Abstract
Background
The cockroach salivary gland consists of secretory acini with peripheral ion-transporting cells and central protein-producing cells, an extensive duct system, and a pair of reservoirs. Salivation is controled by serotonergic and dopaminergic innervation. Serotonin stimulates the secretion of a protein-rich saliva, dopamine causes the production of a saliva without proteins. These findings suggest a model in which serotonin acts on the central cells and possibly other cell types, and dopamine acts selectively on the ion-transporting cells. To examine this model, we have analyzed the spatial relationship of dopaminergic and serotonergic nerve fibers to the various cell types.
Results
The acinar tissue is entangled in a meshwork of serotonergic and dopaminergic varicose fibers. Dopaminergic fibers reside only at the surface of the acini next to the peripheral cells. Serotonergic fibers invade the acini and form a dense network between central cells. Salivary duct segments close to the acini are locally associated with dopaminergic and serotonergic fibers, whereas duct segments further downstream have only dopaminergic fibers on their surface and within the epithelium. In addition, the reservoirs have both a dopaminergic and a serotonergic innervation.
Conclusion
Our results suggest that dopamine is released on the acinar surface, close to peripheral cells, and along the entire duct system. Serotonin is probably released close to peripheral and central cells, and at initial segments of the duct system. Moreover, the presence of serotonergic and dopaminergic fiber terminals on the reservoir indicates that the functions of this structure are also regulated by dopamine and serotonin.
Background
Cockroaches have acinar salivary glands that consist of secretory acini and an extensive duct system [1,2](see Fig. 1a). In addition to the salivary glands proper, the salivary gland complex includes a pair of reservoirs with their ducts, and an extrinsic muscle associated with the orifice of each reservoir [3].
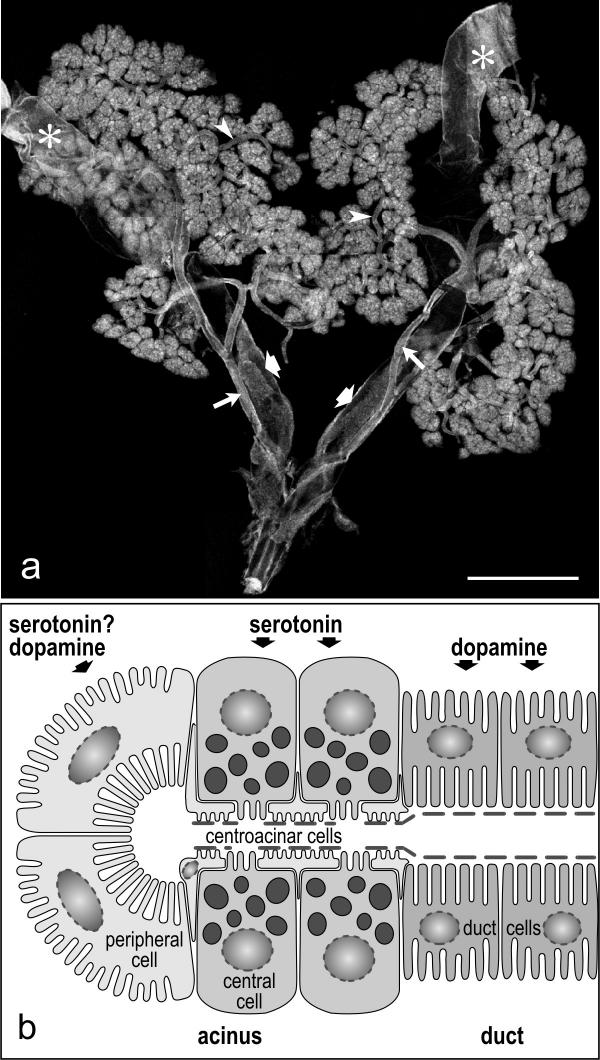
Figure 1.
Morphology of the salivary glands in the cockroach Periplaneta americana a: Low-power micrograph of the salivary gland complex. The salivary glands are paired and consist of several lobes of secretory acini. The ducts (arrowheads) of each gland unite to a single efferent salivary duct (long arrows) that then fuses with the opposite duct to form the main salivary duct. Two reservoirs (asterisks) are associated with the secretory tissue. The reservoirs open into reservoir ducts (broad arrows) that accompany the efferent salivary ducts. b: Schematic representation of the structural organization of secretory acini. Each acinus consists of two peripheral cells with long microvilli and several central cells with numerous secretory granules. The apical surface of the central cells is covered by a sheath of flattened fenestrated centroacinar cells and by a thin discontinuous layer of cuticule. The central cells are stimulated only by serotonin, whereas the peripharal cells respond to dopamine and probably also to serotonin. The duct cells have basal and apical infoldings and are only responsive to dopamine. Scale bar = 2 mm
The physiology of the salivary gland complex and the neuronal and cellular control of salivation are poorly understood. The following picture emerges from currently available evidence. The salivary glands secrete saliva of two different qualities, either with or without proteins [4]. Salivation appears to be mainly controled by direct serotonergic and dopaminergic innervation from the subesophageal ganglion and the stomatogastric nervous system [5-8]. A pair of large dopaminergic neurons located within the subesophageal ganglion and termed SN1 (salivary neuron 1) send their axons via nerves extending along the salivary ducts toward the salivary glands where they ramify extensively [6,8]. This appears to be the only source of dopaminergic innervation of the salivary glands. Serotonergic innervation is achieved via several small axons within the salivary nerves and the esophageal nerve. The latter branches into several thin nerves that form a network over the acinar tissue [8,9]. Despite this general picture, the exact topography of the dopaminergic and serotonergic nerve fibers, their varicosities, and/or their terminals with respect to the various structures and cell types engaged in saliva production and modification is still insufficient for a stringent correlation of innervation and gland physiology.
The acini of cockroach salivary glands are grape-like structures and composed mainly of peripheral cells and central cells [2] (see Fig. 1b). Peripheral cells reside in pairs at the distal end of each acinus, possess long apical microvilli equipped with Na+,K+-ATPase, and are specialized for water and electrolyte transport [10]. Central cells are densely packed with secretory granules and produce the proteinaceous components of the saliva [2,4]. The saliva secreted within the acinar portions of the glands then passes through the salivary ducts composed of a simple epithelial layer. The duct cells have an extensive basal labyrinth carrying Na+,K+-ATPase molecules and apical infoldings studded with vacuolar H+-ATPase molecules [10], suggesting that this cell type modifies the ionic composition and/or the volume of the primary saliva.
Both serotonin and dopamine have been shown to stimulate salivation in isolated salivary glands; however, the quality of the saliva differs upon exposure to these substances [4]. Superfusion of salivary glands with serotonin leads to the exocytosis of secretory granules and the production of a protein-rich saliva, suggesting that at least the central cells are responsive to serotonin. Saliva produced upon dopamine application, in contrast, is completely free of proteins, indicating that this neurotransmitter acts selectively on the ion-transporting cells, viz., the peripheral cells and/or the duct cells. Electrophysiological studies on salivary duct cells have further shown that dopamine induces a slow depolarization, evokes an increase in the intracellular Ca2+ concentration, and elicits an intracellular Na+ elevation and K+ reduction in these cells [11,12]. Serotonin, in contrast, appears to have no effect on salivary duct cells [11].
