cells were quantified using Living Image software (Caliper Life Sciences). A pseudocolor image representing light intensity (blue = least intense and red = most intense) was generated and superimposed over the grayscale reference image. The animals were imaged twice weekly beginning a week after the injection. Mice were euthanized when they met euthanasia criteria (neurological deficits, 20% weight loss, and signs of distress). Survival was analyzed using GraphPad PRISM v8 software.
Immunofluorescence (IF) staining
For histological observations, the brains were removed, fixed with 4% paraformaldehyde for 24 h at 4°C, sectioned at a thickness of 4 μm using an essential microtome (Leica RM2125 RTS), and then subjected to an antigen retrieval process using citrate buffer (pH 6.0). Sections were immunostained overnight at 4°C in a humidified chamber with primary antibodies against CD97 (Abcam), Nestin (BD Transduction Laboratories), ARHGAP1 (Santa Cruz), BZW1 (GeneTex), and BZW2 (Bethyl Laboratories) that had been diluted to the working concentration with antibody diluent buffer (IHC World). Immunoreactive proteins were visualized with the appropriate fluorescent secondary antibodies [Alexa Fluor 488-anti-rabbit IgG (Thermo Fisher Scientific); Alexa Fluor 568 anti-mouse IgG (Thermo Fisher Scientific)]. The nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, Thermo-Fisher Scientific), and fluorescence images were captured using a Zeiss LSM 780 Confocal Laser Scanning Microscope (Carl Zeiss).
Bioinformatics analysis
The mRNA expression of CD97 and other stemness markers in Restall40 and Rechards41 scRNA-Seq cohorts were analyzed using Single Cell Portal (https://singlecell.broadinstitute.org/single_cell).
For the analysis of mRNA expression and survival prognosis, CD97, PCBP1, BSG, ARHGAP1, BZW2, BZW1, and DBN1 mRNA expression and clinical phenotypes were obtained from GBM microarray data in TCGA83,84 (UCSC Xena portal, https://xena.ucsc.edu/public), CGGA85 and REMBRANDT86 (CGGA portal, http://www.cgga.org.cn) databases. All statistical analyses, evaluations of gene expression, and Kaplan‒Meier estimations were performed using GraphPad PRISM v8 software.
Analysis of RNA-sequencing data
RNA-seq libraries were prepared using a TruSeq Stranded mRNA LT Sample Prep kit (Illumina) and were sent for transcriptome resequencing. The phred quality score of the obtained raw FASTQ files was checked using FastQC (v0.11.7). The sequences were mapped onto the GRCh37 human genome using Bowtie2 aligner (v2.3.4.1) and further assembled potential transcript using StringTie (v2.1.3b). For the identification of differentially expressed genes (DEGs), read counts were normalized and quantified using the LPEseq package (v0.99.5), which is designed for nonreplicated samples. Genes with statistical significance (p value and Q value with <0.01) and a fold change ±2.0 were chosen as significant DEGs. Gene Ontology (GO) analysis of downregulated genes in CD97 knockdown GSCs was performed using DAVID (v6.8). GSEA (v4.2) of the hallmark genes was performed using GenePattern.
CAR T cell manufacturing and transduction
PBMCs were isolated from healthy donors, separated by density gradient centrifugation (Ficoll-Paque plus; GE Healthcare), and then cultured with RPMI-1640 (ATCC) supplemented with 10% FBS (HyClone) and 1% P/S (Gibco). CD4+ T cells were prepared by purifying CD4+ T cells from PBMCs using an EasySep Human CD4+ T cell Isolation Kit (STEMCELL Technologies Inc.), followed by activation and polarization into Th9 cells. T cells were activated using two factors: CD3 (1 μg/mL) (BD Biosciences) and CD28 (2 μg/mL) (BD Biosciences). The Th9-polarizing medium was RPMI-1640 medium supplemented with hIL-4 (10 ng/mL, Novoprotein Scientific Inc.), hTGF-β1 (1 ng/mL, Novoprotein Scientific Inc.), and InVivoMAb anti-human-IFN- γ (10 μg/mL, BioXcell) and was incubated with cells for 5 days. The polarized T cell population underwent lentiviral transduction to express a second-generation anti-CD97 CAR and Ctrl vector. Blood samples were collected from healthy donors after obtaining informed written consent and approval from the Ethics Committees at Henan University (HUSOM2020-018).
