Abstract
Genetic disruption of the steroid receptor coactivator (SRC)-1 and transcriptional intermediary factor (TIF)2/SRC-2 in mouse resulted in distinctive mutant phenotypes. To quantify their roles in the function of androgen receptor (AR) transcriptional activity in vivo, we generated a unique transgenic AR-reporter mouse and analyzed the cell-specific contributions of SRC-1 and TIF2 to the activity of AR in mouse testis. Transgenic AR-luciferase and transgenic AR-lacZ mice harbor a recombinant mouse AR gene, ARGAL4DBD, which is functionally coupled with a upstream activation sequence-mediated reporter gene (AR activity indicator). After characterization of these mice in terms of AR function, we further derived bigenic mice by crossing AR activity indicator mice with the SRC-1-/- or TIF2+/- mutant mice. Analyses of the resultant bigenic mice by in vivo imaging and luciferase assays showed that testicular AR activity was decreased significantly in those with the TIF2+/- mutation but not in the SRC-1+/- background, suggesting that TIF2 serves as the preferential coactivator for AR in testis. Immunohistological analysis confirmed that AR and TIF2 coexist in mouse testicular Sertoli cell nuclei under normal conditions. Although SRC-1 concentrates in Sertoli cell nuclei in the absence of TIF2, nuclear SRC-1 is not able to rescue AR activity in the TIF2 mutant background. Interestingly, SRC-1 appears to negatively influence AR activity, thereby counterbalancing the TIF2-stimulated AR activity. Our results provide unique in vivo insights to the multidimensional cell-type-specific interactions between AR and coregulators.
Keywords: transgenic mouse, nuclear receptor
Androgens play critical roles in the regulation of sexual dimorphic development and physiological responses in animals. The biological actions of androgens are mediated through the androgen receptor (AR), a member of the nuclear receptor superfamily that functions as a ligand-responsive transcription factor. AR is encoded by a single gene in the X chromosome (1). AR protein typically forms a homodimer when binding to the androgen responsive element of target gene promoters (2). Mutations in the DNA-binding domain (DBD) and other functional domains of AR resulted in dysfunction of androgen action; such mutations are found in patients with androgen-insensitive syndrome (3). Mouse models generated by conventional gene knockout methods revealed a complete androgen-insensitive syndrome-like phenotype (4, 5), similar to that of testicular-feminized mutant mice carrying a dominant spontaneous mutation on the X chromosome (6). A Sertoli cell-specific knockout of AR reduced testicular size. Although Sertoli cells and spermatogonia were still present, spermatogenesis was arrested at the meiotic prophase, confirming that AR is essential for spermatogenesis (7–9).
AR transcriptional activity is reported to be modulated by an array of coregulators, including ARA70 and ARA55 (10). Among the currently known coregulators, three steroid receptor coactivator (SRC) family members, SRC-1, transcriptional intermediary factor (TIF)2/GRIP-1/SRC-2, and pCIP/RAC3/AIB1/ACTR/TRAM-1/SRC-3, have been extensively characterized (11). Cell transfection studies have shown that all SRC members enhance nuclear receptor transcriptional activity, typically through the interaction of the LXXLL motifs of SRC with the C-terminal domains of most nuclear receptors (12, 13). However, AR is reported to interact with SRC-1, and perhaps also other SRC members, primarily through the AR N terminus; this interaction depends less on SRC LXXLL motifs (14, 15). Instead, the AR protein contains its own LXXLL-like motifs, which mediate an important N- and C-terminal interaction (16, 17).
Animal studies have shown that genetic disruption of SRC-1 in mice results in a partial steroid hormone-resistant phenotype. The androgen-sensitive prostate exhibits a slightly decreased growth and development, whereas compensatory increases of TIF2 were observed in brain and testis (18). Knockout of TIF2 in mice causes hypofertility in both sexes. Analysis of male mice revealed that TIF2 is expressed in testicular Sertoli cells but not in germ cells. Although TIF2+/- heterozygous mice had no significant phenotypic defects, the TIF2-null mutants showed defects of spermiogenesis with abnormal spermatozoa, testicular degeneration, and accumulation of intracellular lipids in Sertoli cells. However, their prostate and seminal vesicles appeared to be normal (19). Moreover, the SRC-1-/+ TIF2-/- compound mutation resulted in a more severe impairment of mouse fertility, and the SRC-1-/- TIF2-/- mutation was lethal, suggesting that SRC-1 and TIF2 have partial functional redundancy in vivo (20). In contrast, loss of SRC-3 had little effect on fertility but revealed general impairment of growth in SRC-3-/- mutant mice (21). These gene knockout studies provided valuable information regarding the in vivo functions of the SRC gene family. Nevertheless, the specific functional relationships among AR and individual SRC members in specific tissues remain unclear.