The above results lead to a model in which salivary duct cells are stimulated exclusively by dopamine and central cells exclusively by serotonin. Peripheral cells may be responsive only to dopamine or to both neurotransmitter substances. In order to examine this model further, we have analyzed the exact spatial relationship of dopaminergic and serotonergic nerve fibers to these cells types by studying anti-dopamine and anti-serotonin immunofluorescence on whole-mount preparations of salivary glands in conjunction with high-resolution confocal microscopy. Close apposition of fiber terminals and/or varicosities to a distinct cell type provides evidence for a selective innervation of the respective cell type. We demonstrate that the innervation pattern is more complex than expected from the above model, but that it essentially supports this working hypothesis. In addition, we have examined the serotonergic and dopaminergic innervation of other structures associated with the salivary glands (see Fig. 1a), viz., the reservoirs, the reservoir ducts, and the muscles attached to the reservoir orifices.
Results
Specificity of antibody labeling
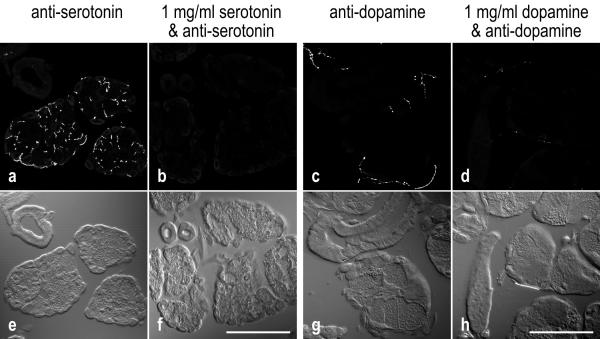
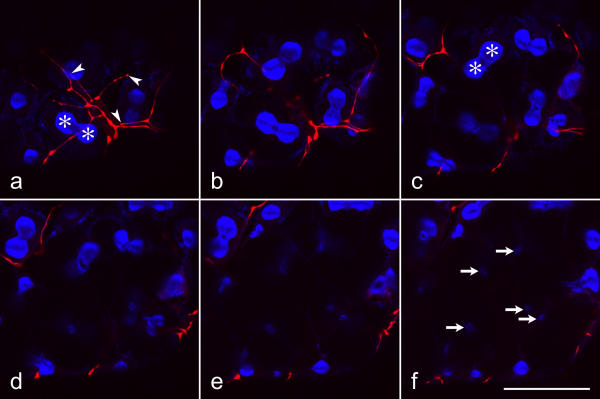
On cryostat sections of cockroach salivary glands, both anti-serotonin and anti-dopamine intensely stained fiber-like structures and individual punctae, the latter probably representing cross-sectioned fibers (Fig. 2a,2c). Specificity of labeling was tested by preabsorption of the primary antibodies with the corresponding antigens, serotonin or dopamine, respectively. Under these conditions, immunoreactivity was highly reduced or absent (Fig. 2b,2d), suggesting that these antibodies identify their appropriate antigens within cockroach salivary glands.
Figure 2.
Specificity of anti-serotonin and anti-dopamine labeling
a-d: Fluorescence confocal images, representing the summarized view of 9-μm-thick image stacks. e-h: Nomarski contrast images of the same areas. a,b: Cryostat sections of salivary glands incubated with anti-serotonin in the absence or in the presence of 1 mg/ml serotonin. c,d: Sections reacted with anti-dopamine in the absence or in the presence of 1 mg/ml dopamine. Immunoreactivity of the tissue is highly reduced in the presence of the corresponding antigen. Scale bars = 100 μm
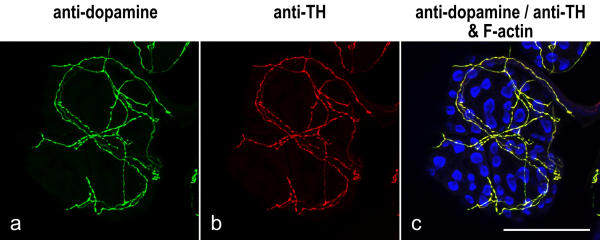
Further support for the specificity of anti-dopamine immunoreactivity was provided by colabeling experiments with affinity-purified antibody against tyrosine hydroxylase (TH), a common probe for dopaminergic neurons in insects [6,8]. TH is the first and rate-limiting enzyme in the synthesis of the catecholamines dopamine, norepinephrine and epinephrine, of which dopamine is the major amine found in insects [13]. When the anti-TH antibody was applied to whole-mounts of salivary glands, it produced a labeling pattern that precisely corresponded to the anti-dopamine-immunoreactive structures (Fig. 3).
Figure 3.
Codistribution of anti-dopamine and anti-TH immunolabeling Whole-mounts of salivary glands were triple-labeled with anti-dopamine (green), anti-TH (red) and BODIPY FL phallacidin (blue), and imaged by confocal microscopy. The image shows a lobule of acinar tissue; the peripheral cells are arranged in pairs and their apical arrays of phallotoxin-stained microvilli appear as "bow ties". A sparse network of fibers resides on the tissue and is labeled by both anti-dopamine and anti-TH. Scale bar = 100 μm
It should be noted that colabeling experiments with anti-dopamine and anti-serotonin were not successful as these antibodies required different protocols for tissue fixation. The anti-dopamine provided specific labeling only in specimens fixed in the presence of at least 0.5% glutaraldehyde. The anti-serotonin, however, only exhibited specific immunoreactivity in tissue fixed without glutaraldehyde.
Distribution of serotonergic and dopaminergic nerve fibers over the secretory acini
The distribution of serotonergic and dopaminergic fibers within the salivary gland complex was probed by confocal fluorescence microscopy of whole-mount preparations stained with anti-serotonin or anti-dopamine. In order to locate the various acinar cells and to provide a spatial reference for the position of the immunoreactive fibers within the tissue, specimens were co-labeled with fluorochrome-tagged phallacidin, a probe for actin filaments [2]. Peripheral cells with their densely packed, long microvilli are arranged in pairs that are visualized as brightly fluorescent "bow ties" in phallotoxin-stained preparations (Figs. 3c, 4, 7, 8). The acinar lumen surrounded by central cells with their short microvilli is delimited by weak labeling with phallotoxin (Figs. 4d,4e,4f, 7d,7e,7f, 8a,8d).
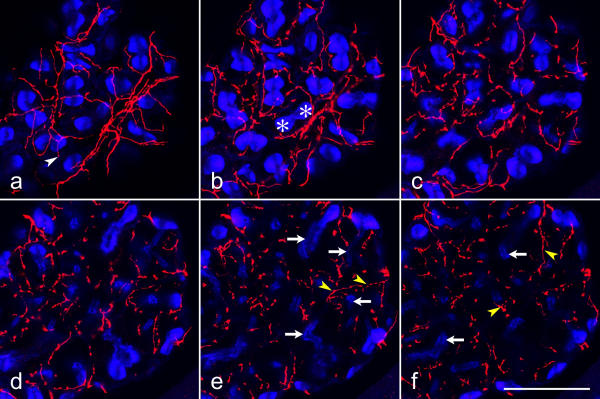
Figure 4.
Distribution of serotonergic nerve fibers on the salivary gland acini Whole-mounts of salivary glands were double-labeled with anti-serotonin (red) and BODIPY FL phallacidin (blue), and imaged by confocal microscopy. Each image shows the sum of 8 consecutive optical sections (inter-section distance 0.35 μm), representing a total thickness of 2.8 μm. Serotonergic fibers and fiber endings (white arrowhead) form a network on the acinar surface (a) over the peripheral cells (asterisks). The fibers extend deep into the acini (yellow arrowheads) between the central cells that are identified by short, phallotoxin-labeled microvilli (arrows) on their luminal surface. Scale bar = 50 μm
Figure 7.