Generation of murine CAR-T cells
Murine CD3+ T cells were purified from C57BL/6J mice splenocytes using the EasySep Mouse T cell Isolation Kit (STEMCELL Technologies Inc.) and stimulated on plates coated with 2 μg/mL mCD3ε (BD Pharmingen) and 2 μg/mL mCD28 mAbs (Biolegend) for 48 h. The stimulated T cell population underwent lentiviral transduction to express a second-generation anti-CD97 CAR T cells were expanded in complete medium RPMI-1640 (ATCC), 10% FBS (Hyclone), 1% Penicillin-Streptomycin (Gibco) with hIL-2 (10 ng/mL; PeproTech.) changing medium every 2 days. On days 6–7, T cells were collected and used for functional assays in vitro and in vivo.
To assess CD97-CAR T cell antitumor activity in vivo, Gl261-Luc-CD97 expression cells (1 × 105) were orthotopically injected into the brains of C57BL/6J mice, as mentioned above. On day 4 after the intracranial tumor injection, CD97-CAR T or Untreated T cells (1 × 106) in 8 μL total volume were stereotactically injected into the brain of mice using the following co-ordinates: the injection coordinates were 2.2 mm to the left of the midline and 0.2 mm posterior to the bregma at a depth of 3.5 mm.
Fluorescence assay of viable cells
CD97-CAR Th9 or Ctrl-CAR Th9 cells were used as effectors (E), and human GSCs or GBM cell lines were used as targets (T) and were previously transduced with luciferase lentiviral vectors. The viability of target cells at different effector-to-target ratios (E:T) was evaluated in 96-well black plates (LABSELECT) using a Spectra Max ID3 Multifunctional Enzyme Labeler (Molecular Devices). Cultures containing the medium alone were used as controls, representing 100% and 0% cell viability, respectively. The average viability was calculated as 100 × (experimental cell fluorescence −0% viable cell fluorescence)/(100% viable cell fluorescence −0% viable cell fluorescence).
Cytotoxicity assay
For analysis of the CAR-expressing T cell killing effect, target cells (GSCs and non-GSCs) were incubated with Celltracker CM-DiI dye (1:1000; Yeasen) at 37°C for 5 min and then incubated at 4°C for 15 min to terminate the staining and washed with PBS and co-cultured with Ctrl-CAR Th9 cells or CD97-CAR Th9 cells in confocal live cell culture dishes. The fluorescence images were captured using a Zeiss LSM 780 Confocal Laser Scanning Microscope.
Isolation of tumor-infiltrating immune cells
Brains were harvested from X01-Luc xenografts on days 2 and 7 after injection of CD97-CAR T cells, followed by enzymatic dissociation, and filtered through a 40 μm cell strainer. The samples were stained with anti-human CD3 antibody (APC, BioLegend), anti-human CD4 antibody (PerCP/Cyanine5.5, BioLegend), and anti-human CD107a antibody (PE, BioLegend) at 4°C for 20 min. Data were acquired using a CytoFLEX (Beckman Coulter, Inc.) and analyzed using CytExpert software v1.0.
Quantification and statistical analysis
The Kaplan‒Meier method was used to plot survival curves. For patients who were alive at the time of the last follow-up, survival records were censored in our analysis. The Statistical Package for the Social Sciences software (v16) was used for statistical analyses. In mouse experiments, the results from multiple datasets were compared using analysis of variance (ANOVA) and the log rank (Mantel‒Cox) test. The results from experiments assessing two datasets were compared using a two-tailed Student’s t test (in the cases where the samples are independent and follow a normal distribution). If the data doesn’t follow a normal distribution, the Mann-Whitney U test should be conducted instead. p values <0.05 were considered to indicate statistical significance; individual p values are provided in the figure legends. Three technical replicates were performed for all experiments for reproducibility.
Published: December 4, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2024.101844.
Contributor Information
Sun Il Choi, Email: sunil.choi@hotmail.com.
Bingyang Shi, Email: bs@henu.edu.cn.
Jinlong Yin, Email: jlyin@henu.edu.cn.
