Abstract
Background and aims
Elevated lipoprotein(a) [Lp(a)], high-sensitivity C-Reactive Protein (hs-CRP), and total homocysteine (tHcy) are associated with atherosclerotic cardiovascular disease (ASCVD) risk. This study investigated the individual and joint associations of Lp(a), hs-CRP and tHcy with coronary heart disease (CHD) and stroke.
Methods
This study was conducted in the Multi-Ethnic Study of Atherosclerosis (MESA) cohort (2000–2017) (CHD analytic N = 6,676; stroke analytic N = 6,674 men and women). Associations between Lp(a) (<50 vs. ≥50 mg/dL), hs-CRP (<2 vs. ≥2 mg/L) and tHcy (<12 vs. ≥12 µmol/L) and CHD and stroke incidence were evaluated individually and jointly using Cox proportional hazards regression.
Results
Individually, elevated tHcy was associated with CHD and stroke incidence, Lp(a) with CHD only and hs-CRP with stroke only. In combined analyses, CHD risk was higher when multiple biomarkers were elevated [hs-CRP+Lp(a), hazard ratio (HR)=1.39, 95 % confidence interval (CI): 1.06, 1.82; hs-CRP+ tHcy, HR = 1.34, 95 % CI: 1.02, 1.75; Lp(a)+ tHcy HR = 1.58, 95 % CI: 1.08, 2.30; hs-CRP+Lp(a)+ tHcy HR = 2.02, 95 % CI: 1.26, 3.24]. Stroke risk was elevated when hs-CRP and either Lp(a) (HR = 1.51, 95 % CI: 1.02, 2.23) or tHcy (HR = 2.10, 95 % CI: 1.44, 3.06) was also high, when all three biomarkers were elevated (HR = 2.99, 95 % CI: 1.61, 5.58), or when hs-CRP and tHcy (HR = 1.79, 95 % CI: 1.16, 2.76) were both high.
Conclusions
Risk of ASCVD was highest with concomitant elevation of tHcy, hs-CRP and Lp(a). Inclusion of tHcy and consideration of biomarker combination rather than individual biomarker levels may help better identify individuals at greatest risk for ASCVD events.
Keywords: High-sensitivity c-reactive protein, Homocysteine, Lipoprotein(a), Stroke, Coronary heart disease
Graphical abstract
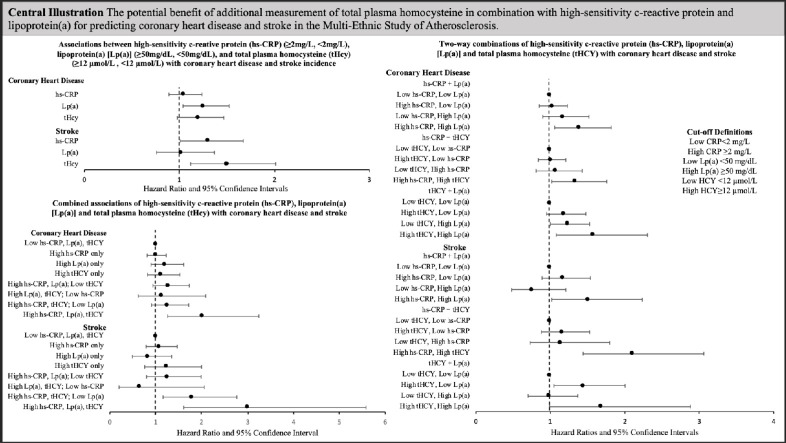
Central Illustration. The potential benefits of additional measurement of total plasma homocysteine in combination with high-sensitivity c-reactive protein and lipoprotein(a) for predicting coronary heart disease and stroke in the Multi-Ethnic Study of Atherosclerosis. Abbreviations: high-sensitivity c-reactive protein, hs-CRP; total plasma homocysteine, tHcy; lipoprotein(a), lp(a). Cox proportional hazards regression hazard ratios and 95 % confidence intervals adjusted for age, sex, race/ethnicity, hypertension, hypertension medication usage, diabetes, pack-years smoking, HDL-C, total cholesterol, triglycerides (log-transformed), BMI and eGFR. Low hs-CRP <2 mg/dL; High hs-CRP ≥2 mg/dL; Low Lp(a) <50 mg/dL; High Lp(a) ≥50 mg/dL; Low tHcy <12 µmol/L; High tHcy=≥12 µmol/L.
1. Introduction
Despite improvements in identifying individuals at risk for atherosclerotic cardiovascular disease (ASCVD), it remains the leading cause of morbidity and mortality worldwide [1]. The integration of non-traditional risk markers, including lipoprotein(a) [Lp(a)], high-sensitivity C-reactive protein (hs-CRP) and plasma total homocysteine (tHcy) may help reduce risk for ASCVD [2]. Lp(a) and hs-CRP are established ASCVD risk markers and are currently listed as risk enhancing factors in the 2019 American Health Association (AHA)/American College of Cardiology (ACC) cardiovascular disease prevention guidelines [3]. tHcy, however, is not currently included in guidelines and research is lacking on the potential benefit of considering biomarker combinations to refine ASCVD risk surveillance.
Lp(a) may influence ASCVD risk through multiple mechanisms including atherosclerosis, thrombosis and inflammation [4,5]. Markers of systemic inflammation have also been associated with ASCVD, including hs-CRP [[6], [7], [8], [9]] and tHcy [[10], [11], [12], [13], [14]]. Elevated tHcy is associated with vascular inflammation and oxidative stress secondary to reactive oxygen species accumulation [15]. It is possible that these inflammatory pathways have synergistic effects and measurements could provide additional information regarding ASCVD risk. Additionally, tHcy and Lp(a) are both associated with increased coagulation and research suggests a possible synergistic effect [[16], [17], [18]].
A previous study in the Multi-Ethnic Study of Atherosclerosis (MESA) found that Lp(a) was only associated with ASCVD risk in individuals with elevated hs-CRP levels [19] and a recent analysis observed that combinations of risk enhancers appeared to better identify intermediate risk individuals who would benefit from treatment [20]. To our knowledge no prior studies have evaluated tHcy in combination with hs-CRP and Lp(a) for ASCVD risk. The present study investigated relationships between Lp(a), hs-CRP, and tHcy with CHD and stroke risk among participants in the MESA prospective cohort. We hypothesized that elevated Lp(a), hs-CRP and tHcy would have additive associations with incident CHD and stroke.
2. Materials and methods
2.1. Study population
The Multi-Ethnic Study of Atherosclerosis (MESA) recruited 6814 men and women aged 45 to 84 years and free of clinically apparent cardiovascular disease, from four ethnic/racial groups (White, Black, Hispanic, Chinese) at six centers in the United States (Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; New York, NY; St. Paul, MN) between July 2000 and August 2002 [21]. The present study utilizes baseline exposure and covariate data and outcome follow-up through 2017. Participants without baseline hs-CRP, Lp(a) or tHcy measurements (N = 118), and without outcome data for CHD (N = 22) and stroke (N = 24) were excluded. Additional participants with Missing covariate data were excluded (N = 107). The analytic sample was 6567 for CHD and 6565 for stroke, with a median follow-up time of 15.8 years.
The study was approved by the institutional review boards for the MESA field centers and all participants provided informed consent.