Distribution of dopaminergic nerve fibers on the salivary gland acini Whole mounts of salivary glands were double-labeled with anti-dopamine (red) and BODIPY FL phallacidin (blue), and imaged by confocal microscopy. Parameters of image acquisition and data presentation are identical to those of Fig. 4. Dopaminergic fibers and their endings (arrowheads) reside on the acinar surface over and between the peripheral cells (asterisks). The inner part of the acinar lobule with the central cells and the acinar lumen (arrows in f) lacks dopaminergic fibers. Scale bar = 50 μm
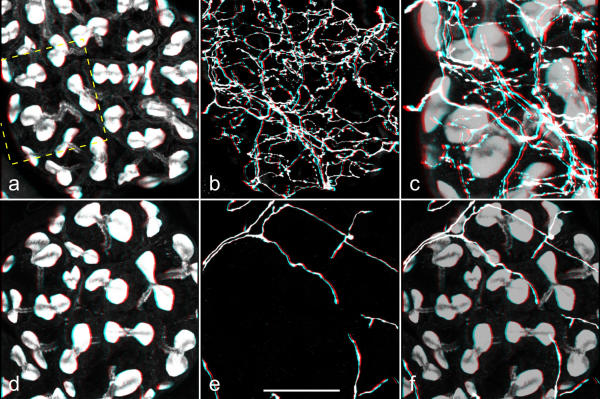
Figure 8.
Three-dimensional (red-green) views of serotonergic and dopaminergic fibers associated with acinar lobules Salivary glands were double-labeled with BODIPY FL phallacidin (a,d) and anti-serotonin (b) or anti-dopamine (e). Stacks of confocal images were recorded, and three-dimensional reconstructions were made by using Carl Zeiss LSM510 software. c,f: The corresponding images of staining with phallotoxin and with antibody were added (a+b or d+e; the phallotoxin image was multiplied with the factor 0.7 to reduce its intensity) in order to present both staining patterns together. The rectangle in a indicates the area that is presented at higher magnification in c. b,c: A dense network of serotonergic fibers extends throughout the entire acinar tissue. e,f: Dopaminergic fibers, in contrast, form a loose network only on the acinar surface. Scale bar = 50 μm
Serotonergic fibers formed a dense network on the surface of the acinar lobules (Fig. 4a). Fibers branched on the lobule surface and displayed either uniform staining over extended stretches or had an irregular beaded appearance. The former fibers appeared thicker in diameter than the varicose fibers and united into bundles that interlinked adjacent lobules (Fig. 5). Moreover, some of these fiber bundles extended away fom the acinar tissue (data not shown); these may represent branches of the esophageal nerve that innervate the acinar tissue and that may have been ruptured during dissection of the salivary gland complex. Other fiber bundles linked the serotonergic network on the acinar tissue to the salivary nerve, supporting the view that both the stomatogastric nervous system and the subesophageal ganglion contribute to the serotonergic innervation of the glandular tissue [8]. Serial confocal sections (Fig. 4a,4b,4c,4d,4e,4f) or cryostat sections (Fig. 2a) through the acinar lobules demonstrated further that serotonergic fibers were not confined to the tissue surface but extended throughout the acini, forming a dense three-dimensional meshwork. These invading fibers were mostly varicose in appearance and resided either below the peripheral cells, suggesting a location between peripheral and central cells, or were localized far deeper than the peripheral cells, suggesting a position among the central cells.
Figure 5.
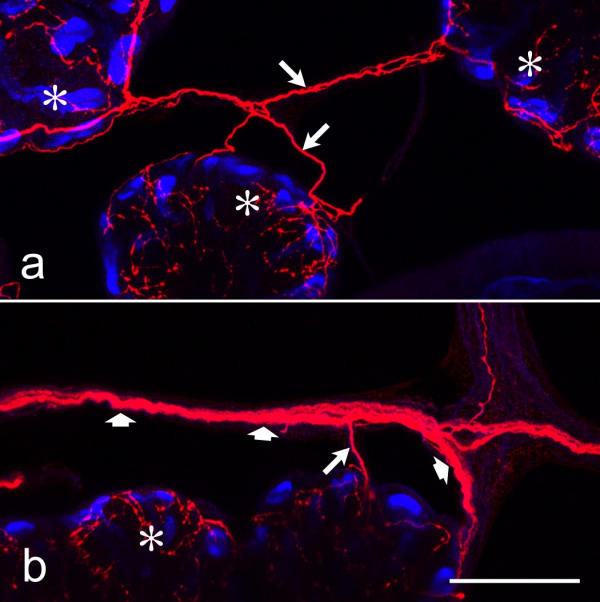
Serotonergic nerve fibers in nerves that interlink adjacent acini Whole-mounts of salivary glands were double-labeled with anti-serotonin (red) and BODIPY FL phallacidin (blue), and imaged by confocal microscopy. Nerves of large (broad arrows) or small (long arrows) diameter interlink the acinar lobules (asterisks) and contain serotonergic fibers. Scale bar = 50 μm
In addition to serotonergic fibers, the nerves interlinking the acinar lobules contained dopaminergic fibers with varicosities and fiber terminals (Fig. 6). In some regions of these nerves, the dopaminergic fibers ramified extensively and had numerous varicosities (Fig. 6b), suggesting that these structures represent neurohemal organs. Individual dopaminergic fibers of these nerves approached acinar lobules and formed a widely spaced network on the lobule surface (Fig. 7). These acinar-tissue-associated dopaminergic fibers had few varicosities irregularly distributed over their length and sidebranches with terminals on the tissue surface (Fig. 7a). Serial confocal sections through acinar lobules demonstrated that, in contrast to serotonergic fibers, dopaminergic fibers did not invade the acinar tissue but were confined to the surface (Fig. 7a,7b,7c,7d,7e,7f). However, extensive cross-linking by the use of glutaraldehyde as a fixative may have prevented penetration of the antibodies into the tissue, and thus the lack of anti-dopamine-immunoreactive structures within the acinar lobules might have been an artefact. Several lines of evidence indicated that this was not the case. First, immunoreactivity was also confined to the surface of the acinar lobules when anti-dopamine was applied to cryostat sections (Fig. 2c). Second, an identical staining pattern was obtained with anti-dopamine on whole-mounts fixed with a low concentration of glutaraldehyde (0.5%; data not shown), and with anti-TH on whole-mounts prepared by the same glutaraldehyde-free fixation protocol as that used for labeling with anti-serotonin (data not shown). Finally, anti-dopamine-positive fibers could be detected not only on the surface, but also within the tissue of other structures of the salivary gland complex (see below).
Figure 6.
