Abstract
Reanalysis and direct accelerator mass spectrometry radiocarbon dating of the cucurbit assemblage from Coxcatlan Cave provide information on the timing and sequence of the initial appearance of three domesticated plants in the Tehuacán Valley (Puebla, Mexico) and allow reassessment of the overall temporal context of plant domestication in Mexico. Cucurbita pepo is the earliest documented domesticate in the cave, dating to 7,920 calibrated calendrical (cal) years B.P. The bottle gourd (Lagenaria siceraria) is dated at 7,200 cal years B.P. Cucurbita argyrosperma does not appear until 2,065 cal years B.P. The earlier identification of Cucurbita moschata specimens is not confirmed. Seventy-one radiocarbon dates, including 23 accelerator mass spectrometry dates on cucurbits, provide ample evidence of postdepositional vertical displacement of organic materials in the western half of Coxcatlan Cave, but they also indicate that the eastern half of the cave was largely undisturbed.
Keywords: archaeology, Mexico, cucurbits, agriculture
The shift from a hunting and gathering lifestyle to economies based on domesticated species of plants and animals marks a major transition in human history. This emergence of food production economies occurred independently in >6 different regions of the world between ≈11,000 and 5,000 years ago, as human societies domesticated a wide range of plant and animal species (1, 2). Research on plant and animal domestication and the associated transition to food production has rapidly expanded in recent years because new perspectives, approaches, and technologies are being used in the analysis of plant and animal remains recovered from archaeological contexts (3).
Rather than focusing on newly excavated sites, recent research has often involved the reexamination of key museum collections of plant and animal remains that were excavated and initially analyzed decades ago. In Mexico, for example, the most significant advances in understanding the transition to food production in this major center of agricultural origin have resulted from the reanalysis of early domesticated plant assemblages that were recovered in the 1950s and 1960s from cave sites in Tamaulipas (Romero's and Valenzuela's Caves) (4), Puebla (Coxcatlan and San Marcos Caves) (5), and Oaxaca (Guilá Naquitz Cave) (6). These five caves have shaped our understanding of the early history of plant domestication and food production economies in Mesoamerica. Reanalyses of maize (Zea mays) (4, 7–13), bean (Phaseolus sp.) (14), and cucurbit (Lagenaria siceraria and Cucurbita sp.) (4, 15, 16) assemblages from these caves have focused on confirming both their domesticated status (on the basis of clearly defined morphological criteria) and their true age [through accelerator mass spectrometry (AMS) radiocarbon dating], in addition to tracing morphological change and crop selection through time.
Direct AMS dating and clear confirmation of domesticated status and taxonomic assignment are also the primary focus for this reanalysis of the cucurbit assemblage from Coxcatlan Cave. The cucurbit assemblage is the final domesticated plant assemblage from the five caves to be reconsidered and, as such, is the final data set necessary for an overall reassessment of the currently available evidence for the spatial and temporal patterns of initial domestication and subsequent diffusion of domesticated plants in Mexico. In addition, the 71 radiocarbon dates now available for Coxcatlan Cave (Table 1) allow clarification of a related and unresolved issue, namely, the extent to which the cave's remarkable record of human occupation may have been subjected to postdepositional disturbance.
Table 1. Seventy-one radiocarbon dates from Coxcatlan Cave.
| Date no. (ref.) | Radiocarbon years B.P. | Calibrated years B.C./A.D. (intercept) | Calibrated years B.C./A.D. (1 σ) | Square | Layer | Zone | Lab no. | Material dated |
|---|---|---|---|---|---|---|---|---|
| 1 AMS | 150 ± 40 | A.D. 1680 | A.D. 1670–1950 | 9 | 1 | II | OS 36646 | L. siceraria rind |
| 2 AMS | 180 ± 25 | A.D. 1670 | A.D. 1665–1680 | 87 | 1 | I | OS 36792 | L. siceraria rind |