2.2. Data collection
2.2.1. Biomarker measurements
Lp(a), hs-CRP and tHcy were measured using 12-hour fasting blood samples collected at the baseline study visit and stored at -70 °C using standardized procedures [21]. High-sensitivity c-reactive protein was measured using the BNII nephelometer (N High Sensitivity CRP; Dade Behring, Inc., Deerfield, IL). Plasma tHcy was measured using high performance liquid chromatography with fluorometric detection. Lp(a) mass concentrations were measured using a latex-enhanced turbidimetric immunoassay that controls for the heterogeneous sizes of apolipoprotein(a) (Health Diagnostics Laboratory, Richmond, VA; Denka Seiken, Tokyo, Japan). Coefficients of variation for measured biomarkers were <5 % (Lp(a)), 3.6 % (hs-CRP), and 3.8 % (tHcy).
2.2.2. Covariates
At the baseline study exam, demographics, personal and medical history and lifestyle behaviors were obtained by interview and questionnaire. Biospecimen samples (12-hour fasting blood and urine) were collected and anthropometrics and blood pressure were measured by study staff. Procedures are outlined in detail elsewhere [21,22]. Hypertension was defined as blood pressure medication usage, diastolic blood pressure ≥90 mmHg or systolic blood pressure ≥140 mmHg and diabetes as taking diabetes medication or a fasting glucose >125 mg/dL.
2.2.3. Primary outcome ascertainment
Incident CHD and stroke events through 2017 were primarily self-reported at study visits or follow-up calls occurring every 9–12 months and verified by medical records, reviewed by trained personnel to confirm events. Records were reviewed by cardiologists or cardiovascular physician epidemiologists for non-neurovascular events, and neurologists for all neurovascular events. Incident CHD was defined as the first occurrence of any of the following: myocardial infarction, resuscitated cardiac arrest, CHD death, or definite angina. Definite angina was defined as symptoms of typical chest pain and physician diagnosis of angina followed by coronary artery bypass graft and percutaneous transluminal coronary angioplasty, evidence of ischemia by stress tests or resting ECG, or ≥70 % obstruction on coronary angiography. Probable angina cases were included if followed by revascularization. Incident stroke was defined as new fatal or non-fatal strokes due to ischemic, hemorrhage, or transient ischemic attack.
2.3. Statistical analysis
Preliminary associations between Lp(a), hs-CRP, tHcy, outcomes of interest and population characteristics were evaluated using Spearman correlation, ANOVA or Wald Χ2. Lp(a), hs-CRP and tHcy were evaluated categorically using standard cut-points [Lp(a): low <50 mg/dL, high ≥50 mg/dL; hs-CRP: low <2mg/L, high ≥2 mg/L; tHcy: low <12 µmol/L, high ≥12 µmol/L) [3,12,23]. Lp(a) was additionally evaluated using the alternatively cut-point 30 mg/dL [Lp(a): low <30 mg/dL, high ≥30 mg/dL].
To evaluate joint effects, all possible combinations of two biomarkers, in addition to all three combined, were evaluated in relation to CHD and stroke incidence. In evaluating two biomarkers together, participants were stratified by high/low status and comparisons were made to individuals with low levels of both biomarkers. Categories for evaluating the combinations of all three biomarkers, again stratified by high/low status were compared to individuals with low hs-CRP, low Lp(a), and low tHcy.
Time to event for CHD and stroke was modeled for hs-CRP, Lp(a) and tHcy separately and by categories of the combined biomarkers of interest using Cox proportional hazards regression. Models were conducted (1) unadjusted; (2) age, sex, race/ethnicity; and (3) age, sex, race/ethnicity, hypertension, hypertension medication usage, diabetes, pack-years smoking, high-density lipoprotein-cholesterol, total cholesterol (HDL-C), triglycerides (log-transformed), body mass index (BMI) and estimated glomerular filtration rate (eGRF). The following variables were also evaluated as covariates, but not included in the final presented models: waist circumference, low-density lipoprotein cholesterol, non-HDL-C, education, income, alcohol intake, Healthy Eating Index diet score, physical activity, aspirin use, and lipid-lowering medication use. For multivariable-adjusted models, a priori chosen factors there were associated with exposure and/or outcome or that changed the association between Lp(a), hs-CRP, tHcy and the outcome(s) were included in the final version of the fully adjusted model. Tests for interaction were conducted using cross-product terms (2-way for two biomarker combinations, 3-way for combination of all three). Multivariable-adjusted survival curves using the same covariates outlined previously were computed for the associations between biomarker combinations and incidence of CHD and stroke. Harrell's Concordance Statistics (c-statistics) were calculated in adjusted models with and without hs-CRP, Lp(a) and tHcy. Sensitivity analyses were conducted using ischemic stroke only. Findings were similar to those using all stroke types, so results are presented using all stroke types. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc) and statistical significance was defined as a two-tailed P value <0.05.
3. Results
There were 669 incident cases of CHD and 294 incident cases of stroke. Baseline population characteristics among individuals with 0/3, 1/3, 2/3 and 3/3 biomarkers above thresholds are presented in Table 1. Among the 6589 participants, 48.0 % (N = 3161) had elevated h-sCRP, 20.1 % (N = 1318) had elevated Lp(a), and 13.3 % (N = 874) had elevated tHcy.
Table 1.
Baseline characteristics of the Multi-Ethnic Study of Atherosclerosis study population by number of biomarkers (high sensitivity c-reactive protein, lipoprotein(a) and homocysteine) above thresholds (N = 6676).
| 0/3 Elevated | 1/3 Elevated | 2/3 Elevated | 3/3 Elevated | P-valuec | |
|---|---|---|---|---|---|
| Overalla | 2406 (36.5) | 3270 (49.6) | 809 (12.3) | 107 (1.6) | — |
| Ageb | 60.0 (52.0, 69.0) | 61.0 (53.0, 69.0) | 68.0 (59.0, 76.0) | 69.0 (64.0, 75.0) | <0.0001 |
| Sex (female)a | 1084 (45.1) | 2072 (63.4) | 276 (34.1) | 50 (46.7) | <0.0001 |
| Race/Ethnicitya | <0.0001 | ||||
| White | 1020 (42.5) | 1118 (36.3) | 311 (38.4) | 22 (20.6) | |
| Black | 415 (17.3) | 1096 (33.5) | 229 (28.3) | 63 (58.9) | |
| Chinese | 485 (20.2) | 201 (6.2) | 101 (12.5) | 1 (0.9) | |
| Hispanic | 483 (20.1) | 785 (24.1) | 168 (20.8) | 21 (19.6) | |
| Hypertensiona | 838 (34.9) | 1548 (47.3) | 481 (59.5) | 79 (74.8) | <0.0001 |
| HTN medicationa | 697 (28.6) | 1263 (38.6) | 418 (51.7) | 74 (69.2) | <0.0001 |
| Diabetesa | 215 (9.0) | 448 (13.7) | 137 (16.9) | 24 (22.04 | <0.0001 |
| Smoking (pack-years)b | 0.0 (0.0, 11.0) | 0.0 (0.0, 16.8) | 0.75 (0.0, 21.8) | 0.50 (0.0, 25.0) | <0.0001 |
| BMI (kg/m2)b | 25.9 (23.3, 28.8) | 29.0 (25.7, 33.0) | 27.4 (24.6, 30.8) | 29.7 (26.4, 32.7) | <0.0001 |
| hs-CRP (mg/L)†b | 0.85 (0.50, 1.31) | 3.78 (2.33, 6.77) | 1.64 (0.74, 3.51) | 4.43 (3.02, 8.94) | <0.0001 |
| Lp(a) (mg/dL)b | 11.6 (5.9, 22.5) | 26.6 (9.7, 62.6) | 13.4 (6.4, 28.8) | 80.9 (66.7, 115.6) | <0.0001 |
| tHcy (µmol/L)b | 8.3 (7.1, 9.7) | 8.3 (7.0, 9.6) | 13.7 (12.6, 15.8) | 14.4 (13.2, 17.5) | <0.0001 |
| HDL-C (mg/dL)b | 49.0 (41.0, 59.0) | 49.0 (41.0, 59.0) | 46.0 (39.0, 57.0) | 46.0 (39.0, 57.0) | 0.0008 |
| Total Cholesterol (mg/dL)b | 189.0 (169.0, 211.0) | 196.0 (174.0, 219.0) | 186.0 (163.0, 210.0) | 197.0 (173.0, 220.0) | <0.0001 |
| Triglycerides (mg/dL)b | 105.0 (74.0, 153.0) | 114.0 (81.0, 165.0) | 112.0 (77.0, 166.0) | 112.0 (84.0, 171.0) | 0.01 |
| eGFRb | 81.0 (71.9, 92.4) | 80.7 (70.7, 92.8) | 71.0 (59.3, 84.1) | 64.6 (50.0, 75.4) | <0.0001 |
Abbreviations: Body mass index, BMI; high sensitivity c-reactive protein, hs-CRP; estimated glomerular filtration rate, eGFR; high-density lipoprotein cholesterol, HDL-C; homocysteine, tHcy; interquartile range, IQR; lipoprotein(a), Lp(a).