Supplemental information
References
- 1.Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKinnon C., Nandhabalan M., Murray S.A., Plaha P. Glioblastoma: clinical presentation, diagnosis, and management. Bmj. 2021;374 doi: 10.1136/bmj.n1560. [DOI] [PubMed] [Google Scholar]
- 3.Gilbertson R.J., Rich J.N. Making a tumour's bed: glioblastoma stem cells and the vascular niche. Nat. Rev. Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 4.FDA . 2017. FDA approves axicabtagene ciloleucel for large B-cell lymphoma.https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-axicabtagene-ciloleucel-large-b-cell-lymphoma [Google Scholar]
- 5.Brown C.E., Alizadeh D., Starr R., Weng L., Wagner J.R., Naranjo A., Ostberg J.R., Blanchard M.S., Kilpatrick J., Simpson J., et al. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N. Engl. J. Med. 2016;375:2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Rourke D.M., Nasrallah M.P., Desai A., Melenhorst J.J., Mansfield K., Morrissette J.J.D., Martinez-Lage M., Brem S., Maloney E., Shen A., et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed N., Brawley V., Hegde M., Bielamowicz K., Kalra M., Landi D., Robertson C., Gray T.L., Diouf O., Wakefield A., et al. HER2-Specific Chimeric Antigen Receptor-Modified Virus-Specific T Cells for Progressive Glioblastoma: A Phase 1 Dose-Escalation Trial. JAMA Oncol. 2017;3:1094–1101. doi: 10.1001/jamaoncol.2017.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh S.K., Clarke I.D., Terasaki M., Bonn V.E., Hawkins C., Squire J., Dirks P.B. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 9.Singh S.K., Hawkins C., Clarke I.D., Squire J.A., Bayani J., Hide T., Henkelman R.M., Cusimano M.D., Dirks P.B. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 10.Liu G., Yuan X., Zeng Z., Tunici P., Ng H., Abdulkadir I.R., Lu L., Irvin D., Black K.L., Yu J.S. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol. Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Y., Stamenkovic I., Yu Q. CD44 attenuates activation of the hippo signaling pathway and is a prime therapeutic target for glioblastoma. Cancer Res. 2010;70:2455–2464. doi: 10.1158/0008-5472.CAN-09-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anido J., Sáez-Borderías A., Gonzàlez-Juncà A., Rodón L., Folch G., Carmona M.A., Prieto-Sánchez R.M., Barba I., Martínez-Sáez E., Prudkin L., et al. TGF-β Receptor Inhibitors Target the CD44(high)/Id1(high) Glioma-Initiating Cell Population in Human Glioblastoma. Cancer Cell. 2010;18:655–668. doi: 10.1016/j.ccr.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 13.Son M.J., Woolard K., Nam D.H., Lee J., Fine H.A. SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell Stem Cell. 2009;4:440–452. doi: 10.1016/j.stem.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erhart F., Blauensteiner B., Zirkovits G., Printz D., Soukup K., Klingenbrunner S., Fischhuber K., Reitermaier R., Halfmann A., Lötsch D., et al. Gliomasphere marker combinatorics: multidimensional flow cytometry detects CD44+/CD133+/ITGA6+/CD36+ signature. J. Cell Mol. Med. 2019;23:281–292. doi: 10.1111/jcmm.13927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li D., Tian Y., Hu Y., Qi Y., Tian N., Li S., Hu P., Wu F., Wei Q., Wei Z., et al. Glioma-associated human endothelial cell-derived extracellular vesicles specifically promote the tumourigenicity of glioma stem cells via CD9. Oncogene. 2019;38:6898–6912. doi: 10.1038/s41388-019-0903-6. [DOI] [PubMed] [Google Scholar]