In the present study, we generated a transgenic mouse model [AR activity indicator (ARAI) mouse] harboring a reporter gene that specifically reflects in vivo AR activity induced by androgens or pharmacologic regulators. Cross-breeding of these ARAI mice with SRC-1 or TIF2 knockout mice enabled us to assess the specific contributions of SRC-1 or TIF2 to AR function in a tissue- and cell-type-dependent manner.
Materials and Methods
Isolation and Modification of Bacterial Artificial Chromosome (BAC) DNA. The BAC clones, 352D1 and 259K23, were isolated from a RPCI-23 BAC library by the probes corresponding to partial sequences of mouse AR exons 1 and 8. Both terminal sequences of the BAC DNA were compared with the mouse genome in the Ensembl server (www.ensembl.org/Mus_musculus). Clone 352D1 that contains the entire AR-coding region with both 5′- and 3′-flanking sequences was used for construction of the AR-reporter system. BAC DNA modification was carried out with a λ prophage recombination system in Escherichia coli strain DY380 (22), kindly provided by D. Court (National Cancer Institute/Frederick Cancer Research and Development Center, Frederick, MD). The methodology has been outlined by Nemoz-Gaillard et al. (23). Our procedure involved two rounds of recombination with multiple steps of Neo marker-mediated recombinant selection and subsequent marker removal in 294Flp or 294Cre strain (24), kindly provided by A. F. Stewart (European Molecular Biology Laboratory, Heidelberg). The intermediate and final recombinant BAC clones of 352D1-G65-lacZ/EGFP and 352D1-G65-luc+ were verified by Southern hybridization and PCR.
Generation of Transgenic Mice. Engineered BAC DNA was linearized by PI-SceI and diluted to 1 ng/ul for microinjection into the C57BL/6J mouse's fertilized oocytes. PCR primers (5′-GGGCATCGGTCGAGCTTGACATTGTA and 5′-GGCAGTGGGAAGTCAGCTAAACTGGT) specific to the 5′ junction of the 352D1 insertion and BAC vector were used in genotyping the ARAI transgenic mice. Transgenes were further confirmed by Southern hybridization. All mice were housed and bred according to National Institutes of Health guidelines and maintained on a constant 12-h light/12-h dark cycle. Male mice (8-wk-old) were used in this study or otherwise, as stated in the text.
RT-PCR. RNA was isolated with TRIzol, treated with DNase I, and further purified with the RNeasy kit (Qiagen, Valencia, CA). RT-PCR was carried out according to the instructions of the manufacturer (Sigma).
In Vivo and ex Vivo Imaging of Luciferase Activity. Animals were anesthetized with a gas mixture of isoflurane/oxygen. Ten minutes before beginning the photon recording, mice were given 50 μl of aqueous solution of 25 mg/ml d-luciferin (Pharmingen) by i.p. injection. Mice were then placed in the chamber of an IVIS imaging system (Xenogen, Alameda, CA), and images were collected for 5 min with the cooled charge-coupled device camera set at the medium sensitivity. Bioluminescent data were presented with a pseudocolor scale of total photon counts. Imaging of the organs from killed mice was performed under the same IVIS setting as that used for in vivo imaging.
Tissue Luciferase Enzymatic Assay. Freshly dissected mouse tissues were homogenized in cold cell lysis reagent (Promega). Super-natant was used for assays of luciferase activity in a luminometer, and the protein concentration was determined with Bradford reagent (Bio-Rad). The luciferase activity was presented as relative light units/10 s per microgram of protein.
Western Blotting. Protein analysis was carried out as described (18). The primary Abs were rabbit anti-AR (PG-21, Upstate Biotechnology, Lake Placid, NY; 1:400 dilution), anti-SRC-1 (sc-8995, Santa Cruz Biotechnology, 1:500), anti-SRC-2/TIF2 [kindly provided by J. Qin (Baylor College of Medicine), 1:10,000], and anti-actin (A-2066, Sigma, 1:500) polyclonal Abs.