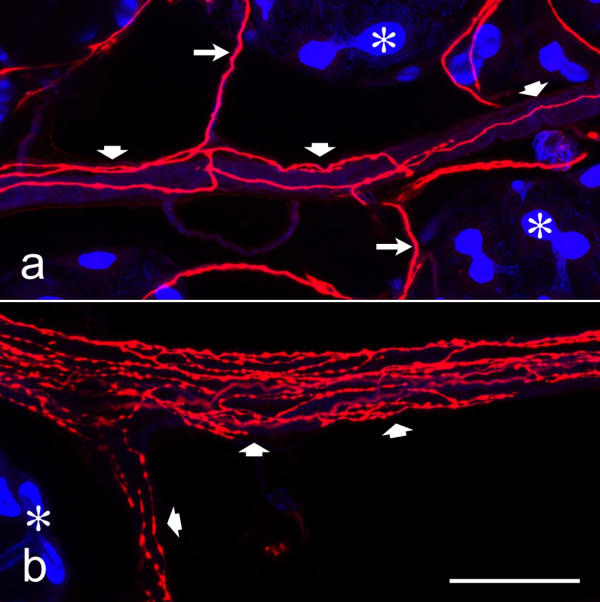
Dopaminergic nerve fibers within nerves that extend between acini Whole mounts of salivary glands were double-labeled with anti-dopamine (red) and BODIPY FL phallacidin (blue), and imaged by confocal microscopy. Acinar lobules (asterisks) are connected by nerves of large (broad arrows) and small (long arrows) diameter, containing dopaminergic fibers. In some of theses nerves, the dopaminergic fibers branch extensively and have numerous varicosities (b), suggesting that these sites represent neurohemal organs. Scale bar = 50 μm
In conclusion, serotonergic and dopaminergic fibers had a dissimilar distribution over the acinar tissue. These differences between serotonergic and the dopaminergic innervation can be directly visualized in Figure 8, presenting three-dimensional views of the two fiber types associated with the acinar lobules. A striking feature of the serotonergic innervation was its richness not only at the lobule surface, but throughout the entire acini. Dopaminergic fibers, in contrast, were sparse and resided only on the surface of the lobules. Moreover, dopaminergic fibers appeared to form neurohemal organs between acinar lobules.
Serotonergic and dopaminergic nerve fibers along the efferent salivary ducts and the reservoir ducts
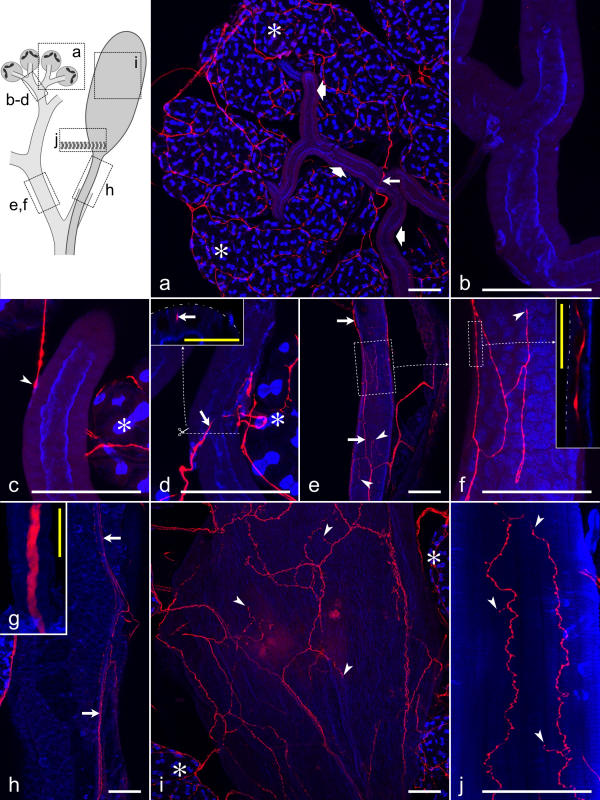
Each of the paired reservoir ducts was accompanied by a large salivary nerve with a 5-μm-thick dopaminergic axon residing at its center (Fig. 9g). The dopaminergic axon extended all the way toward the acinar tissue, supporting the conclusion that it provides the only source of dopaminergic innervation of the salivary gland complex [6,8]. Along the salivary nerves, thin dopaminergic fibers branched off the central axon. These varicose fibers either remained in a superficial position within the nerves, or they left the nerves and spread, either individually or in small bundles, over the outer surface of the reservoir duct (Fig. 9h). Some of these dopaminergic fibers extended from the salivary nerve toward the adjacent efferent salivary duct. Here, varicose fibers and fiber terminals formed a widely spaced network on the outer duct surface (Fig. 9e,9f) and also invaded the epithelium, as demonstrated by vertical optical sections through the ducts (Fig. 9f, inset). It must be noted that, although this dopaminergic innervation was found along almost the entire efferent salivary duct, only a minority of the epithelial cells had intimate contact to dopaminergic fibers.
Figure 9.
Distribution of dopaminergic fibers on salivary ducts, the reservoir, and the reservoir muscle Summarized views of confocal image stacks through whole-mounts double-labeled with anti-dopamine (red) and BODIPY FL phallacidin (blue). The upper left inset presents a scheme of the various structures examined and outlines the areas shown in a-j. Asterisks in a,c,d,i indicate acinar tissue. a: Small salivary ducts (broad arrows) are mostly without dopaminergic fibers (long arrow). b: A small salivary duct without dopaminergic innervation at higher magnification. c: A dopaminergic fiber approaches a small salivary duct and terminates on the duct surface (arrowhead). d: A dopaminergic fiber (arrow) invades the epithelium of a small duct. A vertical section (inset) through the duct at the position indicated by the line in d demonstrates that the dopaminergic fiber (arrow) resides below the duct surface (broken line). e,f: Dopaminergic fibers (arrows) form a loose network on a large salivary duct and terminate on this structure (arrowheads). The inset in f shows a horizontal confocal section through the duct and visualizes a dopaminergic fiber within the duct epithelium, below the duct surface (broken line). g: The salivary nerve coming from the subesophageal ganglion and extending along the reservoir/salivary duct complex contains a single thick dopaminergic axon. h: On the reservoir duct, small dopaminergic varicose fibers reside superficially within the salivary nerve (arrows) or leave the nerve and extend over the duct surface. i: A loose network of dopaminergic fibers with fiber terminals (arrowheads) covers the reservoir. j: Dopaminergic fibers and terminals (arrowheads) within the reservoir muscle. White scale bars = 100 μm; yellow scale bars = 25 μm
In addition to the large dopaminergic axon, the salivary nerves contained several thin serotonergic fibers in a superficial position and with numerous varicosities (Fig. 10g, inset). Moreover, individual serotonergic fibers left the nerves, spread toward the reservoir ducts and terminated on the duct surface (Fig. 10g). In rare cases, serotonergic fibers could be traced to an efferent salivary duct and appeared to end on this structure (data not shown). The majority of efferent salivary ducts, however, was without serotonergic innervation (Fig. 10f).
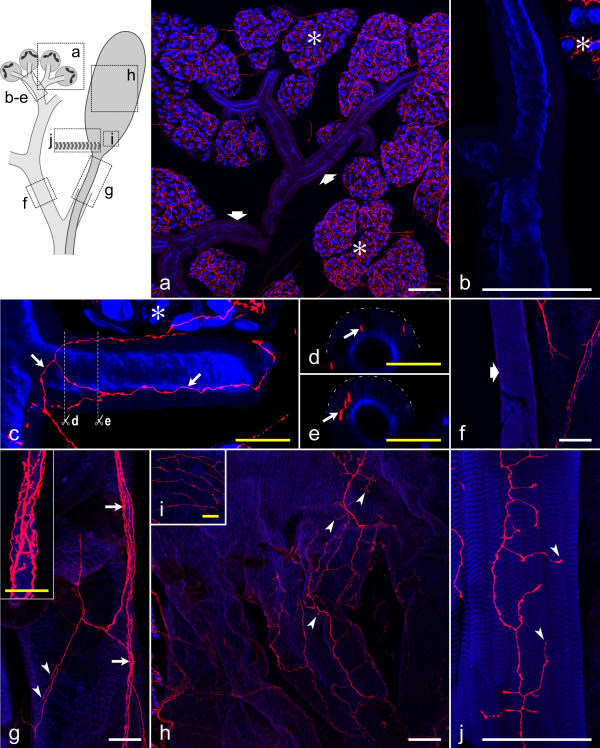
Figure 10.