- 16.Bao S., Wu Q., Li Z., Sathornsumetee S., Wang H., McLendon R.E., Hjelmeland A.B., Rich J.N. Targeting cancer stem cells through L1CAM suppresses glioma growth. Cancer Res. 2008;68:6043–6048. doi: 10.1158/0008-5472.CAN-08-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lathia J.D., Li M., Sinyuk M., Alvarado A.G., Flavahan W.A., Stoltz K., Rosager A.M., Hale J., Hitomi M., Gallagher J., et al. High-throughput flow cytometry screening reveals a role for junctional adhesion molecule a as a cancer stem cell maintenance factor. Cell Rep. 2014;6:117–129. doi: 10.1016/j.celrep.2013.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lathia J.D., Gallagher J., Heddleston J.M., Wang J., Eyler C.E., Macswords J., Wu Q., Vasanji A., McLendon R.E., Hjelmeland A.B., Rich J.N. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell. 2010;6:421–432. doi: 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vora P., Venugopal C., Salim S.K., Tatari N., Bakhshinyan D., Singh M., Seyfrid M., Upreti D., Rentas S., Wong N., et al. The Rational Development of CD133-Targeting Immunotherapies for Glioblastoma. Cell Stem Cell. 2020;26:832–844.e6. doi: 10.1016/j.stem.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Kieliszek A.M., Mobilio D., Upreti D., Bloemberg D., Escudero L., Kwiecien J.M., Alizada Z., Zhai K., Ang P., Chafe S.C., et al. Intratumoral Delivery of Chimeric Antigen Receptor T Cells Targeting CD133 Effectively Treats Brain Metastases. Clin. Cancer Res. 2024;30:554–563. doi: 10.1158/1078-0432.Ccr-23-1735. [DOI] [PubMed] [Google Scholar]
- 21.Prater K.E., Aloi M.S., Pathan J.L., Winston C.N., Chernoff R.A., Davidson S., Sadgrove M., McDonough A., Zierath D., Su W., et al. A Subpopulation of Microglia Generated in the Adult Mouse Brain Originates from Prominin-1-Expressing Progenitors. J. Neurosci. 2021;41:7942–7953. doi: 10.1523/jneurosci.1893-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamann J., Eichler W., Hamann D., Kerstens H.M., Poddighe P.J., Hoovers J.M., Hartmann E., Strauss M., van Lier R.A. Expression cloning and chromosomal mapping of the leukocyte activation antigen CD97, a new seven-span transmembrane molecule of the secretion receptor superfamily with an unusual extracellular domain. J. Immunol. 1995;155:1942–1950. [PubMed] [Google Scholar]
- 23.Martin G.H., Roy N., Chakraborty S., Desrichard A., Chung S.S., Woolthuis C.M., Hu W., Berezniuk I., Garrett-Bakelman F.E., Hamann J., et al. CD97 is a critical regulator of acute myeloid leukemia stem cell function. J. Exp. Med. 2019;216:2362–2377. doi: 10.1084/jem.20190598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward Y., Lake R., Martin P.L., Killian K., Salerno P., Wang T., Meltzer P., Merino M., Cheng S.Y., Santoro M., et al. CD97 amplifies LPA receptor signaling and promotes thyroid cancer progression in a mouse model. Oncogene. 2013;32:2726–2738. doi: 10.1038/onc.2012.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin Y., Xu X., Tang J., Zhang W., Zhangyuan G., Ji J., Deng L., Lu S., Zhuo H., Sun B. CD97 Promotes Tumor Aggressiveness Through the Traditional G Protein-Coupled Receptor-Mediated Signaling in Hepatocellular Carcinoma. Hepatology. 2018;68:1865–1878. doi: 10.1002/hep.30068. [DOI] [PubMed] [Google Scholar]
- 26.Hilbig D., Dietrich N., Wandel E., Gonsior S., Sittig D., Hamann J., Aust G. The Interaction of CD97/ADGRE5 With beta-Catenin in Adherens Junctions Is Lost During Colorectal Carcinogenesis. Front. Oncol. 2018;8:182. doi: 10.3389/fonc.2018.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He Z., Wu H., Jiao Y., Zheng J. Expression and prognostic value of CD97 and its ligand CD55 in pancreatic cancer. Oncol. Lett. 2015;9:793–797. doi: 10.3892/ol.2014.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chidambaram A., Fillmore H.L., Van Meter T.E., Dumur C.I., Broaddus W.C. Novel report of expression and function of CD97 in malignant gliomas: correlation with Wilms tumor 1 expression and glioma cell invasiveness. J. Neurosurg. 2012;116:843–853. doi: 10.3171/2011.11.JNS111455. [DOI] [PubMed] [Google Scholar]
- 29.Safaee M., Clark A.J., Oh M.C., Ivan M.E., Bloch O., Kaur G., Sun M.Z., Kim J.M., Oh T., Berger M.S., Parsa A.T. Overexpression of CD97 confers an invasive phenotype in glioblastoma cells and is associated with decreased survival of glioblastoma patients. PLoS One. 2013;8 doi: 10.1371/journal.pone.0062765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J.K., Lubelski D., Schonberg D.L., Wu Q., Hale J.S., Flavahan W.A., Mulkearns-Hubert E.E., Man J., Hjelmeland A.B., Yu J., et al. Phage display discovery of novel molecular targets in glioblastoma-initiating cells. Cell Death Differ. 2014;21:1325–1339. doi: 10.1038/cdd.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Somasundaram A., Ardanowski N., Opalak C.F., Fillmore H.L., Chidambaram A., Broaddus W.C. Wilms tumor 1 gene, CD97, and the emerging biogenetic profile of glioblastoma. Neurosurg. Focus. 2014;37:E14. doi: 10.3171/2014.9.FOCUS14506. [DOI] [PubMed] [Google Scholar]