Histochemistry and Immunohistochemistry. Mouse tissue β-gal activity staining was performed as described (21). For immunohistochemical staining, paraffin sections were incubated, respectively, with anti-AR (PG-21, 1:200 dilution), anti-SRC-1 (sc-8995, 1:200), and anti-SRC-2/TIF2 (1:1,000) Abs at 4°C overnight. Detection of the antigen–Ab complexes were then carried out by either chromogenic staining with an ABC immunoperoxidase system (Vector Laboratories) or immunofluorescent labeling with FITC conjugates. Microphotographic images of sections were taken with an Axioskope-2 Plus microscope (Zeiss).
Results
Generation of the Transgenic ARAI Mice. We constructed an ARAI mouse-reporter system for readout of the AR-specific activity in vivo. This system contained a modified mouse AR containing a heterogenous GAL4 DBD, which, in response to androgen, targets the chimeric AR to a reporter containing the GAL4 upstream activation sequences (UAS). To achieve an appropriate spatiotemporal expression pattern of the ARGAL4DBD in transgenic mice, we reconstituted this complete reporter system in a BAC DNA containing a mouse genomic AR gene (Fig. 1A). This AR BAC clone (352D1) consisted of an 8.8-kb vector sequence, a 15.2-kb 5′ upstream sequence, a 167.4-kb AR-coding region, and a 10-kb downstream sequence. The 15.2-kb 5′ upstream sequence contains two known mouse AR promoters located at ≈1.6-kb upstream from the ATG translation start codon (25, 26). We swapped the 64-aa-coding sequence of the AR DBD in exons 2 and 3 with a GAL4-DBD cDNA, designated as G65, which encodes 65 aa of the GAL4 zinc finger motif (27). We then inserted an UAS-minimal-promoter-driven reporter, either UAS-luc+ or UAS-lacZ/EGFP, into the BAC vector (Fig. 1 A). The resultant recombinant BAC clones of the ARG65 reporter were verified and used for microinjection into C57BL donor oocytes to generate transgenic mice. Two separate lines of ARAI transgenic mice, the transgenic ARG65-lacZ (TARZ) and the transgenic ARG65-luc+ (TARL), were established from a total of 16 founders. In addition, a line harboring only the transgenic UAS-luc+ (TL) reporter was established and used as a control for the TARL mice. Among the 16 founders, 15 were able to undergo germ line transmission of transgenes, and their offspring showed normal reproductive traits. Southern hybridization of the genomic DNA from the heterozygous F1 progenies showed that TARZ mice carried one to two copies of the integrated ARG65 transgene (Fig. 1B). Further analysis of transgene expression in the tissues of adult male TARZ mice confirmed that ARG65 was expressed in androgen target tissues such as testis, brain, and skin (Fig. 1C). However, the ARG65 expression profile was representative only of endogenous AR expression in certain tissues, suggesting that the AR gene in BAC 352D1 lacks at least one regulatory element(s) that either is located outside this BAC sequence or was lost via excision of intron 2 during the BAC DNA modification. The lacZ-reporter gene transcript was detectable significantly only in testis, cerebellum, and skin (Fig. 1C). Its limited expression is likely influenced by the ARG65 expression levels and the tissue-dependent differential coregulator distributions.
Fig. 1.
Generation of transgenic ARAI mice. (A) Mouse genomic AR gene in the BAC clone 352D1 was modified by DNA recombination. The AR DBD sequence in exons 2 and 3 was first swapped with the GAL4 DBD-65, and then a luciferase or EGFP-tagged lacZ reporter was inserted at the SacB locus in the vector. Neo markers were removed by Flp or Cre after each recombination step. (B) Transgene copy numbers in transgenic mice were determined in the NcoI-digested genomic DNA by hybridization with an AR probe. A representative DNA blot of the F1 progenies of TARZ mice was shown. Copy numbers were estimated by comparison of the band intensity of ARG65 (Tg) to the WT counterpart (Wt), which exists as single-copy gene in the X chromosome in male (m) and two copies in female (f). (C) Transgene expression was analyzed by RT-PCR. RNA were isolated from the TARZ mouse tissues, which were (1) cerebellum, (2) hypothalamus, (3) pituitary, (4) salivary gland, (5) kidney, (6) testis, (7) prostate, (8) epididymis, and (9) skin. PCR was run 24 cycles for GAPDH, 26 cycles for AR, and 32 cycles for ARG65 and lacZ.