| 3 AMS | 200 ± 30 | A.D. 1670 | A.D. 1660–1680 | 69 | 1 | II | OS 36790 | L. siceraria rind |
| 4 AMS | 220 ± 20 | A.D. 1660 | A.D. 1660–1670 | 9 | 1 | II | OS 36791 | L. siceraria rind |
| 5 AMS | 240 ± 40 | A.D. 1655 | A.D. 1645–1665 | 142 | 11 | IX | B 134115 | C. argyrosperma stem |
| 6 AMS | 260 ± 30 | A.D. 1650 | A.D. 1640–1660 | 87 | 1 | I | OS 36909 | L. siceraria rind |
| 7 AMS | 300 ± 30 | A.D. 1640 | A.D. 1630–1650 | 87 | 1 | I | OS 36728 | L. siceraria rind |
| 8 AMS | 340 ± 40 | A.D. 1570 | A.D. 1485–1640 | 70 | 8 | VIII | B 123045 | C. argyrosperma seed |
| 9 AMS (14) | 410 ± 45 | A.D. 1460 | A.D. 1440–1490 | — | — | — | AA 10977 | P. coccineus bean |
| 10 AMS | 420 ± 50 | A.D. 1455 | A.D. 1435–1495 | 70 | 8 | VIII | B 123044 | C. argyrosperma stem |
| 11 AMS (7) | 450 ± 40 | A.D. 1440 | A.D. 1430–1460 | 149 | 5 | XII | AA 3314 | Z. mays cob |
| 12 AMS | 460 ± 50 | A.D. 1440 | A.D. 1425–1460 | 183 | 5 | VII | B 123046 | C. argyrosperma seed |
| 13 AMS | 470 ± 40 | A.D. 1435 | A.D. 1425–1450 | 7 | 9 | XIV | B 123041 | C. argyrosperma seed |
| 14 AMS | 480 ± 50 | A.D. 1430 | A.D. 1415–1445 | 25 | 7 | VII | B 144502 | Cucurbita sp. rind |
| 15 AMS | 480 ± 50 | A.D. 1430 | A.D. 1415–1445 | 37 | 6 | VII | B 144505 | Cucurbita sp. rind |
| 16 AMS (14) | 480 ± 60 | A.D. 1430 | A.D. 1410–1450 | 87 | 5 | III | AA 10982 | P. coccineus bean |
| 17 AMS | 500 ± 50 | A.D. 1425 | A.D. 1410–1455 | 25 | 7 | VII | B 123047 | C. argyrosperma stem |
| 18 AMS | 530 ± 50 | A.D. 1420 | A.D. 1400–1435 | 15 | 8 | XIV | B 123042 | C. argyrosperma seed |
| 19 AMS | 595 ± 30 | A.D. 1340 | A.D. 1310–1370 | 85 | 1 | I | OS 36793 | L. siceraria rind |
| 20 AMS | 600 ± 50 | A.D. 1325 | A.D. 1300–1410 | 7 | 3 | VII | B 144503 | Cucurbita sp. rind |
| 21 | 650 ± 100 | A.D. 1300 | A.D. 1270–1410 | 142 | 2 | II | I 672 | Charcoal |
| 22 | 780 ± 100 | A.D. 1260 | A.D. 1180–1290 | 142 | 3 | II | I 674 | Charcoal |
| 23 | 1050 ± 100 | A.D. 1000 | A.D. 890–1040 | 142 | 4 | III | I 659 | Charcoal |
| 24 | 1050 ± 120 | A.D. 1000 | A.D. 880–1140 | 142 | 2 | II | I 662 | Charcoal |
| 25 | 1625 ± 150 | A.D. 420 | A.D. 250–610 | 142 | 5 | IV | I663 | Charcoal |
| 26 | 1625 ± 120 | A.D. 420 | A.D. 260–570 | 142 | 6 | V | I 671 | Charcoal |
| 27 | 1770 ± 100 | A.D. 250 | A.D. 130–400 | 136 | — | VI | I 656 | Charcoal |
| 28 AMS (7) | 1860 ± 40 | A.D. 130 | A.D. 100–220 | 36 | 8 | XI | AA 3309 | Z. mays cob |
| 29 AMS (7) | 1900 ± 60 | A.D. 100 | A.D. 50–150 | 24 | 11 | XIII | AA 3307 | Z. mays cob |
| 30 | 1900 ± 150 | A.D. 100 | 50 B.C.–A.D. 260 | 142 | 6 | V | I 673 | Charcoal |
| 31 | 1945 ± 200 | A.D. 70 | 180 B.C.–A.D. 320 | 136 | Burial 1 | VI | I 921 | Cloth |
| 32 (19)* | 2050 ± 180 | 50 B.C. | 360 B.C.–A.D. 130 | 141 | 10 | IX | I 677 | Charcoal |
| 33 AMS | 2100 ± 40 | 115 B.C. | 180–55 B.C. | 148 | 3 | VI | B 134116 | C. argyrosperma stem |
| 34 AMS (14) | 2285 ± 60 | 380 B.C. | 400–230 B.C. | 143 | 12 | VIII | AA 5467 | P. vulgaris pod |
| 35 AMS (14) | 2300 ± 50 | 390 B.C. | 400–370 B.C. | 142 | — | VI | AA 10979 | P. acutifolius bean |
| 36 AMS (14) | 2360 ± 50 | 400 B.C. | 420–390 B.C. | 145 | 9 | VII | AA 10978 | P. acutifolius bean |
| 37 AMS (7) | 3740 ± 60 | 2140 B.C. | 2210–2040 B.C. | 74 | 12 | XIII | AA 3313 | Z. mays cob |
| 38 AMS (7) | 4040 ± 100 | 2570 B.C. | 2850–2460 B.C. | 13 | 7 | XI | AA 3312 | Z. mays cob |
| 39 AMS (7) | 4090 ± 50 | 2600 B.C. | 2850–2570 B.C. | 7 | 6 | XIII | AA 3308 | Z. mays cob |
| 40 | 4770 ± 175 | 3570 B.C. | 3710–3360 B.C. | 136 | — | VIII | I 653 | Charcoal |
| 41 (19)* | 4800 ± 200 | 3640 B.C. | 3780–3360 B.C. | 165 | 5 | VII | I 770 | Charcoal |
| 42 | 4950 ± 200 | 3710 B.C. | 3960–3530 B.C. | 141 | — | IX | I 594 | Charcoal |
| 43 (19)* | 5025 ± 180 | 3790 B.C. | 3980–2640 B.C. | 142 | 12 | X | I 654 | Charcoal |
| 44 | 5150 ± 220 | 3960 B.C. | 4240–3700 B.C. | 141 | 9 | VIII | I 593 | Charcoal |
| 45 | 5200 ± 180 | 3980 B.C. | 4240–3790 B.C. | 142 | 10 | IX | I 652 | Charcoal |
| 46 AMS | 5240 ± 60 | 4040 B.C. | 4155–4120 B.C. | 13 | 6 | VI | B 144501 | Cucurbita sp. rind |
| 47 | 5250 ± 200 | 4040 B.C. | 4330–3800 B.C. | 141 | — | IX | I 766 | Charcoal |
| 48 (19)* | 5475 ± 230 | 4340 B.C. | 4530–4040 B.C. | 139 | — | XI | I 664 | Charcoal |