- 32.Mallawaaratchy D.M., Buckland M.E., McDonald K.L., Li C.C.Y., Ly L., Sykes E.K., Christopherson R.I., Kaufman K.L. Membrane proteome analysis of glioblastoma cell invasion. J. Neuropathol. Exp. Neurol. 2015;74:425–441. doi: 10.1097/NEN.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 33.Safaee M., Fakurnejad S., Bloch O., Clark A.J., Ivan M.E., Sun M.Z., Oh T., Phillips J.J., Parsa A.T. Proportional upregulation of CD97 isoforms in glioblastoma and glioblastoma-derived brain tumor initiating cells. PLoS One. 2015;10 doi: 10.1371/journal.pone.0111532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eichberg D.G., Slepak T.I., Pascoini A.L., Komotar R.J., Ivan M.E. Genetic manipulation of adhesion GPCR CD97/ADGRE5 modulates invasion in patient-derived glioma stem cells. J. Neuro Oncol. 2021;153:383–391. doi: 10.1007/s11060-021-03778-8. [DOI] [PubMed] [Google Scholar]
- 35.Safaee M.M., Wang E.J., Jain S., Chen J.S., Gill S., Zheng A.C., Garcia J.H., Beniwal A.S., Tran Y., Nguyen A.T., et al. CD97 is associated with mitogenic pathway activation, metabolic reprogramming, and immune microenvironment changes in glioblastoma. Sci. Rep. 2022;12:1464. doi: 10.1038/s41598-022-05259-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ravn-Boess N., Roy N., Hattori T., Bready D., Donaldson H., Lawson C., Lapierre C., Korman A., Rodrick T., Liu E., et al. The expression profile and tumorigenic mechanisms of CD97 (ADGRE5) in glioblastoma render it a targetable vulnerability. Cell Rep. 2023;42 doi: 10.1016/j.celrep.2023.113374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slepak T.I., Guyot M., Walters W., Eichberg D.G., Ivan M.E. Dual role of the adhesion G-protein coupled receptor ADRGE5/CD97 in glioblastoma invasion and proliferation. J. Biol. Chem. 2023;299 doi: 10.1016/j.jbc.2023.105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L.B., Karpova A., Gritsenko M.A., Kyle J.E., Cao S., Li Y., Rykunov D., Colaprico A., Rothstein J.H., Hong R., et al. Proteogenomic and metabolomic characterization of human glioblastoma. Cancer Cell. 2021;39:509–528.e20. doi: 10.1016/j.ccell.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Q., Hu B., Hu X., Kim H., Squatrito M., Scarpace L., deCarvalho A.C., Lyu S., Li P., Li Y., et al. Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell. 2017;32:42–56.e6. doi: 10.1016/j.ccell.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Restall I.J., Cseh O., Richards L.M., Pugh T.J., Luchman H.A., Weiss S. Brain Tumor Stem Cell Dependence on Glutaminase Reveals a Metabolic Vulnerability through the Amino Acid Deprivation Response Pathway. Cancer Res. 2020;80:5478–5490. doi: 10.1158/0008-5472.CAN-19-3923. [DOI] [PubMed] [Google Scholar]
- 41.Richards L.M., Whitley O.K.N., MacLeod G., Cavalli F.M.G., Coutinho F.J., Jaramillo J.E., Svergun N., Riverin M., Croucher D.C., Kushida M., et al. Gradient of Developmental and Injury Response transcriptional states defines functional vulnerabilities underpinning glioblastoma heterogeneity. Nat. Cancer. 2021;2:157–173. doi: 10.1038/s43018-020-00154-9. [DOI] [PubMed] [Google Scholar]
- 42.Strojnik T., Røsland G.V., Sakariassen P.O., Kavalar R., Lah T. Neural stem cell markers, nestin and musashi proteins, in the progression of human glioma: correlation of nestin with prognosis of patient survival. Surg. Neurol. 2007;68:133–143. doi: 10.1016/j.surneu.2006.10.050. discussion 143-134. [DOI] [PubMed] [Google Scholar]
- 43.Gangemi R.M.R., Griffero F., Marubbi D., Perera M., Capra M.C., Malatesta P., Ravetti G.L., Zona G.L., Daga A., Corte G. SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cell. 2009;27:40–48. doi: 10.1634/stemcells.2008-0493. [DOI] [PubMed] [Google Scholar]