n (%)
Median (IQR)
Wald X2 test for categorical variables; One-way ANOVA for continuous variables.
Associations of individual biomarkers with CHD and stroke are presented in Table 2. Lp(a) was associated with CHD risk while tHcy was positively associated with CHD when evaluated continuously, but not categorically. Hs-CRP was not independently associated with CHD in fully adjusted models. tHcy and hs-CRP were both positively associated with stroke incidence, while Lp(a) was not associated with stroke risk. Results were similar when Lp(a) cut-point of 30 mg/dL was used (Supplementary Table 1).
Table 2.
Individual associations between high sensitivity c-reactive protein (<2 mg/L, ≥2 mg/L), lipoprotein(a) (<50 mg/dL, ≥50 mg/dL), homocysteine (<12 µmol/L, ≥12 µmol/L) with coronary heart disease and stroke (though 2017) in MESA (N = 6676).
| Coronary Heart Disease |
Stroke |
|||||
|---|---|---|---|---|---|---|
| N Cases/ N Total |
Minimally Adjusteda HR (95 % CI) |
Fully Adjustedb HR (95 % CI) |
N Cases/ N Total |
Minimally Adjusteda HR (95 % CI) |
Fully Adjustedb HR (95 % CI) |
|
| hs-C-reactive protein | ||||||
| hs-CRP <2 mg/L | 343/3417 | Reference | Reference | 133/3417 | Reference | Reference |
| hs-CRP ≥2 mg/L | 316/3150 | 1.22 (1.05, 1.43) P = 0.01 |
1.05 (0.89, 1.24) P = 0.53 |
161/3148 | 1.34 (1.06, 1.69) P = 0.02 |
1.30 (1.01, 1.67) P = 0.04 |
| Loghs-CRP (per unit) | 669/6567 | 1.14 (1.07, 1.23) P < 0.0001 |
1.08 (1.00, 1.16) P = 0.05 |
294/6565 | 1.16 (1.05, 1.29) P = 0.004 |
1.15 (1.03, 1.29) P = 0.01 |
| Lipoprotein(a) | ||||||
| Lp(a) <50 mg/dL | 517/5258 | Reference | Reference | 232/5257 | Reference | Reference |
| Lp(a) ≥50 mg/dL | 152/1309 | 1.25 (1.04, 1.51) P = 0.02 |
1.25 (1.04, 1.52) P = 0.02 |
62/1308 | 1.07 (0.80, 1.42) P = 0.66 |
1.02 (0.76, 1.37) P = 0.88 |
| LogLp(a) (per unit) | 669/6567 | 1.06 (0.99, 1.14) P = 0.09 |
1.08 (1.01, 1.17) P = 0.03 |
294/6565 | 1.02 (0.92, 1.14) P = 0.70 |
1.01 (0.91, 1.13) P = 0.81 |
| Homocysteine | ||||||
| tHcy <12 µmol/L | 542/5695 | Reference | Reference | 229/5693 | Reference | Reference |
| tHcy ≥12 µmol/L | 127/872 | 1.25 (1.03, 1.51) P = 0.03 |
1.20 (0.98, 1.47) P = 0.08 |
65/872 | 1.44 (1.09, 1.91) P = 0.01 |
1.50 (1.12, 2.01) P = 0.006 |
| LogtHcy (per unit) | 669/6567 | 1.46 (1.13, 1.89) P = 0.004 |
1.36 (1.02, 1.80) P = 0.03 |
294/6565 | 1.51 (1.03, 2.12) P = 0.03 |
1.66 (1.11, 2.50) P = 0.01 |
Abbreviations: high sensitivity C-reactive protein, hs-CRP; confidence interval, CI; coronary heart disease, CHD; estimated glomerular filtration rate, eGFR; hazard ratio, HR; high-density lipoprotein cholesterol, HDL-C; homocysteine, tHcy; interquartile range, IQR; lipoprotein(a), Lp(a).
Cox proportional hazards regression adjusted for age, sex, race/ethnicity adjusted.
Cox proportional hazards regression adjusted for age, sex, race/ethnicity, hypertension, hypertension medication usage, diabetes, pack-years smoking, HDL-C, total cholesterol, triglycerides (log-transformed), BMI and eGFR.
Two-way combinations of hs-CRP, Lp(a) and tHcy and associations with CHD and stroke are presented in Table 3. Survival curves for the associations are presented in Fig. 1, Fig. 2, respectively. In general, individuals with only one elevated biomarker did not have an increased risk of CHD compared with the reference group where both biomarkers were low. However, CHD incidence was higher when two biomarkers were elevated [high hs-CRP + high Lp(a): hazard ratio (HR)=1.39, 95 % confidence interval (CI): 1.06, 1.82; high hs-CRP + tHcy: HR = 1.34, 95 % CI: 1.02, 1.76; high tHcy + Lp(a):HR = 1.58, 95 % CI: 1.08, 2.30]. Tests for interaction for the combinations of two biomarkers were not statistically significant (P>0.27). Similarly, higher stroke incidence was only observed among individuals with combined high hs-CRP + Lp(a) (HR = 1.51, 95 % CI: 1.02, 2.23, P-interaction=0.07) or combined high hs-CRP + tHcy (HR = 2.10, 95 % CI: 1.44, 3.06, P-interaction=0.11) when compared to individuals with low levels for both biomarkers. High tHcy + Lp(a) were borderline significantly associated with stroke incidence (HR = 1.69, 95 % CI: 0.99, 2.88, P-interaction=0.60), but the sample size in this group was notably smaller than the other groups (n=193). However, individuals with high tHcy only [and low Lp(a)] had increased stroke risk (HR = 1.45, 95 % CI: 1.05, 2.00). Findings were similar when using 30 mg/dL Lp(a) cut-point (Supplementary Table 2).
Table 3.