| 49 | 5560 ± 250 | 4360 B.C. | 4690–4070 B.C. | 8 | — | XI | M 1089 | Pine |
| 50 AMS | 6190 ± 70 | 5205 B.C. | 5270–5035 B.C. | 74 | 7 | XI | B 144504 | L. siceraria rind |
| 51 AMS | 6270 ± 50 | 5280 B.C. | 5310–5320 B.C. | 72 | 12 | XIII | OS 36647 | L. siceraria rind |
| 52 AMS | 6290 ± 50 | 5250 B.C. | 5270–5225 B.C. | 72 | 12 | XIII | B 123043 | L. siceraria rind |
| 53 | 6325 ± 200 | 5310 B.C. | 5480–5040 B.C. | 103 | — | XI | I 459 | Charcoal |
| 54 (19)* | 6500 ± 200 | 5480 B.C. | 5630–5300 B.C. | 130 | 17 | XX | I 661 | Charcoal |
| 55 | 6700 ± 180 | 5630 B.C. | 5740–5480 B.C. | 131 | 13 | XV | I 651 | Charcoal |
| 56 (19)* | 6775 ± 200 | 5670 B.C. | 5840–5510 B.C. | 131 | 13 | XV | I 668 | Charcoal |
| 57 | 6925 ± 200 | 5780 B.C. | 6000–5640 B.C. | 179 | — | XI | I 567 | Charcoal |
| 58 | 7000 ± 220 | 5870 B.C. | 6060–5670 B.C. | 103 | — | XIII | I 457 | Charcoal powder |
| 59 (19)* | 7050 ± 190 | 5910 B.C. | 6070–5730 B.C. | 145 | 15 | XVII | I 655 | Charcoal |
| 60 AMS | 7100 ± 50 | 5960 B.C. | 5980–5880 B.C. | 148 | 11 | XIV | B 123040 | C. pepo seed |
| 61 (19)* | 7150 ± 200 | 6010 B.C. | 6220–5810 B.C. | 103 | — | XXIV | I 460 | Charcoal |
| 62 | 7350 ± 300 | 6220 B.C. | 6460–5910 B.C. | 129 | 14 | XVI | I 675 | Charcoal |
| 63 (19)* | 7520 ± 250 | 6400 B.C. | 6600–6090 B.C. | 130 | 18 | XXI | I 660 | Charcoal |
| 64 (19)* | 7700 ± 250 | 6490 B.C. | 6820–6260 B.C. | 103 | — | XVI | I 458 | Charcoal |
| 65 | 7800 ± 250 | 6630 B.C. | 7050–6420 B.C. | 178 | — | XVI | I 574 | Charcoal |
| 66 (19)* | 7950 ± 250 | 6820 B.C. | 7180–6490 B.C. | 180 | 6 | IX | I 763 | Charcoal |
| 67 (19)* | 8150 ± 340 | 7090 B.C. | 7560–6640 B.C. | 103 | — | XXIII | I 676 | Charcoal |
| 68 | 8375 ± 275 | 7490 B.C. | 7610–7070 B.C. | 127 | 16 | XVIII | I 769 | Charcoal |
| 69 (19)* | 8425 ± 250 | 7520 B.C. | 7620–7140 B.C. | 134 | 20 | XXII | I 764 | Charcoal |
| 70 (19)* | 8550 ± 250 | 7580 B.C. | 7930–7340 B.C. | 103 | — | XVIII | I 461 | Charcoal |
| 71 (19)* | 8625 ± 220 | 7650 B.C. | 7995–7326 B.C. | 105 | 3 | XXV | I 571 | Charcoal |
Coxcatlan Cave
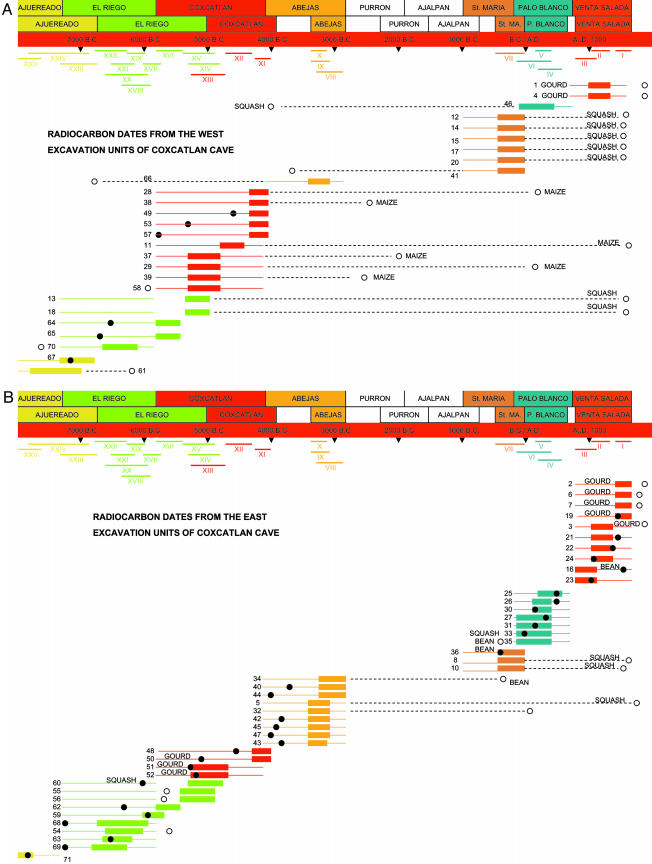
Excavated as part of Richard S. MacNeish's landmark long-term interdisciplinary investigation of agricultural origins and cultural development in the Tehuacán Valley, Coxcatlan Cave was occupied over a span of nearly 10,000 years and has provided one of the most extensive and detailed early records of human cultural history in Mesoamerica (17). Large-scale excavation of the site encompassed ≈150 of the 240 m2 of sheltered area under the overhang (30 m long, 8 m deep) and extended 2–3 m or more to bedrock, exposing an extremely complex horizontal and vertical pattern of 42 discrete occupational episodes. These occupations varied considerably in terms of the size of the occupying group, the seasonal duration of habitation, and the number and range of artifacts and activity areas (18). Activity areas were smaller-scale spatially discrete lenses of occupational debris (e.g., hearths, pit features, scatters of ash, artifacts, and organic refuse). The challenge of assigning individual activity areas to specific occupations and, in turn, establishing the chronological sequence and seasonality of occupations, was accomplished both by tracing stratigraphic associations, to the extent possible, and by comparing the full material culture assemblage of different activity areas and occupations to identify patterns of overall shared similarity and seasonality in tool inventories, organic remains, and activity sets. Comparative analysis of material culture assemblages, with weight given to a range of culturally and temporally sensitive artifact types, was also used to group the 42 occupations into a series of 28 habitation zones (I–XXVIII) and seven cultural phases (Fig. 1), and to further link the occupations, zones, and phases of Coxcatlan Cave to other contemporaneous site components in the Tehuacán Valley and beyond (19, 20). The top seven zones of the cave (zones I–VII), which spanned the ceramic periods of occupation, were separated from the underlying preceramic zones (VIII–XXVIII) by a period of >1,000 years, during which the cave was essentially unoccupied.