- 44.Zbinden M., Duquet A., Lorente-Trigos A., Ngwabyt S.N., Borges I., Ruiz i Altaba A. NANOG regulates glioma stem cells and is essential in vivo acting in a cross-functional network with GLI1 and p53. EMBO J. 2010;29:2659–2674. doi: 10.1038/emboj.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lopez-Bertoni H., Lal B., Li A., Caplan M., Guerrero-Cázares H., Eberhart C.G., Quiñones-Hinojosa A., Glas M., Scheffler B., Laterra J., Li Y. DNMT-dependent suppression of microRNA regulates the induction of GBM tumor-propagating phenotype by Oct4 and Sox2. Oncogene. 2015;34:3994–4004. doi: 10.1038/onc.2014.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beier D., Hau P., Proescholdt M., Lohmeier A., Wischhusen J., Oefner P.J., Aigner L., Brawanski A., Bogdahn U., Beier C.P. CD133(+) and CD133(-) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–4015. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 47.Alcantara Llaguno S., Parada L.F. Cancer stem cells in gliomas: evolving concepts and therapeutic implications. Curr. Opin. Neurol. 2021;34:868–874. doi: 10.1097/WCO.0000000000000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carracedo A., Ma L., Teruya-Feldstein J., Rojo F., Salmena L., Alimonti A., Egia A., Sasaki A.T., Thomas G., Kozma S.C., et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J. Clin. Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palm W., Park Y., Wright K., Pavlova N.N., Tuveson D.A., Thompson C.B. The Utilization of Extracellular Proteins as Nutrients Is Suppressed by mTORC1. Cell. 2015;162:259–270. doi: 10.1016/j.cell.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarbassov D.D., Guertin D.A., Ali S.M., Sabatini D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 51.Sommer E.M., Dry H., Cross D., Guichard S., Davies B.R., Alessi D.R. Elevated SGK1 predicts resistance of breast cancer cells to Akt inhibitors. Biochem. J. 2013;452:499–508. doi: 10.1042/BJ20130342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gasser J.A., Inuzuka H., Lau A.W., Wei W., Beroukhim R., Toker A. SGK3 mediates INPP4B-dependent PI3K signaling in breast cancer. Mol. Cell. 2014;56:595–607. doi: 10.1016/j.molcel.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dong R., Libby K.A., Blaeschke F., Fuchs W., Marson A., Vale R.D., Su X. Rewired signaling network in T cells expressing the chimeric antigen receptor (CAR) EMBO J. 2020;39 doi: 10.15252/embj.2020104730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gudipati V., Rydzek J., Doel-Perez I., Gonçalves V.D.R., Scharf L., Königsberger S., Lobner E., Kunert R., Einsele H., Stockinger H., et al. Inefficient CAR-proximal signaling blunts antigen sensitivity. Nat. Immunol. 2020;21:848–856. doi: 10.1038/s41590-020-0719-0. [DOI] [PubMed] [Google Scholar]
- 55.Majzner R.G., Rietberg S.P., Sotillo E., Dong R., Vachharajani V.T., Labanieh L., Myklebust J.H., Kadapakkam M., Weber E.W., Tousley A.M., et al. Tuning the Antigen Density Requirement for CAR T-cell Activity. Cancer Discov. 2020;10:702–723. doi: 10.1158/2159-8290.CD-19-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu Y., Wang Q., Xue G., Bi E., Ma X., Wang A., Qian J., Dong C., Yi Q. Th9 Cells Represent a Unique Subset of CD4(+) T Cells Endowed with the Ability to Eradicate Advanced Tumors. Cancer Cell. 2018;33:1048–1060.e7. doi: 10.1016/j.ccell.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Speiser D.E., Chijioke O., Schaeuble K., Münz C. CD4(+) T cells in cancer. Nat. Cancer. 2023;4:317–329. doi: 10.1038/s43018-023-00521-2. [DOI] [PubMed] [Google Scholar]