Associations of two-way combinations of high sensitivity c-reactive protein, lipoprotein(a) and homocysteine with coronary heart disease and stroke (through 2017) in MESA (N=6,676)
| Coronary Heart Disease |
Stroke |
|||||
|---|---|---|---|---|---|---|
| N Cases/N Total | Minimally Adjusteda HR (95% CI) |
Fully Adjustedb HR (95% CI) |
N Cases/N Total | Minimally Adjusteda HR (95% CI) |
Fully Adjustedb HR (95% CI) |
|
| hs-CRP and Lp(a)c | ||||||
| Low hs-CRP and Lp(a) | 270/2773 | Reference | Reference | 111/2773 | Reference | Reference |
| High hs-CRP only | 247/2485 | 1.21 (1.01, 1.44) P=0.04 |
1.03 (0.85, 1.23) P=0.79 |
121/2484 | 1.22 (0.94, 1.58) P=0.14 |
1.17 (0.89, 1.54) P=0.27 |
| High Lp(a) only | 73/644 | 1.21 (0.93, 1.58) P=0.15 |
1.17 (0.90, 1.52) P=0.25 |
22/644 | 0.83 (0.53, 1.30) P=0.43 |
0.76 (0.48, 1.21) P=0.24 |
| High hs-CRP, Lp(a) | 79/665 | 1.58 (1.22, 2.06) P=0.0006 |
1.39 (1.06, 1.82) P=0.02 |
40/2484 | 1.59 (1.09, 2.32) P=0.02 |
1.51 (1.02, 2.23) P=0.04 |
| P-Interaction=0.67 | P-Interaction=0.43 | P-Interaction=0.12 | P-Interaction=0.07 | |||
| hs-CRP and tHcyc | ||||||
| Low hs-CRP and tHcy | 282/2956 | Reference | Reference | 109/2956 | Reference | Reference |
| High hs-CRP only | 254/2697 | 1.17 (0.98, 1.39) P=0.08 |
1.01 (0.84, 1.21) P=0.96 |
118/2695 | 1.20 (0.92, 1.56) P=0.18 |
1.16 (0.88, 1.53) P=0.30 |
| High tHcy only | 61/461 | 1.12 (0.85, 1.48) P=0.42 |
1.07 (0.81, 1.43) P=0.62 |
24/461 | 1.09 (0.70, 1.70) P=0.70 |
1.14 (0.73, 1.80) P=0.56 |
| High hs-CRP, HCY | 72/453 | 1.58 (1.22, 2.05) P=0.0006 |
1.34 (1.02, 1.76) P=0.03 |
43/453 | 2.07 (1.44, 2.96) P<0.0001 |
2.10 (1.44, 3.06) P=0.0001 |
| P-Interaction=0.33 | P-Interaction=0.27 | P-Interaction=0.11 | P-Interaction=0.11 | |||
| tHcy and Lp(a)c | ||||||
| Low tHcy and Lp(a) | 416/4534 | Reference | Reference | 181/4533 | Reference | Reference |
| High tHcy only | 101/724 | 1.23 (0.99, 1.53) P=0.07 |
1.18 (0.94, 1.48) P=0.15 |
51/724 | 1.40 (1.02, 1.92) P=0.04 |
1.45 (1.05, 2.00) P=0.03 |
| High Lp(a) only | 120/1119 | 1.24 (1.00, 1.51) P=0.05 |
1.24 (1.00, 1.53) P=0.05 |
46/1118 | 1.03 (0.74, 1.42) P=0.87 |
0.98 (0.70, 1.37) P=0.91 |
| High tHcy, Lp(a) | 32/190 | 1.62 (1.22, 2.33) P=0.01 |
1.58 (1.08, 2.30) P=0.02 |
16/190 | 1.67 (0.99, 2.80) P=0.05 |
1.69 (0.99, 2.88) P=0.05 |
| P-Interaction=0.78 | P-Interaction=0.73 | P-Interaction=0.65 | P-Interaction=0.60 | |||
Abbreviations: high sensitivity C-reactive protein, hs-CRP; confidence interval, CI; coronary heart disease, CHD; estimated glomerular filtration rate, eGFR; hazard ratio, HR; high-density lipoprotein cholesterol, HDL-C; homocysteine, tHcy; interquartile range, IQR; lipoprotein(a), Lp(a).
Cox proportional hazards regression adjusted for age, sex, race/ethnicity adjusted.
Cox proportional hazards regression adjusted for age, sex, race/ethnicity, hypertension, hypertension medication usage, diabetes, pack-years smoking, HDL-C, total cholesterol, triglycerides (log-transformed), BMI and eGFR.
Low hs-CRP <2 mg/L; High hs-CRP ≥2 mg/L; Low Lp(a) <50 mg/dL; High Lp(a) ≥50 mg/dL; Low tHcy <12 µmol/L; High tHcy=≥12 µmol/L.
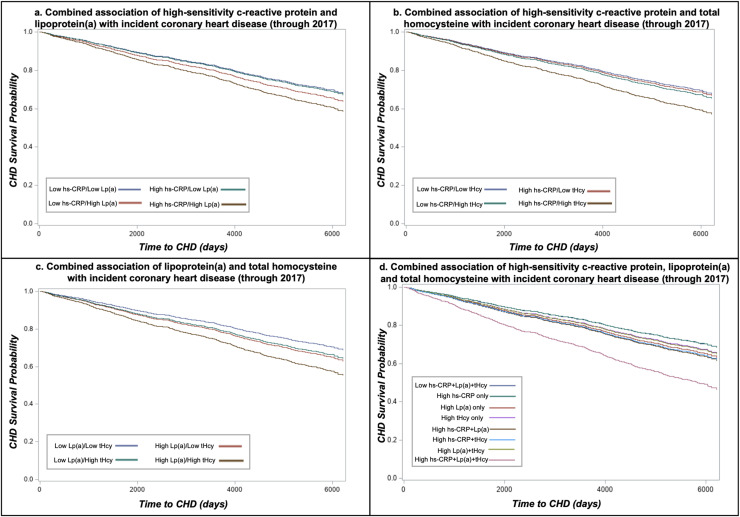
Fig. 1.
Combined associations of high-sensitivity c-reactive protein, lipoprotein(a) and total homocysteine with incident coronary heart disease in the Multi-Ethnic Study of Atherosclerosis.
Abbreviations: high-sensitivity c-reactive protein, hs-CRP; coronary heart disease (CHD) total plasma homocysteine, tHcy; lipoprotein(a), LPA. Cox proportional hazards regression survival curves adjusted for age, sex, race/ethnicity, hypertension, hypertension medication usage, diabetes, pack-years smoking, HDL-C, total cholesterol, triglycerides (log-transformed), BMI and eGFR. Low hs-CRP <2 mg/dL; High hs-CRP ≥2 mg/dL; Low Lp(a) <50 mg/dL; High Lp(a) ≥50 mg/dL; Low tHcy <12 µmol/L; High tHcy=≥12 µmol/L.
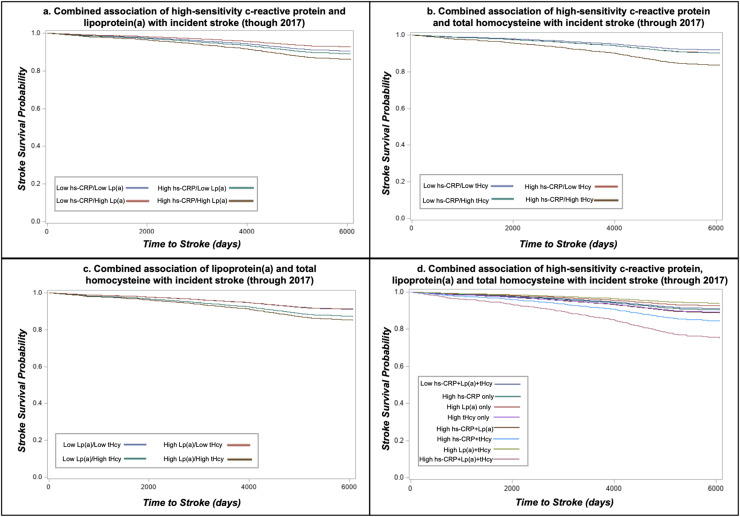
Fig. 2.