Fig. 1.
Radiocarbon age determinations from Coxcatlan Cave, compared with the initial temporal placement of the samples based on cultural stratigraphic association. (A and B) Dates from excavation units on the west (A) and east (B) sides of the cave. The time spans of cultural phases are expressed in radiocarbon years in the lower bar and in calibrated calendar years in the upper bar (19). The time spans of cultural zones are shown in radiocarbon years, and the time span of cultural phases, in calibrated calendar years.
One of the most important aspects of the long history of human habitation documented in Coxcatlan Cave involves the initial appearance of domesticated plants in the cave's cultural deposits and the evidence they provide for the associated transition to a food production economy. Establishing the cultural and temporal context of when maize, bottle gourd, squashes, and beans were added to the diet of the occupants of Coxcatlan Cave involved the straightforward documentation of when each of these crop plants first appeared in the site's long and complex vertical sequence of occupation levels, zones, and phases. Thirty-seven radiocarbon dates obtained on charcoal samples carefully selected from undisturbed contexts in different locations and levels throughout the cave, along with additional dates on similar material culture assemblages from other Tehuacán sites, were used to establish the age and duration of the cultural zones and phases of Coxcatlan Cave (18, 19). However, some of these initial 37 standard radiocarbon dates and many of the 34 subsequently obtained small sample AMS dates (Table 1), did not fit within the overall chronological framework developed for the site, raising the possibility of postdepositional disturbance of cultural deposits (see Discussion) and underscoring the value of confirming initial age estimates for early domesticated plants by direct AMS age determinations.
The Coxcatlan Cave Cucurbit Assemblage
Plant materials were recovered in far greater abundance from the ceramic period cultural zones of Coxcatlan Cave (I–VII) than from preceramic zones (VIII–XXVIII) (Table 2). Whereas the ceramic zones consisted of layers of compressed and well preserved plant remains with abundant artifacts, the preceramic deposits were largely composed of ash deposits capped by thin floors of organic material, with the ash likely representing burned plant material similar to the unburned material remaining in the upper zones (18). Parallel to this overall difference in plant preservation between the ceramic and preceramic zones, the relative contribution of domesticated crop plants to the total plant inventory was also significantly reduced in the lower levels of the cave, with domesticated plants representing ≈45% of the food plants recovered from ceramic contexts and only ≈2% of the plant assemblages from preceramic cultural zones.
Table 2. Food plant remains recovered from Coxcatlan Cave.
|
Z. mays
|
Cucurbita sp.
|
C. pepo
|
C. argyrosperma
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zone | Food plants total* | Total† | Cobs† | P. vulgaris‡ | P. lunatus‡ | L. siceraria rind fragments | Peduncle fragments | Rind fragments | Peduncles | Seeds | Peduncles and rind§ | Seeds |
| I | 2,522 | 1,608 | 1,380 | 2 | 1 | 103 | 2 | 1 | 9 | 2 | ||
| II | 5,895 | 2,737 | 2,273 | 5 | 3 | 298 | 2 | 1 | 34 | 24 | ||
| III | 7,230 | 4,879 | 4,115 | 3 | 1 | 1 | 64 | 1 | 20 | 134 | ||
| IV | 5,929 | 2,127 | 1,793 | 1 | 2 | 61 | 10 | 6 | ||||
| V | 3,140 | 685 | 639 | 2 | 1 | 35 | 2 | 6 | ||||
| VI | 6,787 | 2,497 | 2,302 | 2 | 72 | 10 | 23 | |||||
| VII | 3,290 | 719 | 592 | 1 | 20 | 1 | 1 | |||||
| Ceramic zones total | 34,793 | 15,254 | 13,094 | 6 | 1 | 10 | 8 | 653 | 4 | 3 | 86 | 190 |
| VIII | 1,572 | 24 | 15 | 1 | 7 | 1 | 3 | |||||
| IX | 1,070 | 33 | 27 | 1 | 1 | |||||||
| X | 155 | 2 | 2 | |||||||||
| XI | 2,685 | 33 | 25 | 1 | 2 | 1 | 10 | |||||
| XII | 1,443 | 5 | 5 | |||||||||
| XIII | 302 | 18 | 18 | 1 | ||||||||
| XIV | 203 | 1 | 2 | |||||||||
| XV–XXVI | 392 | 1 | ||||||||||
| Preceramic zones total | 7,822 | 115 | 92 | 2 | 0 | 3 | 1 | 18 | 0 | 1 | 2 | 6 |
| Grand total | 42,615 | 15,369 | 13,186 | 8 | 1 | 13 | 10 | 671 | 4 | 4 | 88 | 196 |
The results of reanalysis of the cucurbit collections from Coxcatlan Cave (summarized in Table 2) differed in a few important respects from those reached in the initial analysis carried out by Cutler and Whitaker 40 years ago (24). Perhaps most significantly, none of the 32 seeds and 2 peduncles (fruit stems) identified as Cucurbita moschata in the initial study were recognized in the analysis reported here, and they may have been separated from the rest of the collection before reanalysis. Because none of the specimens initially assigned to C. moschata were illustrated in the original report and the cucurbits in the restudied collection rarely carried original taxonomic identifications, it was not possible to reassess species assignments specimen by specimen. If still present in the assemblage, the seeds and peduncles earlier identified as C. moschata were either assigned to a different species in the current study, or more likely, included with the other fragmentary specimens identified only to genus level and listed under Cucurbita sp. in Table 2.