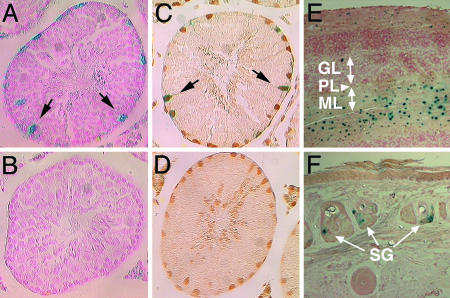
Characterization of Tissue- and Cell-Type-Specific AR-Reporter Activities. To examine whether the expressions of ARG65 and its reporter correlate with AR distribution in a tissue context-dependent manner, we used β-gal activity staining and immunohistochemistry to visualize the lacZ reporter and AR in testis, cerebellum, and skin tissues from TARZ mice. In testis, reporter activity was observed mostly in Sertoli cells along the seminiferous tubules at stages VII and VIII (Fig. 2A) but not in germ cells (Fig. 2B). Overlaying the AR immunohistochemical stains on β-gal activity staining confirmed that lacZ-reporter activity was colocalized with the AR-positive Sertoli cells (Fig. 2 C and D). Similarly, immunostaining of the GAL4 DBD positively identified the transgene product in Sertoli cells (data not shown). In addition to Sertoli cells, weak reporter activity was observed in AR-positive peritubular myoid cells but not in Leydig cells. In the cerebellum, β-gal activity was clearly identified in the molecular layer, specifically in stellate and basket interneurons (Fig. 2E). The AR-specific function in the cerebellum is currently unknown. In skin samples, the lacZ-positive cells were localized in the sebocytes of sebaceous glands (Fig. 2F). The dermal sebaceous gland has been known to be sensitive to androgen abnormalities, which result in acne and other skin diseases (28). Our histological analyses confirmed that the reporter was coexpressed with AR in this subset of expected tissues and cell types.
Fig. 2.
Localization of the AR-reporter expression in transgenic mouse tissues. The lacZ-reporter activities in the testes of TARZ (A and C) and WT (B and D) mice were detected by β-gal activity staining. Some sections were further processed for immunostaining of the AR (C and D). Representative Sertoli cells are marked by arrows. In the TARZ mouse cerebellum (E), lacZ activity was shown in the molecular layer (ML) but not in the granular layer (GL) or Purkinje cell layer (PL). In the skin (F), the sebaceous glands (SG) were positively stained in TARZ mice. (Original photographic magnifications are ×200.)
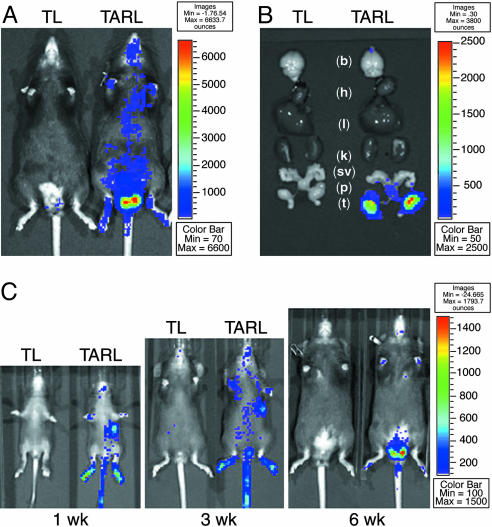
Although the lacZ reporter was useful for mapping cellular AR activity in tissue sections of TARZ mice, it could not be used easily for organ analyses in living animals. For this purpose, we used in vivo imaging in the TARL mice that expressed the transgenic ARG65 and a luciferase reporter. As a control, TL mice harboring only the luciferase reporter were assessed in the live detection procedure. Mice were anesthetized and given luciferase substrate d-luciferin by i.p. injection before imaging in an IVIS system. Our results revealed that the highest photon intensive signals were localized at the lower abdomen of TARL mice (Fig. 3A). To verify the signal source in the transgenic mice, we killed mice after in vivo imaging and dissected the organs for ex vivo imaging; we confirmed that the testis was the organ emitting the strongest luminescent light (Fig. 3B). In addition to testis, other tissues such as skin and brain showed lower signals in ex vivo imaging (data not shown). While monitoring luciferase activity in mice during the first postnatal week to the eighth week, we found that the testicular signals markedly increased with sexual maturation (Fig. 3C), driven by androgen production. In contrast, the signals emanating from stomach and skin of the foot pad and tail decreased after weaning.