Distribution of serotonergic fibers on salivary ducts, the reservoir, and the reservoir muscle The upper left inset indicates the structures shown in a-j. a-c, f-j: Summarized views of confocal image stacks through whole-mounts, double-labeled with anti-serotonin (red) and BODIPY FL phallacidin (blue). Asterisks in a,b,c indicate acinar tissue. a: A dense network of serotonergic fibers is associated with the acini (asterisks), whereas small salivary ducts (broad arrows) are mostly without serotonergic fibers. b: A small salivary duct without serotonergic innervation at higher magnification. c: A small salivary duct with a network of serotonergic fibers (arrows). d,e: Vertical sections through the salivary duct shown in c (planes indicated by white lines), demonstrating that the serotonergic fibers (arrows) reside below the duct surface (broken lines). f: A large salivary duct (broad arrow) without serotonergic innervation. g: The reservoir duct is accompanied by a nerve (arrows and inset) containing several serotonergic fibers. Fibers in a superficial position within the nerve have numerous varicosities (inset). Individual fibers also extend over the reservoir duct and have terminals (arrowheads) associated with this structure. h: A loose network of serotonergic fibers, with their terminals (arrowheads), covers the middle part of the reservoir. i: At the orifice, the reservoir has a relatively dense network of serotonergic fibers on its surface. Note that i is enlarged twofold compared with h. j: The reservoir muscle contains numerous serotonergic fiber terminals (arrowheads). White scale bars = 100 μm; yellow scale bars = 25 μm
Distribution of serotonergic and dopaminergic nerve fibers over small salivary ducts
Most of the salivary duct system upstream of the paired efferent salivary ducts was exclusively, but only locally, innervated by dopaminergic fibers (Figs. 9a,9b,9c,9d). The varicose fibers and fiber terminals formed a loose network on the outer duct surface and often invaded the epithelium (Fig. 9d, inset). On duct segments next to the acinar tissue, however, both dopaminergic and serotonergic varicose fibers extended from the acinar lobules to the duct surface and deep into the epithelium (Fig. 10c,10d,10e). Again, the innervation of these most proximal segments of the duct system was only local, and on the majority of these small salivary ducts close to the acinar tissue, no serotonergic or dopaminergic fibers could be detected at all (Figs. 9a,9b, 10a,10b).
Association of serotonergic and dopaminergic nerve fibers with the reservoir system
The paired reservoirs reside amidst the acinar tissue (Fig. 1a). Nerve fibers that entangled the acinar tissue extended toward the reservoirs, and both serotonergic and, as reported previously [6], dopaminergic fibers were detected on the surface of the reservoirs. However, we noticed differences in the distribution of serotonergic and dopaminergic fibers over this structure. Dopaminergic fibers branched and formed a loose network over the entire reservoir. These fibers had a varicose morphology and terminated on the reservoir (Fig. 9i). The serotonergic innervation pattern, in contrast, varied along the reservoir. On the distal half of the reservoir, serotonergic fibers appeared to be absent (data not shown). Its middle section had a loose network of varicose serotonergic fibers on the outer surface (Fig. 10h); these fibers were linked to acinar-tissue-associated serotonergic fibers via small nerves, indicating that they originated in the stomatogastric nervous system and/or the subesophageal ganglion. Finally, the basal part of the reservoir next to the orifice into the reservoir duct had a relatively dense network of varicose serotonergic fibers and fiber terminals on its surface (Fig. 10i). These serotonergic fibers could be traced back directly to the salivary nerve accompanying the reservoir duct, suggesting that they originated in the subesophageal ganglion.
The reservoir muscle is attached near the orifice of each reservoir [3]. Both serotonergic and dopaminergic fibers were detected within this muscle (Figs. 9j, 10j). The fibers branched extensively and had numerous varicosities and nerve terminals.
Discussion
In the present study, serotonergic and dopaminergic nerve fibers were identified by immunolabeling of the cockroach salivary gland complex with anti-serotonin/anti-dopamine antibodies and confocal fluorescence imaging. The results of these analyses are summarized in a schematic manner in Figure 11 and demonstrate that:
Figure 11.
Schematic presentation of the distribution of serotoneric and dopaminergic fibers over the salivary gland complex The salivary gland is innervated by the salivary nerve (1) and via branches of the esophageal nerve (2). The salivary nerve accompanies the reservoir duct and contains one thick dopaminergic axon. Moreover, several serotonergic fibers ramify within the nerve and have numerous varicosities. Nerves containing numerous dopaminergic varicose fibers (3) link the acinar lobules and may function as neurohemal organs.
1. Serotonergic varicose fibers are associated with the lobule surface and invade each acinus to form a dense network over and within the entire acinar lobule. Thus, serotonergic varicosities and fiber terminals are found next to peripheral cells and central cells.
2. Dopaminergic varicose fibers form a loose network only on the surface of the acinar tissue, being closely positioned to peripheral cells.
3. In nerves interlinking adjacent acinar lobules, dopaminergic fibers ramify extensively and have numerous varicosities (Fig. 11, #3), suggesting that these structures represent sites for the neurohemal release of dopamine.
4. Segments of the salivary duct system immediately adjacent to the acini are sparsely innervated by both serotonergic and dopaminergic fibers. Segments of the duct system further downstream are exclusively associated with dopaminergic fibers. These fibers reside on the outer surface of the ducts and invade the epithelium where they terminate between the duct cells.
5. The entire reservoir system, composed of the reservoir, the reservoir duct, and the reservoir muscle, is innervated by dopaminergic and serotonergic fibers. Within the salivary nerve along the reservoir duct, these serotonergic fibers branch and form varicosities (Fig. 11, #1), suggesting that this portion of the nerve serves the neurohemal release of serotonin.
Innervation of the acinar tissue
The innervation of the cockroach salivary gland has been investigated previously by light microscopy of methylene-blue stained preparations and by electron microscopical techniques [5,7]. These studies have established that the salivary gland receives innervation via the salivary nerves emerging form the subesophageal ganglion and via the esophageal nerves of the stomatogastric nervous system. By labeling with anti-serotonin and anti-TH, evidence has been provided that each of the paired salivary nerves contains a single dopaminergic axon and several thin serotonergic axons, whereas the stomatogastric nervous system provides only serotonergic innervation of the salivary gland [6,8,9]. Although the focus of the present study has not been on the origin of the innervation, our results are generally in agreement with the conclusions of the aforementioned studies. The acinar tissue thus appears to have a dual innervation by serotonergic fibers, whereas dopaminergic innervation is provided by the salivary nerve only. This situation raises the question as to whether serotonergic fibers of stomatogastric and of subesophageal origin have a different distribution over the acinar tissue, or in other words, whether they innervate different cell types. However, because of the density and complexity of the serotonergic fiber network associated with the acinar tissue, individual fibers could not be traced back to their source, and therefore this question has to remain unanswered for now.