This Cucurbita sp. category, which makes up more than two-thirds of the 986 cucurbit specimens included in the reanalysis reported here, consists of 10 peduncle fragments and 671 uncarbonized rind fragments. The rind fragments could be distinguished from the rind of bottle gourd (L. siceraria) on the basis of their lighter color and distinctive cross-sectional cell structure (4).
Of the 296 Cucurbita seeds and peduncles that could be identified to the species level, a total of only eight specimens (four peduncles and four seeds) could be assigned to the species C. pepo. Recovered from scattered contexts across the two uppermost habitation zones of the cave, the C. pepo peduncles range from 14 to 33 mm in maximum basal diameter and exhibit 10 very prominent, ropy, and rounded parallel ridges (often alternating large and small). These ridges are oriented perpendicular to the base, are separated by deep intervening creases or furrows, and have basal lobes that round under before attachment with the fruit (4, 16, 25). Although the C. pepo seeds assigned to the top three habitation zones of Coxcatlan Cave were considerably larger (18.7 × 8.5, 23.8 × 10.4, and 25.0 × 10.4 mm) than the single seed recovered from the preceramic El Riego phase zone XIV (12.3 × 6.1 mm), all four exhibited the seed outline, margin configuration, and marginal hair pattern characteristic for the species (4, 15, 16).
Whereas the El Riego phase seed clearly exhibits the key morphological characteristics that are diagnostic for C. pepo, Cutler and Whitaker (24) were appropriately cautious in their initial analysis, given its small size, suggesting that the zone XIV seed “may be an early form of pepo, or it could be a seed from a well-developed fruit of a wild species.” The recently proposed 11- to 12-mm seed length minimum for domesticated C. pepo, however, along with the documentation of domesticated C. pepo at Guilá Naquitz Cave in Oaxaca (15, 16, 26) allows for the identification here of the El Riego seed as being from a domesticated plant. A direct AMS date of 5,960 calibrated calendrical (cal) years B.C. was obtained on the seed (Table 1, date 60), pushing back the initial appearance of C. pepo in the Tehuacán valley by 5,000 years, and providing the earliest evidence for a domesticated plant in the region. It is important to point out that although the current reanalysis reclassifies the El Riego C. pepo seed as representing a domesticated plant, the direct AMS date obtained on the specimen agrees quite closely with the original age estimate of 5,800 cal years B.C. assigned to the seed and to the terminal El Riego phase (ref. 19, p. 5).
Although the 13 bottle gourd (L. siceraria) rind fragments identified here fall short of the 60 counted in the initial analysis (24), few from preceramic levels were identified in either study. Ten of the 13 bottle gourd rind fragments identified in the current reanalysis came from excavation units along the east central portion of the back wall of the cave. Six of these bottle gourd fragments provided a total of 10 AMS dates from two different laboratories, all of which were in general agreement with their assigned cultural phase (Table 1 and Fig. 1). Although most of the bottle gourd rind and most of the dates came from late ceramic Venta Salada phase period occupation zones, three AMS dates on two of the three rind fragments recovered from preceramic Coxcatlan phase occupations (zones XI and XIII) documented the presence of this domesticated plant in Coxcatlan Cave by 5,200 cal years B.C. (Table 1 and Fig. 1). This direct age determination on the earliest bottle gourd rind from Coxcatlan Cave is also in general agreement with the initial early Coxcatlan phase calibrated age estimates for the initial appearance of the species (5800–4150 B.C.) (19).
The vast majority of the Cucurbita specimens recovered from Coxcatlan Cave were identified as Cucurbita argyrosperma (previously Cucurbita mixta) on the basis of very distinctive morphological characteristics. Ranging in maximum basal diameter from 16 to 54 mm, the 66 C. argyrosperma peduncles were cylindrical to bulbous in cross section and cork-like in appearance. Peduncle ridges were wide and flat to gently rounded, with central grooves giving the impression that each ridge consisted of two parallel conjoined ridges, each of which in turn has a distinctive vertical line or central appliqué strip (4, 25). Reflecting substantial postdepositional vertical displacement, the only two peduncles recovered from preceramic habitation zones (Table 1, date 5, zone IX; date 10, zone VIII), along with the single peduncle recovered from zone VII (Table 1, date 17) produced AMS age determinations ranging from A.D. 1425 to A.D. 1655. A peduncle with a maximum basal diameter of 27 mm recovered from overlying zone VI however, yielded a cal intercept of 115 B.C., providing the oldest date for C. argyrosperma from the cave (Table 1, date 33). This initial appearance of C. argyrosperma in the cultural deposits of Coxcatlan Cave is ≈5,000 years more recent than originally estimated (24).
The 196 C. argyrosperma seeds recovered from Coxcatlan Cave were similarly classified on the basis of distinctive morphology (16, 24, 25) such as narrow willow leaf outline with length/width ratios of >2.0 (seed size range 18.3 × 9.2 to 28.5 × 10.8 mm), white pulpy porous seed body, nonuniform, and often golden-colored seed margin with long marginal hairs extending to the midline. As was the case for the AMS-dated peduncles of the species, the C. argyrosperma seeds from preceramic contexts that were selected for AMS age determination (Table 1, date 8, zone VIII; dates 13 and 18, zone XIV), along with a seed from zone VII (Table 1, date 12) all yielded 15th and 16th century A.D. dates and ranged in age from A.D. 1420 to A.D. 1570.
Radiocarbon Dates and the Stratigraphic Integrity of Coxcatlan Cave
The detailed excavation records maintained for Coxcatlan Cave allow the exact vertical and horizontal location to be established for 70 of the 71 organic samples that yielded radiocarbon dates (Table 1). When the location of these samples is taken into consideration, several patterns emerge that provide some clarification and resolution of questions regarding the nature and extent of postdepositional disturbance of cultural materials within the cave.