Combined associations of high-sensitivity c-reactive protein, lipoprotein(a) and total homocysteine with incident stroke in the Multi-Ethnic Study of Atherosclerosis.
Abbreviations: high-sensitivity c-reactive protein, hs-CRP; total plasma homocysteine, tHcy; lipoprotein(a), LPA. Cox proportional hazards regression survival curves adjusted for age, sex, race/ethnicity, hypertension, hypertension medication usage, diabetes, pack-years smoking, HDL-C, total cholesterol, triglycerides (log-transformed), BMI and eGFR. Low hs-CRP <2 mg/dL; High hs-CRP ≥2 mg/dL; Low Lp(a) <50 mg/dL; High Lp(a) ≥50 mg/dL; Low tHcy <12 µmol/L; High tHcy=≥12 µmol/L.
Results for combinations of all three biomarkers in relation to incidence of CHD and stroke are presented in Table 4 with survival curves in Figs. 1d and Fig. 2d. When evaluated as the number of biomarkers over the corresponding clinical thresholds, incidence of both CHD (HR = 1.99, 95 % CI: 1.24, 3.20) and stroke (HR = 2.95, 95 % CI: 1.58, 5.49) were highest when all three biomarkers were above clinical cut-points and hazard ratios increased and associations strengthened which each additional elevated. There was a significant increased risk of CHD only in individuals with combined high Lp(a), hs-CRP and tHcy (HR = 2.02, 95 % CI: 1.26, 3.24, P-interaction=0.58). Similarly, individuals classified as high for all three biomarkers were at an increased risk for stroke (HR = 2.99, 95 % CI: 1.61, 5.58, P-interaction=0.34). However, individuals with both high hs-CRP and tHcy but low Lp(a) also had a statistically significantly higher incidence of stroke relative to individuals with the combination of low hs-CRP, tHcy and Lp(a) (HR = 1.79, 95 % CI: 1.16, 2.76). Results using Lp(a) cut-point of 30 mg/dL are presented on Supplementary Table 3 and are similar to findings using 50 mg/dL). C-statistics for models with and without hs-CRP, Lp(a) and tHcy were similar [CHD models c-statistic range: 0.7274 (no biomarkers) to 0.7294 (all three biomarkers); stroke models c-statistic range: 0.7329 (no biomarkers and Lp(a) only) to 0.7374 (all three biomarkers) Supplementary Table 4.
Table 4.
Combined associations of high sensitivity c-reactive protein (<2 mg/dL, ≥2 mg/dL), lipoprotein(a) (<50 mg/dL, ≥50 mg/dL) and homocysteine (<12 µmol/L, ≥12 µmol/L) with coronary heart disease and stroke (though 2017) in MESA (N = 6676).
| Coronary Heart Disease |
Stroke |
|||||
|---|---|---|---|---|---|---|
| N Cases/ N Total |
Minimally Adjusteda HR (95 % CI) |
Fully Adjustedb,d HR (95 % CI) |
N Cases/ N Total |
Minimally Adjusteda HR (95 % CI) |
Fully Adjustedb,d HR (95 % CI) |
|
| Overallc | ||||||
| 0:3 Elevated | 220/2396 | Reference | Reference | 90/2396 | Reference | Reference |
| 1:3 Elevated | 316/3257 | 1.23 (1.03, 1.48) P = 0.02 |
1.07 (0.89, 1.29) P = 0.44 |
137/3255 | 1.12 (0.85, 1.46) P = 0.43 |
1.04 (0.79, 1.38) P = 0.77 |
| 2:3 Elevated | 112/808 | 1.30 (1.03, 1.64) P = 0.03 |
1.18 (0.93, 1.49) P = 0.18 |
54/808 | 1.38 (0.98, 1.94) P = 0.07 |
1.40 (0.98, 1.98) P = 0.06 |
| 3:3 Elevated | 21/106 | 2.38 (1.51, 3.76) P = 0.0002 |
1.99 (1.24, 3.20) P = 0.005 |
13/106 | 2.98 (1.64, 5.39) P = 0.0003 |
2.95 (1.58, 5.49) P = 0.007 |
| Individual Combinationsc | ||||||
| Low hs-CRP, Lp(a), tHCY | 220/2369 | Reference | Reference | 90/2396 | Reference | Reference |
| High hs-CRP only | 196/2138 | 1.18 (0.97, 1.44) P = 0.09 |
1.00 (0.81, 1.23) P = 0.98 |
91/2137 | 1.13 (0.84, 1.52) P = 0.42 |
1.08 (0.79, 1.47) P = 0.62 |
| High Lp(a) only | 62/560 | 1.27 (0.95, 1.68) P = 0.11 |
1.21 (0.90, 1.61) P = 0.21 |
19/560 | 0.90 (0.56, 1.46) P = 0.67 |
0.82 (0.49, 1.35) P = 0.43 |
| High tHcy only | 50/377 | 1.18 (0.87, 1.61) P = 0.29 |
1.12 (0.82, 1.53) P = 0.48 |
21/377 | 1.18 (0.73, 1.90) P = 0.50 |
1.23 (0.76, 2.00) P = 0.40 |
| High hs-CRP, Lp(a); Low tHcy | 58/559 | 1.45 (1.08, 1.96) P = 0.01 |
1.28 (0.94, 1.73) P = 0.12 |
27/558 | 1.34 (0.86, 2.08) P = 0.19 |
1.26 (0.80, 1.99) P = 0.31 |
| High Lp(a), tHcy; Low hs-CRP | 11/84 | 1.16 (0.63, 2.14) P = 0.62 |
1.13 (0.62, 2.09) P = 0.69 |
3/84 | 0.65 (0.20, 2.05) P = 0.46 |
0.65 (0.20, 2.06) P = 0.46 |
| High hs-CRP, tHcy; Low Lp(a) | 51/347 | 1.49 (1.10, 2.02) P = 0.01 |
1.25 (0.91, 1.72) P = 0.16 |
30/347 | 1.78 (1.17, 2.70) P = 0.007 |
1.79 (1.16, 2.76) P = 0.008 |
| High hs-CRP, Lp(a), tHcy | 21/106 | 2.40 (1.52, 3.80) P < 0.0001 |
2.02 (1.26, 3.24) P = 0.004 |
13/106 | 2.99 (1.65, 5.41) P = 0.0003 |
2.99 (1.61, 5.58) P = 0.0006 |
Abbreviations: high sensitivity C-reactive protein, hs-CRP; confidence interval, CI; coronary heart disease, CHD; estimated glomerular filtration rate, eGFR; hazard ratio, HR; high-density lipoprotein cholesterol, HDL-C; homocysteine, tHcy; interquartile range, IQR; lipoprotein(a), Lp(a).
Cox proportional hazards regression adjusted for age, sex, race/ethnicity adjusted.
Cox proportional hazards regression adjusted for age, sex, race/ethnicity, hypertension, hypertension medication usage, diabetes, pack-years smoking, HDL-C, total cholesterol, triglycerides (log-transformed), BMI and eGFR.
Low hs-CRP < 2 mg/L; High hs-CRP ≥2 mg/L; Low Lp(a) <50 mg/dL; High Lp(a) ≥50 mg/dL; Low tHcy <12 µmol/L; High tHcy=≥12 µmol/L.