Over and within the acinar lobules, serotonergic fibers form a dense three-dimensional plexus with numerous varicosities. On the acinar surface, each peripheral cell appears to have a neighboring serotonergic fiber. Likewise, although we have no marker at hand that permits the identification of individual central cells, the density of the serotonergic fiber network within the acinar tissue suggests that every central cell has immediate contact to a serotonergic fiber. The bouton-like structures along these fibers possibly represent sites of neurotransmitter release, similar to the situation at the Drosophila neuromuscular junction [14]. Moreover, by transmission electron microscopy of cockroach acinar tissue, axonal profiles with numerous synaptic vesicles have been observed not only on the acinar surface but also embedded between central cells [5,15]. At these sites, the axonal profiles are without glial wrappings and occasionally have an electron-dense plaque on the axonal membrane, indicating an active zone. Finally, preliminary data suggest that the serotonin-positive varicosities as well as the dopamine-positive varicosities associated with the acinar tissue colocalize with a marker for synapses (O. Baumann, D. Kühnel, P. Dames and B. Walz, in preparation). It may be concluded that serotonin is liberated both on the surface of the acini, next to each peripheral cell, and deep within the acini, next to each central cell.
Physiological studies have demonstrated that central cells are responsive to serotonin, and that serotonin application stimulates the exocytosis of secretory granules [4]. For peripheral cells, in contrast, direct evidence for a physiological response to serotonin is lacking. The close spatial relationship of serotonergic varicosities to peripheral cells however indicates that serotonin also acts on this cell type. We suggest that serotonin stimulates electrolyte and water transport across peripheral cells in order to flush the secretory products of the central cells out of the acini.
Dopaminergic fibers are confined to the surface of the acini and form a relatively loose network. Thus, not every pair of peripheral cells has a dopaminergic varicose fiber in its immediate vicinity. Moreover, within the nerves interlinking adjacent acinar lobules, dopaminergic fibers ramify extensively and have numerous swellings, indicating that these structures serve the neurohemal release of dopamine. This confinement of dopaminergic fibers to the periphery of the acinar lobules is in agreement with the suggestion that only the peripheral cells are sensitive to dopamine [4]. The sparsity of dopaminergic fibers in association with the acinar tissue and the presence of putative sites of neurohemal release of dopamine suggest further that dopamine acts not as a neurotransmitter, but is released into the hemolyph to function as a paracrine substance or neurohormone.
Dopaminergic neurohemal organs have not been described in insects so far, whereas serotonergic, octopaminergic, histaminergic and peptidergic neurohemal organs seem to be quite common in the peripheral nervous system [e.g., [9,16-18]]. It must be admitted, however, that the presence of varicose fibers detected by light microscopy can only be taken as an indication of neurosecretion, and thickened fiber sites could also result from an accumulation of cell organelles, such as mitochondria. Unequivocal identification of these structures as neurohemal organs requires confirmation by use of other techniques. Therefore, a detailed analysis of the distribution of a synapse-specific protein and of the ultrastructure of the fibers associated with salivary gland complex is in progress (O. Baumann, D. Kühnel, P. Dames and B. Walz, in preparation). Preliminary data suggest an enrichment of a synapse-specific protein within these varicosites, providing further support for the conclusion that these structures serve as neurohemal organs.
Innervation of the salivary duct system
Although the innervation of the cockroach salivary gland has been studied previously by various techniques, an association of nerve fibers with the salivary duct system has not been reported so far, except for the paired efferent salivary ducts [5]. The reason for this may be that smaller duct segments are embedded between the acinar lobules and are thus not immediately accessible to conventional light-microscopic techniques, and that nerve fibers are sparse along the duct system and therefore detectable by electon microscopy in serial sections only. By confocal fluorescence microscopy, however, it is possible to determine the exact spatial relationship between fluorescently labeled fibers and the duct epithelium.
Dopaminergic fibers are present over the entire length of the duct system but innervate only small areas. Thus, only a small number of duct epithelial cells resides in close apposition to dopaminergic fibers. Surprisingly, rather than remaining on the outer epithelial surface, varicose fibers invade the epithelium, suggesting that dopamine is released deep within the epithelial layer.
Physiological studies have demonstrated that the duct epithelial cells are responsive to dopamine [11,12]. However, how do all duct cells become stimulated when only a fraction of them has intimate contact to dopaminergic varicosities? One possibility is that the putative neurohemal structures at the acinar periphery represent the major source of dopamine acting on the salivary duct cells. An alternative, but not exclusive, possibility is that direct stimulation of only a few epithelial cells is sufficient to activate ion transport mechanisms in the entire epithelium, because the cells are extensively coupled by gap junctions [19], and second messengers may diffuse through gap junctions from the dopamine-activated cells to their neighbors. This suggestion is directly supported by ratiometric imaging of dopamine-induced spatiotemporal intracellular Ca2+ changes in the salivary duct epithelial cells loaded with Fura-2. Dopamine stimulates a Ca2+ elevation in duct cells at several points along the ducts, and from there, the increase in intracellular Ca2+ spreads over the duct as a Ca2+ wave at a velocity of 3.7 μm s-1[11].
The presence of serotonergic varicose fibers on some duct segments may seem to contradict the results of previous physiological studies, showing that duct cells are unresponsive to serotonin [11]. However, serotonergic innervation is restricted to segments immediately adjacent to the acini and to the efferent salivary duct. Our physiological studies on the cockroach salivary duct, in contrast, have been performed on areas in between these segments [11] and, thus, on areas that are only associated with dopaminergic fibers. The identification of serotonergic varicose fibers only on distinct segments of the duct system indicates that the various segments differ in their properties and functions. This hypothesis is in line with results on the morphological characteristics of the duct segments. Whereas secretory granules have been detected in the duct cells next to the secretory acini, cells in the major portion of the duct system lack granules but have an extensive basal labyrinth and numerous mitochondria [1,20].
The reservoir complex – innervation and possible functions
The functions and the physiology of the reservoir system are still enigmatic. It has been demonstrated that ligation of the salivary ducts prevents the filling of the reservoirs [3], suggesting that the acinar tissue is the source of at least part of the reservoir content, and that the reservoirs may become filled by back pressure of the secreted fluid when the hypopharynx is closed. The contracted reservoir muscle may serve as an occlusor of the reservoir orifice, and when the muscle relaxes, pressure of the hemolymph on the reservoir walls may cause emptying of the reservoirs [3]. In this scenario, the reservoir would play a primarily passive role and serve as a storage compartment for watery saliva. The reservoir content may be released during ingestion in order to moisten and digest the food [3]. Moreover, the reservoir may have some osmoregulatory function and satisfy the water requirements of the animal in times of water shortage.
The present study demonstrates that both serotonergic and dopaminergic varicose fibers are associated with the reservoir wall and the reservoir duct, and that the pattern of serotonergic innervation varies over the length of these structures. These findings indicate that the reservoir and the adjoining duct serve not only as a passive storage compartment or passageway, respectively, but have some active functions that may be regulated by dopamine and serotonin. For example, the epithelium of the reservoir wall may modify the composition of the primary fluid made within the glandular tissue. In agreement with this hypothesis is the finding that creatinine and urea have been detected in the contents of the reservoir but not in homogenized glandular tissue, suggestive of an excretory function for the reservoir [20]. Moreover, the epithelial cells of the reservoir wall are intensely stained for Na+,K+-ATPase, indicating that these cells are active in ion transport across the reservoir wall (W. Blenau and O. Baumann, unpublished results). Preliminary results suggest further that not only the serotonergic innervation but also the cellular architecture varies along the length of the reservoir (W. Blenau and O. Baumann, unpublished results), supporting the view that the various regions of the reservoir differ in their physiological properties.