The possibility of disturbance of habitation layers and vertical displacement of organic material was recognized during the initial formulation of the chronological framework and occupational history of the site. Ten of the 37 initial large sample radiocarbon dates were rejected because they were either not in agreement with other dates from the same zone or not in correct sequence with dates from adjacent zones (19, 20). Nine of the 10 rejected dates came from the preceramic occupations of the cave, with only 18 of the 27 samples obtained from zones VIII–XXV producing acceptable age determinations. Five more of the initial radiocarbon dates from preceramic contexts in Coxcatlan Cave were recently reclassified as unacceptable (20), bringing the percentage of rejected standard large sample dates from the preceramic zones >50% (14 of 27) (Table 1). The triage of initially available radiocarbon dates focused on the all-important challenge of constructing an accurate chronological framework for the occupational history of Coxcatlan Cave and set aside the issue of what might have caused so many of the samples drawn from the preceramic zones of the site to be unacceptable (19).
The most likely explanation for the anomalous radiocarbon results from Coxcatlan Cave is that the organic samples that yielded the radiocarbon dates had been vertically displaced up or down because of any number of different human activities or natural intrusion vectors (e.g., pit-digging, rodent-burrowing). Postdepositional vertical displacement has been increasingly implicated in recent years as the cause of out-of-sequence radiocarbon dates, and rodent disturbance and associated intrusion of charcoal from higher levels was identified as a likely explanation for why the three dates from the lowest zones of Coxcatlan Cave were younger than the expected age of the associated extinct Pleistocene fauna (27). Interestingly, recent reassessments of the overall stratigraphic integrity of Romero's, Valenzuela's, and Guilá Naquitz Caves, involving additional direct AMS dates combined with reanalysis of extant radiocarbon dates and available stratigraphic and provenience information, indicated very little evidence of postdepositional disturbance and vertical displacement of organic material (4, 15, 16).
In contrast to these studies, however, the first set of direct AMS radiocarbon dates from Coxcatlan Cave, obtained on six corn cobs selected from secure and well dated preceramic contexts, all produced dates considerably younger than the temporal span of the cultural zones and phases from which they were drawn, further increasing the percentage of anomalous preceramic dates and providing further evidence for postdepositional disturbance (7) (Fig. 1, dates 11, 28, 29, 37–39).
Seventy-one radiocarbon dates are now available for Coxcatlan Cave (Table 1 and Fig. 1), including 34 direct AMS radiocarbon dates on domesticated crop plants, 23 that provide temporal context for the cucurbit assemblage analyzed here. This large set of dates both confirms that considerable postdepositional vertical displacement of organic materials occurred and provides several finer scale indications of the spatial patterns of disturbance. When calibrated from radiocarbon years to calendar years and compared with the calibrated calendar year correction table developed for Coxcatlan Cave (ref. 19, p. 5), only 36 of 70 dates fall within the projected time span of their phase of origin (Fig. 1). However, if Coxcatlan Cave is divided down the central north–south grid line, the west side of the cave clearly experienced substantially more disturbance and vertical displacement. Only 6 of the 27 radiocarbon dates from the west half of the cave fall within their phase of origin (Fig. 1 A). In contrast, 30 of 43 dates from the east half of the cave matched the time span of their phase of origin (Fig. 1B). In addition, of the 13 dates from the east half of the cave that fell outside of their assigned phase, 3 of 7 from the preceramic zones and 5 of 7 from ceramic zones were relatively close to their phase assignments, whereas 16 of the 21 anomalous dates from the west half of the cave were a thousand years or more outside of their phase assignments.
Although the 70 radiocarbon dates shown in Fig. 1 are open to alternative and more focused analyses, a number of basic points relevant to the current study are clear: (i) there has been considerable postdepositional disturbance and vertical displacement of organic material within the cave; (ii) 75% of the disturbance involves downward displacement; (iii) 25 of the 34 direct AMS dates obtained on domesticated plants fell outside of their assigned phase; and (iv) vertical displacement was far more common and more serious in the western half of the cave, where anomalous dates were more abundant and fell much farther outside their assigned phase.
This final point is significant in that the relatively limited evidence for east side disturbance, particularly for displacement from ceramic down into preceramic zones, indicates considerable stratigraphic integrity of cultural deposits and provides strong support for the general chronological and cultural developmental frameworks that have been established for Coxcatlan Cave and the Tehuacán Valley. At the same time, the three substantially displaced east side radiocarbon samples (dates 5, 32, 34) indicate that vertical displacement of organic materials from the overlying ceramic zones down into the preceramic occupations also occasionally occurred in the east excavation units and underscores the need for direct AMS radiocarbon dates on any domesticated plant specimens used in focused time-sensitive analyses.
For example, a recent study focused on measuring early evolutionary rates in maize based on analysis of a temporally ordered series of 26 maize cobs from San Marcos and Coxcatlan Caves in Tehuacán (9). Rather than obtaining direct AMS dates on all of the cobs included in the study, the majority was dated by association. As part of the analysis, three AMS dates obtained on west side maize cobs from Coxcatlan Cave (Table 1, dates 28, 29, 39) were used to establish the age of an additional 10 “contemporaneous specimens from different provenience units” (9) in the two caves. However, the excavation units that yielded dates 28, 29, and 39 exhibit considerable evidence of disturbance, making any dating by association extremely problematic. For example, date 39 (2600 B.C.) came from a square that produced a much younger sample (date 13, A.D. 1435) from the next lower zone, and immediately adjacent squares produced much older samples from overlying zones (dates 49, 4360 B.C. and 57, 5780 B.C.). Similarly, a square immediately adjacent to the squares that yielded dates 28 (A.D. 130) and 29 (A.D. 100) produced much older dates from overlying zones (dates 38, 2570 B.C. and 46, 4040 B.C.). Given these glaring indications of vertical displacement, the outward projection of contemporaneity from the three AMS-dated cobs to embrace additional unidentified maize cobs in other squares of Coxcatlan and San Marco Caves is not justified, and the subsequent detailed and time-sensitive analysis of rates of evolutionary change is rendered meaningless.