Fig. 3.
In vivo and ex vivo imaging of luciferase-reporter activity in transgenic mice. (A) Detection of the ARG65-luciferase activity in the 8-wk-old male TARL mice was performed in an IVIS-100 imaging system. A representative image of the TARL and TL mice was shown. The relative luminescent intensity was indicated by a color scale bar. (B) Tissue-specific activities of the luciferase reporter were examined by ex vivo imaging. White lowercase letters mark the brain (b), heart (h), liver (l), kidney (k), seminal vesicles (sv), prostate (p), and testis (t). (C) Under the same imaging conditions, a series of luminescent data was recorded chronically from the living TARL and TL mice during the postnatal 1–8 wk. Three representative images of different time points were shown with a normalized luminescent intensity scale.
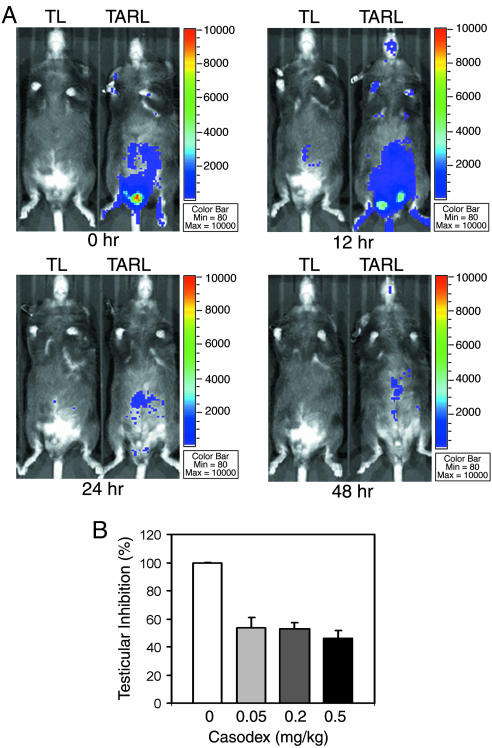
Readout of Pharmacological Responses from the AR Reporter. At the onset of puberty in male animals, an increased secretion of pituitary gonadotropins stimulates testicular production of testosterone (29). To counterbalance gonadotropin stimulation, there is a feedback inhibition of gonadotropin secretion by circulating testosterone at the hypothalamus-pituitary level (30). Pharmacological studies demonstrated that continuous administration of gonadotropin-releasing hormone analogs, such as leuprolide and goserelin, causes a transient elevation of testosterone level followed by a subsequent strong suppression of testosterone production (31). Similarly, administration of AR partial antagonists, such as casodex and flutamide, also causes a temporary elevation of testosterone, which likely results from inhibition of the negative feedback loop at the hypothalamus-pituitary axis (32, 33). To assess pharmacological response in the ARAI mice, we treated 8-wk-old male TARL and TL mice with 25 ng/kg leuprolide (by multiple s.c. injections), followed by in vivo imaging to record the testicular luminescent signal changes in the same groups of experimental mice. We found that testicular luciferase activity indeed increased transiently at 12 h and then decreased by 24 h of treatment (Fig. 4A), indicating that the transgenic AR reporter is responsive to the effect of the gonadotropin-releasing hormone agonist drug. In testing the acute response to casodex, we observed that luciferase-reporter activity decreased in TARL mice after treatments with 0.05–0.5 mg/kg casodex for 21 h (Fig. 4B). However, chronic use of high doses of casodex (5–40 mg/kg) resulted in an increase of serum testosterone, which increased reporter activity (data not shown), suggesting that the antiandrogenic effect could be compromised by a feedback compensation mechanism in intact animals in the absence of leuprolide.
Fig. 4.