Innervation of the salivary gland complex by other sources
Electron microscopy [5] and immunofluorescence staining with a neuron-specific marker (our unpublished data) visualized that the salivary nerve contains the axons of the giant neurons SN1 and SN2, and several small axons. The present study confirms that one of the large axons (SN1) is dopaminergic, and that most, if not all, small axons are serotonergic [6,8]. The second large axon (SN2) must thus contain a different, yet unidentified neurotransmitter or neurohormone, and serotonergic and dopaminergic neurons do not provide the only innervation of the salivary gland complex. Moreover, we should not dismiss the possibility that the salivary gland complex is innervated by neurons that are located in other parts of the nervous system than the subesophageal ganglion and that contain neither dopamine nor serotonin. In locusts, evidence has been presented that neuronal processes with FMRFamide-related peptides extend from the prothoracic and mesothoracic ganglia via transverse nerves to the salivary glands and ramify over the acinar tissue [8,21]. The physiological roles of FMRFamide-related peptides in this system are unknown; it has been proposed that these neurotransmitters may modulate rather than activate salivation in locust salivary glands [21]. In order to obtain a complete view on the innervation pattern and the neuronal control of salivation in the cockroach, several issues are yet to be solved: (1) the neurotransmitter content of the SN2 neuron, (2) the spatial relationship of the SN2 axon terminals to the various cell types, (3) the functional role of SN2 in salivation, and (4) the possibility of innervation by other sources.
Conclusions
Earlier research in our laboratory established the importance of serotonin and dopamine in salivation by the cockroach salivary gland. The present data extend these findings by determining the exact spatial relationship of the serotonergic and dopaminergic fiber endings and varicosities to the various structures and cell types composing the salivary gland complex. Close apposition of fiber terminals and/or varicosities to a distinct cell type provides evidence for a selective innervation of the respective cell type.
The distribution pattern of serotonergic and dopaminergic varicose nerve fibers over and within the acinar tissue supports the concept that central cells are stimulated by serotonin only, whereas peripheral cells are responsive to both serotonin and dopamine. The salivary duct system, previously thought to be regulated by dopamine only, may vary in functions along its length, as the initial acinar-close segments have a dopaminergic and a serotonergic innervation. Finally, the finding of a complex serotonergic and dopaminergic innervation pattern of the reservoirs, the adjoining reservoir ducts and the reservoir muscles warrants further investigations of the physiology of these structures.
Materials and Methods
Animals and preparation
A colony of the American cockroach (Periplaneta americana) was maintained at 25°C under a 12-h light:12-h dark regime and with free access to food and water. Young male and female imagines were sacrificed, and the salivary glands were dissected under physiological saline (160 mM NaCl, 10 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 10 mM glucose, 10 mM TRIS, pH 7.4), as described previously [2].
Antibodies
Anti-serotonin was obtained from Sigma (Taufkirchen, Germany; Product No. S5545); this antiserum was made in rabbits against serotonin conjugated to bovine serum albumin. Anti-dopamine, raised in goats against glutaraldehyde-conjugated dopamine, was provided by H.W.M. Steinbusch (Maastricht University, Maastricht, The Netherlands). Affinity-purified rabbit antibody against rat TH was purchased from Chemicon (Temecula, CA; Product No. AB152). This antibody has been reported previously to cross-react with TH of an invertebrate, Aplysia[22]. Secondary antibodies conjugated to Cy3 or Cy5 were obtained from Rockland (Gilbertsville, PA) and Dianova (Hamburg, Germany).
Fixation protocols
For labeling with anti-serotonin, salivary glands were fixed for 2 hours at room temperature with 2% paraformaldehyde, 0.075% lysine-HCl, 10 mM Na-periodate in 0.1 M phosphate buffer (PB), pH 7.0 [10,23]. Specimens were washed for 10 minutes in PB and treated further as described below.
For labeling with anti-dopamine, salivary glands were fixed for 30 minutes on ice with 5% glutaraldehyde in PB supplemented with 10 mM ascorbic acid (PB/AA). For colabeling with anti-dopamine and anti-TH, 0.5% glutaraldehyde, 3% paraformaldehyde in PB/AA was used as a fixative. After fixation, specimens were washed for 10 min on ice in PB/AA, treated for 30 min with 0.5% sodium borohydride in PB/AA to reduce free aldehyde groups, and washed again for 10 min in PB/AA.
Immunofluorescence labeling
Fixed salivary glands were either directly used for immunolabeling or processed for cryostat sectioning. In the latter case, preparations were incubated with 10% sucrose in PB or PB/AA for 30 minutes on ice, infiltrated with 25% sucrose in PB or PB/AA overnight at 4°C, and then shock-frozen in melting isopentane. Sections (8–10 μm thick) were cut at -30°C in a cryostat, collected on poly-L-lysine-coated coverslips, air-dried, and stored at 4°C until use.
For labeling with anti-serotonin, salivary glands or cryosections were permeabilized with 0.01% Tween 20 in PBS, reacted with 50 mM NH4Cl in phosphate-buffered saline (PBS), washed in PBS, and blocked with 1% normal goat serum, 0.8% bovine serum albumin, 0.1% fish gelatine, and 0.5% Triton X-100 in PBS. After being labeled overnight at 4°C with anti-serotonin (diluted 1:10,000 in the above blocking solution), specimens were washed in PBS and reacted for 1 hour (cryostat sections) or 3 hours (whole-mounts) with Cy3-conjugated goat anti-rabbit-IgG. In case of whole-mount preparations, the F-actin probe BODIPY FL phallacidin (Molecular Probes, Eugene, OR) was added to the secondary antibody solution. After a final extensive wash in PBS, specimens were mounted in Mowiol 4.88 (Farbwerke Hoechst, Frankfurt, Germany), containing 2% n-propyl-gallate as an anti-fading reagent.
For labeling with anti-dopamine, entire salivary glands or cryosections were permeabilized and blocked with a solution consisting of 1% normal donkey serum, 0.8% bovine serum albumin, 0.1% fish gelatine, and 0.5% Triton X-100 in PBS supplemented with 10 mM ascorbic acid (PBS/AA). Preparations were then labeled overnight at 4°C with anti-dopamine (diluted 1:8,000 in blocking solution), washed extensively with PBS/AA, and reacted with Cy3-conjugated donkey anti-goat-IgG and (in the case of whole-mounts) BODIPY FL phallacidin in PBS/AA. For double-labeling of whole-mount preparations with anti-dopamine and anti-TH, both primary antibodies were applied together (anti-TH diluted 1:200); the tissue was then washed, incubated with Cy3-conjugated donkey anti-goat-IgG, washed again, and reacted with Cy5-conjugated goat anti-rabbit IgG and BODIPY FL phallacidin.
Confocal microscopy
Specimens were examined with a Zeiss LSM 510 confocal laser-scanning microscope (Carl Zeiss, Jena, Germany) equipped with a 488-nm Argon laser, a 543-nm Helium-Neon laser, a 633-nm Helium-Neon laser, and differential interference contrast optics. Low-magnification images were recorded with a Fluar 10x/0.5, images at higher magnification either with a Plan-Neofluar 40x/1.4 or with a C-Apochromat 40x/1.2 W. In the case of double-labeled specimens, BODIPY FL and Cy3 were excited sequentially with the 488-nm and the 543-nm laser lines, by using the multitracking function of the LSM 510 software, and detected through 505–530-nm bandpass and 560-nm longpass filters. In the case of triple-labeled specimens, BODIPY FL and Cy5 were excited simultaneously at 488 nm and 633 nm and detected through 505–550-nm bandpass and 650-nm longpass filters; subsequently, Cy3 fluorescence was imaged through a 560–615-nm bandpass filter by using the 543-nm laser line for illumination. Specimens labeled with only one fluorochome and viewed with the instrument settings as used for double- or triple-labeled preparations demonstrated that there was no bleed-through between the detector channels under these recording conditions.