Discussion
In Mexico, as in other regions of the world, the transition from a hunting and gathering way of life to food-producing economies was a long and complex developmental process. It involved the time-transgressive and differential adoption of crop plants in different combinations in different regions, as human societies found a range of different solutions to local environmental and cultural settings (28). Accurate determination of when key crop plants initially appear in the archaeological record of different areas represents a basic but essential initial framework from which to begin to understand this transition. Direct AMS radiocarbon dating of domesticates from Guilá Naquitz and the caves of Ocampo and Tehuacán provides a starting point for considering the early history of farming economies in Mexico and substantially alters earlier spatial and temporal frameworks for the initial appearance and subsequent diffusion of key crop plants (Table 3).
Table 3. Direct AMS radiocarbon dates of earliest domesticates from fives caves in Mexico.
| Species | Oaxaca | Tehuacán | Tamaulipas |
|---|---|---|---|
| C. moschata | Not present ≈5000 B.C. | 800 B.C. (4) 3,400 yr younger | |
| C. argyrosperma | 115 B.C. 4,900 yr younger | 3085 B.C. (4) 2,900 yr younger | |
| P. vulgaris | 147 B.C. (14) | 380 B.C. (14) 4,600 yr younger | A.D. 730 (14) 3,400 yr younger |
| Z. mays | 4280 B.C. (10) | 3540 B.C. (7) 600 yr younger | 2455 B.C. (4) 400 yr older |
| L. siceraria | 7970 B.C. (16) no change | 5250 B.C. 1,300 yr younger | 4490 B.C. (4) 500 yr younger |
| C. pepo | 8025 B.C. (15) no change | 5960 B.C. 5,000 yr older | 4360 B.C. (4) 2,800 yr older |
Results are presented as dendrocalibrated calendar time scale intercept in years B.C. (ref. no.), followed by change in age from initial estimate.
However, reconsideration of the plant assemblages from these caves also clearly indicates that the amount of information currently available for even the most basic preliminary assessment of plant domestication and agricultural origins in Mexico is extremely limited. A total of 128 specimens representing the primary domesticated species (maize, beans, and cucurbits) were recovered from the preceramic habitation zones of Coxcatlan Cave (Table 2). In addition, of the 14 directly dated domesticates from preceramic zones, 11 yielded ceramic period dates (Table 1). Similarly, the domesticated plant assemblage from the preceramic habitation zones of Guilá Naquitz Cave consists of 3 maize cob fragments, 3 bottle gourd rind fragments, and 13 seeds, peduncles, and fruit end-fragments of C. pepo (11, 15, 16). The early occupations of Romero's Cave (Occupations 1–7, pre-2400 B.C.) yielded 9 maize fragments and 69 domesticated cucurbit specimens, whereas the early levels of Valenzuela's Cave (Occupations 1–6, pre-2300 B.C.) produced 7 maize specimens and 48 domesticated cucurbit fragments (4). Taken together, the four caves that provided the essential core data set for plant domestication in Mexico have yielded a total of only 280 specimens of domesticated plants from preceramic cultural contexts.
In addition to being small in terms of specimen count, this core data set also represents only three isolated regional data points on the vast cultural and environmental landscape of pre-Columbian Mexico. Romero's, Valenzuela's, Coxcatlan, and Guilá Naquitz Caves thus constitute a clear present-day research challenge: to recover comparably well documented and carefully interpreted preceramic records of cultural development and subsistence change from other regions of Mesoamerica, particularly in lowland environmental zones. Excavation of waterlogged deposits at the marshy Gulf Coast San Andrés site (29) has recently indicated the existence of remarkably well preserved archaeobotanical assemblages in lowland settings. San Andrés also underscores the potential difficulties involved in adequately documenting and dating the initial appearance of domesticated plants (e.g., projecting the age and domesticated status of Zea pollen from sediment cores) and the Faustian lure of ephemeral interpretive overreach. The San Andrés achene and seed identified as Helianthus annuus, for example, were cited by David Lentz as evidence that sunflower was first domesticated not in eastern North America, but in Mexico, and that, as a result, the status of eastern North America as an independent center of plant domestication could be called into question (30). Genetic analysis, however, has confirmed eastern North America as the source of all modern domesticated sunflower varieties, which relegates the Tabasco sunflower to the status of either an introduction from eastern North America or a secondary domestication event that quickly went extinct (31).
Along with building detailed records of the shift to greater reliance on crop plants in, as yet, poorly documented regions of Mexico, there are also obvious gaps in our knowledge in locations that have been the focus of most of the research to date. Although several general trends can be recognized in the south to north timing and sequence of initial appearance of L. siceraria and C. pepo, followed by Z. mays, and much later Phaseolus, in the caves of Oaxaca, Puebla, and Tamaulipas (Table 3) (32), it is also important to appreciate the obvious gaps in these regional sequences. With the exception of a single seed dated to 5960 cal B.C., for example, there is no record of C. pepo in Coxcatlan Cave until after A.D. 1000. Similarly, the very late appearance of C. argyrosperma in the Tehuacán sequence, when compared with Tamaulipas, strongly suggests that its absence, like that of C. moschata, may be a function of small sample size rather than actual dietary absence. And the apparent late domestication and diffusion of P. vulgaris across Mexico, although certainly in sequence with its late appearance in eastern North America, could eventually be moved back substantially in time as more sites in more regions of Mexico are excavated. Clearly there is a great deal still to be learned regarding the transition to food production economies in Mexico, both from additional reanalyses of extant museum archaeobotanical collections and through the recovery of additional data sets through excavation.