Pharmacological responses in transgenic mice. (A) Effect of the gonadotropin-releasing hormone analog Leuprolide on AR activity was monitored by in vivo imaging of the same pair of TARL and TL mice. The mice were given 25 ng/kg leuprolide solution by s.c. injection at 0, 6, 12, 24, and 36 h and imaging at 0, 12, 24, and 48 h, respectively. The images were normalized by the same luminescent intensity scale. (B) The antiandrogenic effect of casodex was examined by luciferase enzymatic assay. The mice were administered casodex in 0.5% Tween 80 suspension by s.c. injection at 0 and 12 h and were killed at 21 h. The testicular samples were collected and homogenized for measurement of luciferase activity by luminometer.
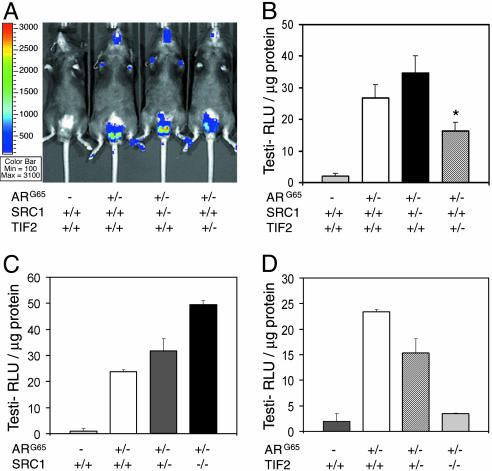
Dissection of the in Vivo Contributions of SRC-1 and TIF2 to AR Activity in Testes. AR coregulators are thought to be crucial in determining tissue-selective AR activity in animals. Currently, >30 coregulators have been suggested to be involved in AR activity regulation (10). SRC-1 and TIF2 are well studied coactivators that enhance AR-mediated transcriptional activity in cultured cells, but their effects on AR function under physiological conditions have not been well defined. To explore their functions in vivo, we generated two lines of bigenic mice by crossing the TARL mice (ARG65+/- luc+/-) with the previously established SRC-1 or TIF2 defective mouse lines (18, 19). The derived heterozygous mice (ARG65+/- luc+/- SRC-1+/- or ARG65+/- luc+/- TIF2+/-) were used for analysis of AR activity. By imaging luciferase activity in living mice and subsequently confirming it in tissue extracts, we compared the AR-reporter activities in 8-wk-old male mice. We observed that loss of one allele of SRC-1 did not reduce ARG65-luciferase activity in the testis; instead, the activity was slightly increased in the heterozygous mutant background (Fig. 5 A and B). Strikingly, much higher ARG65-luciferase activity was detected in the testes of SRC-1-null mice in comparison to WT or SRC-1 heterozygous littermates (Fig. 5C), indicating that SRC-1 plays a suppressive role in counteracting AR activity in the testis. In contrast to the SRC-1 mutants, loss of one allele of TIF2 in bigenic mice caused a significant decrease in AR-reporter activity (Fig. 5 A and B). Although bigenic mice with a TIF2-null mutant background showed a further decrease in ARG65-luciferase activity (Fig. 5D), the effect may be compounded by partial testicular atrophy, although Sertoli cells still remain in the seminiferous tubules. Our results generally agree with the previous observations that SRC-1-null mutants have normal fertility but TIF2-null mutants are semifertile due to severely impaired spermatogenesis (18, 19). The later phenotype resembles the Sertoli cell-specific knockout of AR in mouse (7–9). Thus, it is likely that AR and TIF2 act in the same regulatory pathway to control spermatogenesis.
Fig. 5.
Effects of SRC-family members on the AR activity in transgenic mice. (A) In vivo imaging of 8-wk-old male bigenic mice with age-matched TARL and TL mice. The representative image showed luminescent signals among these mice with or without heterozygous SRC-1 or TIF2 mutation. (B) After the death of these mice, testicular luciferase activities in tissue extracts were measured by luminometer. Bars are average values, n = 8. *, P < 0.01, as compared with the TARL mice with a WT SRC background. (C) SRC-1 effects were determined among the bigenic littermates. (D) Similarly, the TIF2 effects were also compared among the bigenic littermates. Age-matched TL mice were used as the negative control in luciferase activity assays.