- 58.Xue G., Zheng N., Fang J., Jin G., Li X., Dotti G., Yi Q., Lu Y. Adoptive cell therapy with tumor-specific Th9 cells induces viral mimicry to eliminate antigen-loss-variant tumor cells. Cancer Cell. 2021;39:1610–1622.e9. doi: 10.1016/j.ccell.2021.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tian Y., Sette A., Weiskopf D. Cytotoxic CD4 T Cells: Differentiation, Function, and Application to Dengue Virus Infection. Front. Immunol. 2016;7:531. doi: 10.3389/fimmu.2016.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brown C.E., Hibbard J.C., Alizadeh D., Blanchard M.S., Natri H.M., Wang D., Ostberg J.R., Aguilar B., Wagner J.R., Paul J.A., et al. Locoregional delivery of IL-13Rα2-targeting CAR-T cells in recurrent high-grade glioma: a phase 1 trial. Nat. Med. 2024;30:1001–1012. doi: 10.1038/s41591-024-02875-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Donovan L.K., Delaidelli A., Joseph S.K., Bielamowicz K., Fousek K., Holgado B.L., Manno A., Srikanthan D., Gad A.Z., Van Ommeren R., et al. Locoregional delivery of CAR T cells to the cerebrospinal fluid for treatment of metastatic medulloblastoma and ependymoma. Nat. Med. 2020;26:720–731. doi: 10.1038/s41591-020-0827-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Du H., Hirabayashi K., Ahn S., Kren N.P., Montgomery S.A., Wang X., Tiruthani K., Mirlekar B., Michaud D., Greene K., et al. Antitumor Responses in the Absence of Toxicity in Solid Tumors by Targeting B7-H3 via Chimeric Antigen Receptor T Cells. Cancer Cell. 2019;35:221–237.e8. doi: 10.1016/j.ccell.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zheng N., Fang J., Xue G., Wang Z., Li X., Zhou M., Jin G., Rahman M.M., McFadden G., Lu Y. Induction of tumor cell autosis by myxoma virus-infected CAR-T and TCR-T cells to overcome primary and acquired resistance. Cancer Cell. 2022;40:973–985.e7. doi: 10.1016/j.ccell.2022.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dirks P.B. Brain tumour stem cells: the undercurrents of human brain cancer and their relationship to neural stem cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008;363:139–152. doi: 10.1098/rstb.2006.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Galdieri L., Jash A., Malkova O., Mao D.D., DeSouza P., Chu Y.E., Salter A., Campian J.L., Naegle K.M., Brennan C.W., et al. Defining phenotypic and functional heterogeneity of glioblastoma stem cells by mass cytometry. JCI Insight. 2021;6 doi: 10.1172/jci.insight.128456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hatakeyama J., Wald J.H., Printsev I., Ho H.Y.H., Carraway K.L., 3rd Vangl1 and Vangl2: planar cell polarity components with a developing role in cancer. Endocr. Relat. Cancer. 2014;21:R345–R356. doi: 10.1530/erc-14-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ghosh S., Mukherjee S., Sengupta A., Chowdhury S., Sarkar S., Keswani T., Bhattacharyya A. CD4(+)IL9(+) (Th9) cells as the major source of IL-9, potentially modulate Th17/Treg mediated host immune response during experimental cerebral malaria. Mol. Immunol. 2022;152:240–254. doi: 10.1016/j.molimm.2022.11.005. [DOI] [PubMed] [Google Scholar]
- 68.Gomes-Silva D., Srinivasan M., Sharma S., Lee C.M., Wagner D.L., Davis T.H., Rouce R.H., Bao G., Brenner M.K., Mamonkin M. CD7-edited T cells expressing a CD7-specific CAR for the therapy of T-cell malignancies. Blood. 2017;130:285–296. doi: 10.1182/blood-2017-01-761320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen D., Varanasi S.K., Hara T., Traina K., Sun M., McDonald B., Farsakoglu Y., Clanton J., Xu S., Garcia-Rivera L., et al. CTLA-4 blockade induces a microglia-Th1 cell partnership that stimulates microglia phagocytosis and anti-tumor function in glioblastoma. Immunity. 2023;56:2086–2104.e8. doi: 10.1016/j.immuni.2023.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kruse B., Buzzai A.C., Shridhar N., Braun A.D., Gellert S., Knauth K., Pozniak J., Peters J., Dittmann P., Mengoni M., et al. CD4(+) T cell-induced inflammatory cell death controls immune-evasive tumours. Nature. 2023;618:1033–1040. doi: 10.1038/s41586-023-06199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meyran D., Zhu J.J., Butler J., Tantalo D., MacDonald S., Nguyen T.N., Wang M., Thio N., D'Souza C., Qin V.M., et al. T(STEM)-like CAR-T cells exhibit improved persistence and tumor control compared with conventional CAR-T cells in preclinical models. Sci. Transl. Med. 2023;15 doi: 10.1126/scitranslmed.abk1900. [DOI] [PubMed] [Google Scholar]