Interaction p-values from fully-adjusted Cox proportional hazards regression cross-product term: CHD hs-CRP category*Lp(a) category*tHcy category P = 0.58; stroke hs-CRP category*Lp(a) category*tHcy category P = 0.34.
4. Discussion
The results of this study suggest it may be beneficial to consider biomarker combinations and to include tHCY measurements in assessing ASCVD risk. When considering combinations of two biomarkers, significantly higher CHD risk was only seen when both biomarkers were concomitantly elevated, and not with elevation of single biomarkers. Similarly, elevated stroke risk was only seen with elevated hs-CRP in combination with elevated Lp(a) or tHcy; only tHcy alone conferred significant stroke risk individually. In three-way analyses, CHD risk was elevated only when all three biomarkers were at or above clinical cut-points; stroke incidence was increased only when all three biomarkers were elevated, or when both hs-CRP and tHcy were elevated. Additionally, both CHD and stroke risk appeared highest when all three biomarkers were above clinical thresholds, and associations increased with each additional biomarker over the corresponding threshold.
Lp(a) [5,[24], [25], [26], [27]], hs-CRP [[6], [7], [8], [9]] and tHcy [[10], [11], [12], [13], [14]] have all been shown to be associated with ASCVD risk. Two prior studies, one involving primary and the other secondary prevention of ASCVD have evaluated Lp(a) and hs-CRP in combination. A secondary analysis in the prevention trial ACCELERATE demonstrated that hs-CRP may modulate Lp(a)-associated CVD risk [12]. More recently, a study of MESA participants not on statins demonstrated Lp(a) was associated with greater risk of ASCVD only when hs-CRP was >2 mg/L [19]. While Lp(a) was individually associated with CHD and hs-CRP and tHcy were individual associated with stroke when evaluated separately, these biomarkers were not individually associated with CHD when considering multiple risk factors. This may be due to smaller numbers in those categories, or the potential residual effect of the other coinciding risk factors. Our study aligns with and expands on these results by demonstrating that when considering combinations of biomarkers, the CHD risk associated with Lp(a) was only present when an individual also had elevated hs-CRP and/or tHcy. Similarly, the association between stroke risk and hs-CRP was only present when Lp(a) and/or tHcy was concomitantly elevated. In contrast, tHcy was associated with stroke risk without elevation in Lp(a), but when considering hs-CRP measurements was only associated with increased risk when hs-CRP was also elevated. The combination of elevated tHcy and Lp(a) was only of borderline significance in its association with stroke which may be due to the limited numbers of cases and participants with this biomarker combination. However, Lp(a) was not independently associated with stroke risk in this study, and these results suggest a more complicated relationship, possibly owing to the more heterogeneous nature of stroke. Using the lower clinical threshold of Lp(a) <30 mg/dL did not change study findings overall and tended to weaken associations, which may be due to the inconsistent associations observed with ASCVD risk when using a threshold of 30 mg/dL in MESA [28,29]. While risk was highest when all three biomarkers were elevated, benefits of additional measurement for risk prediction remain unclear. C-statistics suggested limited additional predictive value in study models with the additional of hs-CRP, Lp(a) and tHcy, however, future studies are needed to further explore the potential benefits of adding these biomarkers to study models as study sample size likely limited out ability to detect incremental, but still meaningful improvements in risk stratification [30,31].
In our study, the finding that elevated tHcy significantly enhances risk for CHD or stroke when combined with either hs-CRP and/or Lp(a) represents a novel finding. Numerous studies have demonstrated that systemic inflammation plays an important role as a CVD risk factor [32]. While hs-CRP is the most validated biomarker of inflammation, Lp(a) and tHcy are also known to play important roles in inflammation [12,33]. The current study demonstrated that in addition to hs-CRP, tHcy also plays a role in modulating the Lp(a)-associated ASCVD risk. Additionally, research suggests Lp(a) and tHcy are both associated with increased coagulation and appear to have a synergistic effect [[16], [17], [18]].
Homocysteine is not currently recognized as a risk-enhancing factor by the AHA/ACC guidelines. Although homocysteine has been shown to be a risk factor for ASCVD since the mid-1990s, interest in this biomarker as a CHD risk factor has diminished in the ensuing decades due in part to the failure of homocysteine-lowering intervention trials [17]. However, many of these trials had short treatment and follow up periods (≤ 2 years), and/or enlisted participants with high CHD risk, and therefore the impact of long-term homocysteine-lowering on CHD risk in low to intermediate risk individuals has not been determined. In support of tHcy risk-enhancing role, a recent publication from our group demonstrated that elevated tHcy was associated with increased risk of prevalent and incident coronary artery and descending thoracic aorta calcification and calcification progression in the MESA cohort [12].
Several recent publications have demonstrated the important role played by tHcy as a risk factor for stroke [[34], [35], [36], [37]]. Research suggests lowering tHcy concentrations may have a greater impact on reducing stroke incidence, compared with myocardial infarction [38,39]. The MTHFR C677T genotype, which is associated with higher tHcy levels is associated with small vessel disease, which is more strongly associated with stroke than CHD and hyperhomocysteinemia has been shown to be more strongly associated with risk of stroke compared to CHD [38,39]. In view of the decreased focus on tHcy in recent years as a risk factor/risk enhancer, more studies are needed to determine whether measurement of tHcy, either individually or in combination with hs-CRP and Lp(a), may offer value in assessing CHD and stroke risk in the routine care and risk assessment of patients for primary prevention of CHD and stroke.
Our study has implications for future clinical care as well as avenues for future investigation. In addition to their usefulness for assessing overall risk to CHD and stroke, all three biomarkers may be potential therapeutic targets and pathways to address residual ASCVD risk. Plasma total homocysteine levels can be lowered with B vitamins including folic acid [40,41] and has been shown to lower risk of stroke [36,42,43]. Systemic inflammation, reflected in hs-CRP levels, can be effectively lowered with lifestyle interventions [44] and medications, including statins [45] and other lipid lowering therapy, such as bempedoic acid therapy [46]. Drugs that lower inflammation such as canakinumab and colchicine have been demonstrated to improve cardiovascular outcomes in patients with established ASCVD [47,48]. While effective therapy for lowering Lp(a) is currently not available, anti-sense oligonucleotide therapy has shown promise in lowering Lp(a) [49,50]. Pelacarsen was shown to lower Lp(a) by more than 80 % in the phase II HORIZON trial with Phase III currently ongoing [51]. Additionally, all three biomarkers are currently widely available as FDA-approved assays on high-throughput testing platforms in clinical laboratories at a relatively low cost (<$20 each), and thus there would be minimal logistical or practical barriers to incorporating these biomarkers into routine CVD risk assessment.
While this study has important strengths, including the availability of all three biomarkers for evaluating combined associations and rigorous data collection and follow-up procedures, it also has some important limitations. While MESA is a relatively large study, there were small groups of participants in certain categories of combined biomarkers, particularly in the stroke analyses which has a smaller number of events than CHD, which may have limited our ability to detect a true association. It may also have limited our ability to detect statistically significant interactions which require greater statistical power. An additional limitation is that biomarkers were only measured at baseline and repeated measures were not available. While tHcy and Lp(a) have been demonstrated to be relatively stable in prior studies, hs-CRP can vary substantially across repeated measures which could not be accounted for in the present study [[52], [53], [54]].
5. Conclusions
In this study, CHD and stroke risk were highest only when Lp(a), hs-CRP and tHCY were all elevated, suggesting the additional measurement of tHCY, and consideration of combinations of multiple ASCVD risk biomarkers, may help refine identification of individuals are highest risk for CHD and stroke. Future research should consider whether adding biomarker combinations to risk prediction assessments may be beneficial for improving stratifying CHD and stroke risk.