Acknowledgments
I thank Terry Martin (Illinois State Museum, Springfield, IL) for facilitating the loan of the Tehuacán cucurbit collections; Noreen Tuross (Harvard University, Cambridge, MA) for the Woods Hole AMS dates on Coxcatlan bottle gourd; and Kent Flannery, Joyce Marcus, and Melinda Zeder for commenting on the manuscript.
Author contributions: B.D.S. designed research, performed research, contributed new reagents/analytic tools, analyzed data, and wrote the paper.
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on April 29, 2003.
Abbreviations: cal, calibrated calendrical; AMS, accelerator mass spectrometry.
See accompanying Profile on page 9435.
References
- 1.Smith, B. D. (1998) The Emergence of Agriculture (Freeman, New York).
- 2.Piperno, D. R., Ranere, A. J., Holst, I. & Hansell, P. (2000) Nature 407, 894-897. [DOI] [PubMed] [Google Scholar]
- 3.Zeder, M., Bradley, D., Emshwiller, E. & Smith, B. D., eds. Documenting Domestication: New Genetic and Archaeological Paradigms (Univ. of California Press, Berkeley), in press.
- 4.Smith, B. D. (1997) Lat. Am. Antiq. 8, 342-383. [Google Scholar]
- 5.MacNeish, R. S., ed. (1967) The Prehistory of the Tehuacán Valley (Univ. of Texas Press, Austin), Vol. 5.
- 6.Flannery, K. V. (1986) Guilá Naquitz (Academic, New York).
- 7.Long, A., Benz, B., Donahue, J., Jull, A. & Toolin, L. (1989) Radiocarbon 31, 1035-1040. [Google Scholar]
- 8.Benz, B. F. & Iltis, H. (1990) Am. Antiq. 55, 500-511. [Google Scholar]
- 9.Benz, B. F. & Long, A. (2000) Curr. Anthropol. 41, 459-465. [PubMed] [Google Scholar]
- 10.Piperno, D. R. & Flannery, K. V. (2001) Proc. Natl. Acad. Sci. USA 98, 2101-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benz, B. F. (2001) Proc. Natl. Acad. Sci. USA 98, 2104-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaenicke-Després, V., Buckler, E. S., Smith, B. D., Gilbert, M. T., Cooper, A., Doebley, J. & Pääbo, S. (2003) Science 302, 1206-1208. [DOI] [PubMed] [Google Scholar]
- 13.Després, V. & Smith, B. D. (2006) in The Histories of Maize, eds. Staller, J., Tykot, R. & Benz, B. (Elsevier, San Diego), in press.
- 14.Kaplan, L. & Lynch, T. (1999) Econ. Bot. 53, 261-272. [Google Scholar]
- 15.Smith, B. D. (1997) Science 276, 932-934. [Google Scholar]
- 16.Smith, B. D. (2000) in Cultural Evolution, eds. Feinman, G. & Manzanilla, L. (Plenum, New York), pp. 15-59.
- 17.Flannery, K. V. (2001) Anc. Mesoamerica 12, 149-156. [Google Scholar]
- 18.Fowler, M. & MacNeish, R. S. (1972) in The Prehistory of the Tehuacán Valley, ed. MacNeish, R. S. (Univ. of Texas Press, Austin), Vol. 5, pp. 219-339. [Google Scholar]
- 19.Johnson, F. & MacNeish R. S. (1972) in The Prehistory of the Tehuacán Valley, ed. MacNeish, R. S. (Univ. of Texas Press, Austin), Vol. 4, 3-58. [Google Scholar]
- 20.MacNeish, R. S. (1997) Curr. Anthropol. 38, 663-672. [Google Scholar]
- 21.Smith, C. E. (1967) in The Prehistory of the Tehuacán Valley, ed. MacNeish, R. S. (Univ. of Texas Press, Austin), Vol. 5, pp. 220-255. [Google Scholar]
- 22.Mangelsdorf, P., MacNeish, R. S. & Galinat, W. (1967) in The Prehistory of the Tehuacán Valley, ed. MacNeish, R. S. (Univ. of Texas Press, Austin), Vol. 5, pp. 178-201. [Google Scholar]
- 23.Kaplan, L. (1967) in The Prehistory of the Tehuacán Valley, ed. MacNeish, R. S. (Univ. of Texas Press, Austin), Vol. 5, pp. 201-211. [Google Scholar]
- 24.Cutler, H. & Whitaker, T. (1967) in The Prehistory of the Tehuacán Valley, ed. MacNeish, R. S. (Univ. of Texas Press, Austin), Vol. 5, pp. 212-219. [Google Scholar]
- 25.Cutler, H. & Whitaker, T. (1961) Am. Antiq. 26, 469-485. [Google Scholar]
- 26.Smith, B. D. in Documenting Domestication, eds. Zeder, M., Bradley, D., Emshwiller, E. & Smith, B. D. (Univ. of California Press, Berkeley), Chap. 2, in press.
- 27.MacNeish, R. S. (1967) in The Prehistory of the Tehuacán Valley, ed. Byers, R. (Univ. of Texas Press, Austin), Vol. 1, pp. 14-25. [Google Scholar]
- 28.Smith, B. D. (2001) J. Archaeol. Res. 9, 1-43. [Google Scholar]
- 29.Pope, K. O., Pohl, M. E. D., Jones, J. G., Lentz, D. L., von Nagy, C., Vega, F. J. & Quitmyer, I. R. (2001) Science 292, 1370-1373. [DOI] [PubMed] [Google Scholar]
- 30.Lentz, D., Pohl, M., Pope, K. & Wyatt, A. (2001) Econ. Bot. 55, 370-376. [Google Scholar]
- 31.Harter, A., Gardner, K., Falush, D., Lentz, D., Bye, R. & Rieseberg, H. (2004) Nature 430, 201-205. [DOI] [PubMed] [Google Scholar]
- 32.Smith, B. D. (2001) Proc. Natl. Acad. Sci. USA 98, 1324-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]