- 72.Choi S.I., Yin J. Prospective approaches to enhancing CAR T cell therapy for glioblastoma. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1008751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cohnen A., Chiang S.C., Stojanovic A., Schmidt H., Claus M., Saftig P., Janßen O., Cerwenka A., Bryceson Y.T., Watzl C. Surface CD107a/LAMP-1 protects natural killer cells from degranulation-associated damage. Blood. 2013;122:1411–1418. doi: 10.1182/blood-2012-07-441832. [DOI] [PubMed] [Google Scholar]
- 74.Aghajanian H., Rurik J.G., Epstein J.A. CAR-based therapies: opportunities for immuno-medicine beyond cancer. Nat. Metab. 2022;4:163–169. doi: 10.1038/s42255-022-00537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Milone M.C., Xu J., Chen S.J., Collins M.A., Zhou J., Powell D.J., Jr., Melenhorst J.J. Engineering enhanced CAR T-cells for improved cancer therapy. Nat. Cancer. 2021;2:780–793. doi: 10.1038/s43018-021-00241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aust G., Zheng L., Quaas M. To Detach, Migrate, Adhere, and Metastasize: CD97/ADGRE5 in Cancer. Cells. 2022;11 doi: 10.3390/cells11091538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ward Y., Lake R., Yin J.J., Heger C.D., Raffeld M., Goldsmith P.K., Merino M., Kelly K. LPA receptor heterodimerizes with CD97 to amplify LPA-initiated RHO-dependent signaling and invasion in prostate cancer cells. Cancer Res. 2011;71:7301–7311. doi: 10.1158/0008-5472.Can-11-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walter D.M., Venancio O.S., Buza E.L., Tobias J.W., Deshpande C., Gudiel A.A., Kim-Kiselak C., Cicchini M., Yates T.J., Feldser D.M. Systematic In Vivo Inactivation of Chromatin-Regulating Enzymes Identifies Setd2 as a Potent Tumor Suppressor in Lung Adenocarcinoma. Cancer Res. 2017;77:1719–1729. doi: 10.1158/0008-5472.Can-16-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin W., Niu R., Park S.M., Zou Y., Kim S.S., Xia X., Xing S., Yang Q., Sun X., Yuan Z., et al. IGFBP5 is an ROR1 ligand promoting glioblastoma invasion via ROR1/HER2-CREB signaling axis. Nat. Commun. 2023;14:1578. doi: 10.1038/s41467-023-37306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yin J., Kim S.S., Choi E., Oh Y.T., Lin W., Kim T.H., Sa J.K., Hong J.H., Park S.H., Kwon H.J., et al. ARS2/MAGL signaling in glioblastoma stem cells promotes self-renewal and M2-like polarization of tumor-associated macrophages. Nat. Commun. 2020;11:2978. doi: 10.1038/s41467-020-16789-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Patel A.P., Tirosh I., Trombetta J.J., Shalek A.K., Gillespie S.M., Wakimoto H., Cahill D.P., Nahed B.V., Curry W.T., Martuza R.L., et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344:1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yin J., Jung J.E., Choi S.I., Kim S.S., Oh Y.T., Kim T.H., Choi E., Lee S.J., Kim H., Kim E.O., et al. Inhibition of BMP signaling overcomes acquired resistance to cetuximab in oral squamous cell carcinomas. Cancer Lett. 2018;414:181–189. doi: 10.1016/j.canlet.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 83.Verhaak R.G.W., Hoadley K.A., Purdom E., Wang V., Qi Y., Wilkerson M.D., Miller C.R., Ding L., Golub T., Mesirov J.P., et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Y., Qian T., You G., Peng X., Chen C., You Y., Yao K., Wu C., Ma J., Sha Z., et al. Localizing seizure-susceptible brain regions associated with low-grade gliomas using voxel-based lesion-symptom mapping. Neuro Oncol. 2015;17:282–288. doi: 10.1093/neuonc/nou130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Madhavan S., Zenklusen J.C., Kotliarov Y., Sahni H., Fine H.A., Buetow K. Rembrandt: helping personalized medicine become a reality through integrative translational research. Mol. Cancer Res. 2009;7:157–167. doi: 10.1158/1541-7786.Mcr-08-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
RNA-seq data have been deposited at Gene Expression Omnibus and are publicly available as of the date of publication. Accession numbers are listed in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.