Financial support
This research was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). HSB was supported by National Institutes of Health Grant 1KL2TR001444.
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
CRediT authorship contribution statement
Sarah O. Nomura: Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. Harpreet S. Bhatia: Writing – original draft, Writing – review & editing. Parveen K. Garg: Writing – original draft, Writing – review & editing. Amy B. Karger: Writing – original draft, Writing – review & editing. Weihua Guan: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. Jing Cao: Writing – original draft, Writing – review & editing. Michael D. Shapiro: Methodology, Writing – original draft, Writing – review & editing. Michael Y. Tsai: Conceptualization, Methodology, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2024.100903.
Contributor Information
Sarah O. Nomura, Email: oppe0020@umn.edu.
Harpreet S. Bhatia, Email: hsbhatia@health.ucsd.edu.
Parveen K. Garg, Email: parveeng@med.usc.edu.
Amy B. Karger, Email: karge026@umn.edu.
Weihua Guan, Email: wguan@umn.edu.
Jing Cao, Email: jing.cao2@utsouthwestern.edu.
Michael D. Shapiro, Email: mdshapir@wakehealth.edu.
Michael Y. Tsai, Email: tsaix001@umn.edu.
Appendix. Supplementary materials
References
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the american heart association. Circulation. 2019;139(10):e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM. How common is residual inflammatory risk? Circ Res. 2017;120(4):617–619. doi: 10.1161/CIRCRESAHA.116.310527. [DOI] [PubMed] [Google Scholar]
- 3.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;74(10):1376–1414. doi: 10.1016/j.jacc.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kronenberg F, Mora S, Stroes ESG, Ference BA, Arsenault BJ, Berglund L, et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European Atherosclerosis Society consensus statement. Eur Heart J. 2022;43(39):3925–3946. doi: 10.1093/eurheartj/ehac361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsimikas S, Hall JL. Lipoprotein(a) as a potential causal genetic risk factor of cardiovascular disease: a rationale for increased efforts to understand its pathophysiology and develop targeted therapies. J Am Coll Cardiol. 2012;60(8):716–721. doi: 10.1016/j.jacc.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 6.Cao JJ, Arnold AM, Manolio TA, Polak JF, Psaty BM, Hirsch CH, et al. Association of carotid artery intima-media thickness, plaques, and C-reactive protein with future cardiovascular disease and all-cause mortality: the Cardiovascular Health Study. Circulation. 2007;116(1):32–38. doi: 10.1161/CIRCULATIONAHA.106.645606. [DOI] [PubMed] [Google Scholar]
- 7.Puri R, Nissen SE, Arsenault BJ, St John J, Riesmeyer JS, Ruotolo G, et al. Effect of c-reactive protein on lipoprotein(a)-associated cardiovascular risk in optimally treated patients with high-risk vascular disease: a prespecified secondary analysis of the accelerate trial. Jama Cardiol. 2020;5(10):1136–1143. doi: 10.1001/jamacardio.2020.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ Res. 2016;118(1):145–156. doi: 10.1161/CIRCRESAHA.115.306656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong ND, Budoff MJ, Ferdinand K, Graham IM, Michos ED, Reddy T, et al. Atherosclerotic cardiovascular disease risk assessment: an american society for preventive Cardiology clinical practice statement. Am J Prev Cardiol. 2022;10 doi: 10.1016/j.ajpc.2022.100335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bostom AG, Rosenberg IH, Silbershatz H, Jacques PF, Selhub J, D'Agostino RB, et al. Nonfasting plasma total homocysteine levels and stroke incidence in elderly persons: the framingham study. Ann Intern Med. 1999;131(5):352–355. doi: 10.7326/0003-4819-131-5-199909070-00006. [DOI] [PubMed] [Google Scholar]
- 11.Bostom AG, Silbershatz H, Rosenberg IH, Selhub J, D'Agostino RB, Wolf PA, et al. Nonfasting plasma total homocysteine levels and all-cause and cardiovascular disease mortality in elderly Framingham men and women. Arch Intern Med. 1999;159(10):1077–1080. doi: 10.1001/archinte.159.10.1077. [DOI] [PubMed] [Google Scholar]
- 12.Karger AB, Steffen BT, Nomura SO, Guan W, Garg PK, Szklo M, et al. Association between homocysteine and vascular calcification incidence, prevalence, and progression in the MESA cohort. J Am Heart Assoc. 2020;9(3) doi: 10.1161/JAHA.119.013934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shai I, Stampfer MJ, Ma J, Manson JE, Hankinson SE, Cannuscio C, et al. Homocysteine as a risk factor for coronary heart diseases and its association with inflammatory biomarkers, lipids and dietary factors. Atherosclerosis. 2004;177(2):375–381. doi: 10.1016/j.atherosclerosis.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 14.Stampfer MJ, Malinow MR, Willett WC, Newcomer LM, Upson B, Ullmann D, et al. A prospective study of plasma homocyst(e)ine and risk of myocardial infarction in US physicians. JAMA. 1992;268(7):877–881. [PubMed] [Google Scholar]
- 15.Fu Y, Wang X, Kong W. Hyperhomocysteinaemia and vascular injury: advances in mechanisms and drug targets. Br J Pharmacol. 2018;175(8):1173–1189. doi: 10.1111/bph.13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coppola A, Davi G, De Stefano V, Mancini FP, Cerbone AM, Di Minno G. Homocysteine, coagulation, platelet function, and thrombosis. Semin Thromb Hemost. 2000;26(3):243–254. doi: 10.1055/s-2000-8469. [DOI] [PubMed] [Google Scholar]
- 17.Klein JH, Hegele RA, Hackam DG, Koschinsky ML, Huff MW, Spence JD. Lipoprotein(a) is associated differentially with carotid stenosis, occlusion, and total plaque area. Arterioscler Thromb Vasc Biol. 2008;28(10):1851–1856. doi: 10.1161/ATVBAHA.108.169292. [DOI] [PubMed] [Google Scholar]
- 18.Spence JD, Koschinsky M. Mechanisms of lipoprotein(a) pathogenicity: prothrombotic, proatherosclerotic, or both? Arterioscler Thromb Vasc Biol. 2012;32(7):1550–1551. doi: 10.1161/ATVBAHA.112.251306. [DOI] [PubMed] [Google Scholar]
- 19.Zhang W, Speiser JL, Ye F, Tsai MY, Cainzos-Achirica M, Nasir K, et al. High-sensitivity c-reactive protein modifies the cardiovascular risk of lipoprotein(a): multi-ethnic study of atherosclerosis. J Am Coll Cardiol. 2021;78(11):1083–1094. doi: 10.1016/j.jacc.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akintoye E, Afonso L, Bengaluru Jayanna M, Bao W, Briasoulis A, Robinson J. Prognostic utility of risk enhancers and coronary artery calcium score recommended in the 2018 ACC/AHA multisociety cholesterol treatment guidelines over the pooled cohort equation: insights from 3 large prospective cohorts. J Am Heart Assoc. 2021;10(12) doi: 10.1161/JAHA.120.019589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 22.Tsai MY, Johnson C, Kao WH, Sharrett AR, Arends VL, Kronmal R, et al. Cholesteryl ester transfer protein genetic polymorphisms, HDL cholesterol, and subclinical cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2008;200(2):359–367. doi: 10.1016/j.atherosclerosis.2007.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmussen K, Moller J, Lyngbak M, Pedersen AM, Dybkjaer L. Age- and gender-specific reference intervals for total homocysteine and methylmalonic acid in plasma before and after vitamin supplementation. Clin Chem. 1996;42(4):630–636. [PubMed] [Google Scholar]