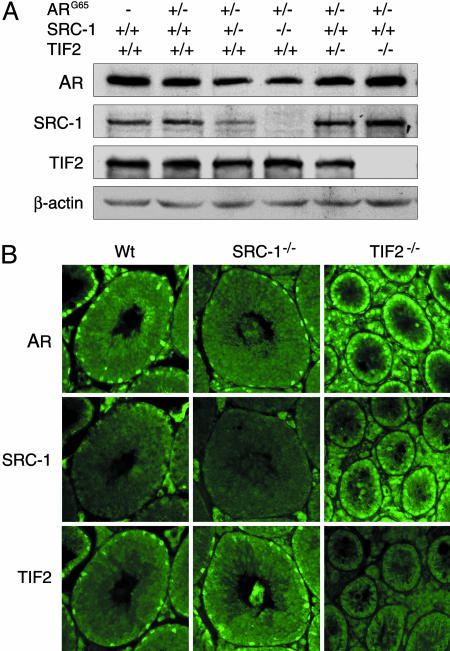
We examined the protein levels of AR and coregulators in the bigenic mouse testicular extracts by Western blotting. In the SRC-1-null mutant, the testicular AR protein was slightly reduced but TIF2 remained at a similar level (Fig. 6A). In contrast, the testicular protein levels of AR and SRC-1 were slightly elevated in TIF2-null mice (Fig. 6A), perhaps to compensate for decreased AR activity. Although we could not draw a firm conclusion from these protein level changes without consideration of the cellular and subcellular context of these factors, we suggest that SRC-1 and TIF2 both can influence AR expression levels in testis.
Fig. 6.
Effects of SRC-family members on AR expression and cellular localization in mouse testis. (A) Western blotting of proteins prepared from testicular samples of transgenic mouse littermates and age-matched control mice. The same blot was probed and reprobed sequentially with the Abs against AR, SRC-1, TIF2, and β-actin. (B) Localization of AR, SRC-1, and TIF2 in the testes of WT and the mutants was determined by immunohistochemistry. (Original photographic magnifications are ×200.)
Because AR-reporter activity was located primarily in Sertoli cells in the testis (Fig. 2A), we examined whether AR and the SRC coregulators reside in the same cell types by immunohistochemistry. Results showed that AR and TIF2 were indeed localized in Sertoli cell nuclei (Fig. 6B), suggesting they may interact directly in the cell nucleus. AR and TIF2 have been reported in these cells by others (19, 34). SRC-1 staining was faint and perinuclear in the WT mouse testis but stronger in the Sertoli cell nuclei of TIF2-null mice (Fig. 6B). This may be due to translocation of the existing SRC-1 in Sertoli cell cytoplasm to the nucleus in the absence of TIF2. However, it is notable that the presence of SRC-1 in Sertoli cell nuclei could not rescue AR-reporter activity in the testis of TIF2-null mice. We conclude that, under physiological conditions in vivo, AR preferentially functions with TIF2 rather than SRC-1 in this androgen-responsive cell type to achieve its transcriptional activity and functions.
Discussion
Tissue-selective nuclear receptor activity, either as a natural phenomenon of hormonal regulation or as an induced pharmacological response, has been the topic of intensive investigation. There is current need for in vivo readout systems to effectively measure tissue-selective nuclear receptor (NR) activities. Particularly for the AR, in vivo readout of its specific functional activity in different tissues in response to a ligand challenge has not been established. Only a few AR target genes have been characterized, mostly from prostate, such as human prostate-specific antigen and mouse probasin. The important characteristic of these “direct target genes” is that their promoters or enhancers should contain androgen-responsive elements, which can discriminate androgen-induced AR binding from other NRs (35). However, differing degrees of crossreactivity with glucocorticoid and progesterone receptors were found in these promoter- or enhancer-mediated transcriptional assays (35–39). Although these crossreactions in animals have not been clearly defined, even the probasin composite promoter has been shown to be responsive to glucocorticoids in transgenic mice (40).
In the present study, we established a transgenic mouse model for assessing AR-specific activity and for functional analysis of AR coregulators in vivo. This ARAI model contains a unique reporter that depends on the transgenic ARGAL4DBD interacting with an UAS-composite promoter located upstream from reporter genes, thus avoiding promoter crossreaction with other nuclear receptors such as glucocorticoid and progesterone receptors. To direct the reporter expression in a tissue and cell-type manner where endogenous AR is expressed, this reporter system was constructed within mouse genomic DNA that contains the native AR gene promoter and enhancers to control tissue expression of the ARGAL4DBD.