Controls for labeling specificity
Specificity of antibody binding was checked by treating cryostat sections in the described fashion except that primary antibodies were omitted from the procedure. No fluorescence was detected when these control specimens were viewed under the same instrumental settings as used for imaging sections that had been reacted with primary antibody. As a further control, primary antibody solutions were supplemented with 1 mg/ml dopamine or 1 mg/ml serotonin, preincubated for 30 minutes, and then used for immunofluorescence labeling of cryostat sections.
Abbreviations used
PB – phosphate buffer
PB/AA – phosphate buffer with ascorbic acid
PBS – phosphate-buffered saline
PBS/AA phosphate-buffered saline with ascorbic acid
SN1 – salivary neuron 1
SN2 – salivary neuron 2
TH – tyrosine hydroxylase
Authors' contributions
O.B. participitated in the design and coordination of the study, carried out part of the image analysis, and drafted the manuscript. P.D. and D.K. carried out the immunolabeling experiments and did the confocal imaging of the specimens. B.W. proposed the project and participated in the design and coordination of the study.
Acknowledgments
Acknowledgements
We should like to thank Dr. Wolfgang Blenau and Mrs. Katja Rietdorf for the maintenance of the cockroach culture and for stimulating discussions, and Mrs. Bärbel Wuntke for technical assistance.
Contributor Information
Otto Baumann, Email: obaumann@rz.uni-potsdam.de.
Petra Dames, Email: pittys@lycos.de.
Dana Kühnel, Email: Kuehnel@rz.uni-potsdam.de.
Bernd Walz, Email: walz@rz.uni-potsdam.de.
References
- Bland KP, House CR. Function of the salivary glands of the cockroach, Nauphoeta cinerea. J Insect Physiol. 1971;17:2069–2084. doi: 10.1016/0022-1910(71)90168-5. [DOI] [PubMed] [Google Scholar]
- Just F, Walz B B. Salivary glands of the cockroach, Periplaneta americana: new data from light and electron microscopy. J Morphol. 1994;220:35–46. doi: 10.1002/jmor.1052200105. [DOI] [PubMed] [Google Scholar]
- Sutherland DJ, Chillseyzn JM. Function and operation of the cockroach salivary reservoir. J Insect Physiol. 1968;14:21–31. doi: 10.1016/0022-1910(68)90130-3. [DOI] [PubMed] [Google Scholar]
- Just F, Walz B. The effects of serotonin and dopamine on salivary secretion by isolated cockroach salivary glands. J Exp Biol. 1996;199:407–413. doi: 10.1242/jeb.199.2.407. [DOI] [PubMed] [Google Scholar]
- Whitehead AT. The innervation of the salivary gland in the American cockroach: light and electron microscopic observations. J Morphol. 1971;135:483–506. doi: 10.1002/jmor.1051350405. [DOI] [PubMed] [Google Scholar]
- Elia AJ, Ali DW, Orchard I. Immunochemical staining of tyrosine hydroxylase(TH)-like material in the salivary glands and ventral nerve cord of the cockroach, Periplaneta americana (L.). J Insect Physiol. 1994;40:671–683. doi: 10.1016/0022-1910(94)90094-9. [DOI] [Google Scholar]
- Bowser-Riley F. The salivary glands of the cockroach Nauphoeta cinerea (Olivier). Cell Tissue Res. 1978;187:525–534. doi: 10.1007/BF00229617. [DOI] [PubMed] [Google Scholar]
- Ali DW. The aminergic and peptidergic innervation of insect salivary glands. J Exp Biol. 1997;200:1941–1949. doi: 10.1242/jeb.200.14.1941. [DOI] [PubMed] [Google Scholar]
- Davis NT. Serotonin-immunoreactive visceral nerves and neurohemal system in the cockroach Periplaneta americana (L.). Cell Tissue Res. 1985;240:593–600. [Google Scholar]
- Just F, Walz B. Immunocytochemical localization of Na+/K+-ATPase and V-H+-ATPase in the salivary glands of the cockroach, Periplaneta americana. Cell Tissue Res. 1994;278:161–170. doi: 10.1007/s004410050203. [DOI] [PubMed] [Google Scholar]
- Lang I, Walz B. Dopamine stimulates salivary duct cells in the cockroach Periplaneta americana. J Exp Biol. 1999;202:729–738. doi: 10.1242/jeb.202.6.729. [DOI] [PubMed] [Google Scholar]
- Lang I, Walz B. Dopamine-induced epithelial K+ and Na + movements in the salivary ducts of Periplaneta americana. J Insect Physiol. 2001;47:465–474. doi: 10.1016/S0022-1910(00)00134-7. [DOI] [PubMed] [Google Scholar]
- Evans PD. Biogenic amines in the insect nervous system. Adv Insect Physiol. 1980;15:317–473. [Google Scholar]
- Prokop A. Integrating bits and pieces: synapse structure and formation in Drosophila embryos. Cell Tissue Res. 1999;297:169–186. doi: 10.1007/s004410051345. [DOI] [PubMed] [Google Scholar]
- Maxwell DJ. Fine structure of axons associated with the salivary apparatus of the cockroach, Nauphoeta cinerea. Tissue Cell. 1978;10:699–706. doi: 10.1016/0040-8166(78)90056-3. [DOI] [PubMed] [Google Scholar]
- Nässel DR, Elekes K. Serotonergic terminals in the neural sheath of the blowfly nervous system: electron microscopical immunocytochemistry and 5,7-dihydroxytryptamine labelling. Neuroscience. 1985;15:293–307. doi: 10.1016/0306-4522(85)90136-8. [DOI] [PubMed] [Google Scholar]
- Helle J, Dircksen H, Eckert M, Nässel DR, Sporhase-Eichmann U, Schürmann FW. Putative neurohemal areas in the peripheral nervous system of an insect, Gryllus bimaculatus, revealed by immunocytochemistry. Cell Tissue Res. 1995;281:43–61. doi: 10.1007/s004410050399. [DOI] [PubMed] [Google Scholar]
- Miksys S, Lange AB, Orchard I, Wong V. Localization and neurohemal release of FMRFamide-related peptides in the stick insect Carausius morosus. Peptides. 1997;18:27–40. doi: 10.1016/S0196-9781(96)00245-8. [DOI] [PubMed] [Google Scholar]
- Lang I, Walz B. Dye-coupling between cells of the salivary glands in the cockroach Periplaneta americana. Cell Tissue Res. 1999;298:357–360. doi: 10.1007/s004419900108. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri DN, Ghosh SK. Study of the histophysiology of the salivary apparatus of Periplaneta americana Linn. Zool Anz. 1963;173:227–237. [Google Scholar]
- Fusé M, Ali DW, Orchard I. The distribution and partial characterization of FMRFamide-related peptides in the salivary glands of the locust, Locusta migratoria. Cell Tissue Res. 1996;284:425–433. doi: 10.1007/s004410050603. [DOI] [PubMed] [Google Scholar]
- Srivatsan M, Peretz B. Acetylcholinesterase promotes regeneration of neurites in cultured adult neurons of Aplysia. Neuroscience. 1997;77:921–931. doi: 10.1016/S0306-4522(96)00458-7. [DOI] [PubMed] [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative: a new fixative for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]