- 24.Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361(26):2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 25.Emerging Risk Factors C, Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302(4):412–423. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301(22):2331–2339. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 27.Willeit P, Ridker PM, Nestel PJ, Simes J, Tonkin AM, Pedersen TR, et al. Baseline and on-statin treatment lipoprotein(a) levels for prediction of cardiovascular events: individual patient-data meta-analysis of statin outcome trials. Lancet. 2018;392(10155):1311–1320. doi: 10.1016/S0140-6736(18)31652-0. [DOI] [PubMed] [Google Scholar]
- 28.Guan W, Cao J, Steffen BT, Post WS, Stein JH, Tattersall MC, et al. Race is a key variable in assigning lipoprotein(a) cutoff values for coronary heart disease risk assessment: the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35(4):996–1001. doi: 10.1161/ATVBAHA.114.304785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steffen BT, Thanassoulis G, Duprez D, Stein JH, Karger AB, Tattersall MC, et al. Race-based differences in Lipoprotein(a)-associated risk of carotid atherosclerosis. Arterioscler Thromb Vasc Biol. 2019;39(3):523–529. doi: 10.1161/ATVBAHA.118.312267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhiman P, Ma J, Qi C, Bullock G, Sergeant JC, Riley RD, et al. Sample size requirements are not being considered in studies developing prediction models for binary outcomes: a systematic review. BMC Med Res Method. 2023;23(1):188. doi: 10.1186/s12874-023-02008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pencina MJ, D'Agostino RB. Sr. Evaluating discrimination of risk prediction models: the C statistic. JAMA. 2015;314(10):1063–1064. doi: 10.1001/jama.2015.11082. [DOI] [PubMed] [Google Scholar]
- 32.Dhindsa DS, Sandesara PB, Shapiro MD, Wong ND. The evolving understanding and approach to residual cardiovascular risk management. Front Cardiovasc Med. 2020;7:88. doi: 10.3389/fcvm.2020.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dangas G, Mehran R, Harpel PC, Sharma SK, Marcovina SM, Dube G, et al. Lipoprotein(a) and inflammation in human coronary atheroma: association with the severity of clinical presentation. J Am Coll Cardiol. 1998;32(7):2035–2042. doi: 10.1016/s0735-1097(98)00469-0. [DOI] [PubMed] [Google Scholar]
- 34.Iso H, Moriyama Y, Sato S, Kitamura A, Tanigawa T, Yamagishi K, et al. Serum total homocysteine concentrations and risk of stroke and its subtypes in Japanese. Circulation. 2004;109(22):2766–2772. doi: 10.1161/01.CIR.0000131942.77635.2D. [DOI] [PubMed] [Google Scholar]
- 35.Zhang T, Jiang Y, Zhang S, Tie T, Cheng Y, Su X, et al. The association between homocysteine and ischemic stroke subtypes in Chinese: A meta-analysis. Medicine (Baltimore) 2020;99(12):e19467. doi: 10.1097/MD.0000000000019467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao M, Wang X, He M, Qin X, Tang G, Huo Y, et al. Homocysteine and stroke risk: modifying effect of methylenetetrahydrofolate reductase C677T polymorphism and folic acid intervention. Stroke. 2017;48(5):1183–1190. doi: 10.1161/STROKEAHA.116.015324. [DOI] [PubMed] [Google Scholar]
- 37.Larsson SC, Traylor M, Markus HS. Homocysteine and small vessel stroke: a mendelian randomization analysis. Ann Neurol. 2019;85(4):495–501. doi: 10.1002/ana.25440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rutten-Jacobs LC, Traylor M, Adib-Samii P, Thijs V, Sudlow C, Rothwell PM, et al. Association of MTHFR C677T genotype with ischemic stroke is confined to cerebral small vessel disease subtype. Stroke. 2016;47(3):646–651. doi: 10.1161/STROKEAHA.115.011545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spence JD. Homocysteine-lowering therapy: a role in stroke prevention? Lancet Neurol. 2007;6(9):830–838. doi: 10.1016/S1474-4422(07)70219-3. [DOI] [PubMed] [Google Scholar]
- 40.Collaboration HLT. Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomised trials. Homocysteine Lowering Trialists' Collaboration. BMJ. 1998;316(7135):894–898. [PMC free article] [PubMed] [Google Scholar]
- 41.Wald DS, Bishop L, Wald NJ, Law M, Hennessy E, Weir D, et al. Randomized trial of folic acid supplementation and serum homocysteine levels. Arch Intern Med. 2001;161(5):695–700. doi: 10.1001/archinte.161.5.695. [DOI] [PubMed] [Google Scholar]
- 42.Huo Y, Li J, Qin X, Huang Y, Wang X, Gottesman RF, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015;313(13):1325–1335. doi: 10.1001/jama.2015.2274. [DOI] [PubMed] [Google Scholar]
- 43.Marti-Carvajal AJ, Sola I, Lathyris D, Dayer M. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2017;8(8) doi: 10.1002/14651858.CD006612.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuczmarski MF, Mason MA, Allegro D, Zonderman AB, Evans MK. Diet quality is inversely associated with C-reactive protein levels in urban, low-income African-American and white adults. J. Acad. Nutrit. Dietetics. 2013;113(12):1620–1631. doi: 10.1016/j.jand.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM, Kastelein JJP, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 46.Ballantyne CM, Laufs U, Ray KK, Leiter LA, Bays HE, Goldberg AC, et al. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur J Prev Cardiol. 2020;27(6):593–603. doi: 10.1177/2047487319864671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ, et al. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391(10118):319–328. doi: 10.1016/S0140-6736(17)32814-3. [DOI] [PubMed] [Google Scholar]
- 48.Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381(26):2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 49.Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, Tardif JC, Baum SJ, Steinhagen-Thiessen E, et al. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med. 2020;382(3):244–255. doi: 10.1056/NEJMoa1905239. [DOI] [PubMed] [Google Scholar]
- 50.Viney NJ, van Capelleveen JC, Geary RS, Xia S, Tami JA, Yu RZ, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388(10057):2239–2253. doi: 10.1016/S0140-6736(16)31009-1. [DOI] [PubMed] [Google Scholar]
- 51.Tsimikas S, Moriarty PM, Stroes ES. Emerging RNA therapeutics to lower blood levels of Lp(a): JACC focus seminar 2/4. J Am Coll Cardiol. 2021;77(12):1576–1589. doi: 10.1016/j.jacc.2021.01.051. [DOI] [PubMed] [Google Scholar]
- 52.Garg UC, Zheng ZJ, Folsom AR, Moyer YS, Tsai MY, McGovern P, et al. Short-term and long-term variability of plasma homocysteine measurement. Clin Chem. 1997;43(1):141–145. [PubMed] [Google Scholar]
- 53.Trinder M, Paruchuri K, Haidermota S, Bernardo R, Zekavat SM, Gilliland T, et al. Repeat measures of Lipoprotein(a) molar concentration and cardiovascular risk. J Am Coll Cardiol. 2022;79(7):617–628. doi: 10.1016/j.jacc.2021.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bogaty P, Dagenais GR, Joseph L, Boyer L, Leblanc A, Belisle P, et al. Time variability of C-reactive protein: implications for clinical risk stratification. PLoS One. 2013;8(4):e60759. doi: 10.1371/journal.pone.0060759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.