Analyses of our transgenic mice showed that tissue expression levels of ARG65 were generally lower than native AR with the exception of testis and few other tissues. This is possibly due to unknown missing AR regulatory elements that locate beyond 15 kb upstream or 10 kb downstream of the AR-coding region or in the intron 2 that was deleted during modification of the BAC DNA (Fig. 1 A). Earlier studies found there are two promoters located within 1.6 kb upstream of the mouse AR translational start codon; they resemble certain housekeeping genes containing no TATA or CAAT boxes but having two perfect GC boxes and a long GC-rich region (25, 26). In addition, a repressor element and a cAMP response element were identified within this 1.6-kb region (41, 42). These elements, together with multiple androgen responsive elements and a Myc site in the AR-coding region, appear to modulate expression of AR in its target tissues (43). Our transgenic mice showed detectable AR-reporter activities only in certain AR-positive tissues and cell types such as testicular Sertoli cells, cerebellar stellate and basket cells, and dermal sebacytes. Because AR function in testicular Sertoli cells has been well documented (7–9), it was chosen for testing of our AR reporter.
Our ARAI model proved to be effective for the study of hormonal responses in living animals when we used the testis as a primary target tissue. In principle, it has the same fundamental mechanism of AR action as in other tissues, and reporter activity can be easily captured by quantitative imaging because of its superficial and confined anatomic position. However, the intratesticular testosterone concentration is higher than in other tissues (44), making it more difficult for intratesticular AR to be blocked by low-affinity AR antagonists.
We used our mouse model to explore the in vivo interactions of AR and SRC-1/SRC-2 coactivators. We found that AR activity in testis was suppressed by loss of TIF2/SRC-2 but not by loss of SRC-1. Our results substantiated that mouse fertility defects in TIF2 gene knockout mutants are at least partly a result of impaired AR activity in testis, and that spermatogenesis is not affected by loss of SRC-1. Many published transient cell transfection assays have shown that AR can be coactivated by both SRC-1 and TIF2 (15, 45), but only one study showed that AR activity is suppressed by SRC-1 (16). There also are in vitro studies indicating that the N-terminal glutamine-rich region of AR physically interacts with both SRC-1 and TIF2 (14, 15), but that the C-terminal domain preferentially binds to TIF2 (13). Clearly, the in vivo interactions of AR and coregulators depend on tissue- and cell-type context and animal physiology. Nevertheless, our study of testicular AR in the transgenic ARAI mouse model revealed that TIF2 functions as the preferential coactivator for AR-mediated transcription in Sertoli cells. Interestingly, SRC-1 can serve as a counterbalancing factor that negatively influences AR activity under physiologic conditions. Our study not only has validated select previous findings derived from in vitro or transient transfection assays but also serves as an initiative for understanding the differential functions of coregulators in regulating AR activity in vivo. In addition, it provides proof of concept that future models can be devised to target a specific nuclear receptor (NR)–coregulator interface to inhibit or activate the NR in a tissue-specific and ligand-independent manner that would be useful in pharmaceutical analyses.
Acknowledgments
We thank Dr. Pierre Chambon (Institut de Génétique et de Biologie Moléculaire et Cellulaire et Institut Clinique de la Souris, Illkirch, France) for providing the TIF2+/- mouse, Dr. Eric Nemoz-Gaillard for helping in BAC recombination, Dr. Frederic Pereira for advice on histology, and Dr. David Spencer (Baylor College of Medicine) for providing in vivo imaging. This work was supported in part by funding from the National Institutes of Health (Grants DK59820 and HD-07857 to B.W.O.) and a postdoctoral fellowship from the Department of Defense (Grant PC010231 to X.Y.).
Author contributions: B.W.O. designed research; X.Y. performed research; S.J.H., F.J.D., J.X., and B.W.O. contributed new reagents/analytic tools; X.Y., S.Y.T., and M.-J.T. analyzed data; and X.Y. wrote the paper.
Abbreviations: AR, androgen receptor; SRC, steroid receptor coactivator; TIF, transcriptional intermediary factor; UAS, upstream activation sequence; DBD, DNA-binding domain; BAC, bacterial artificial chromosome; ARAI, AR activity indicator; TARL, transgenic ARG65-luc+; TARZ, transgenic ARG65-lacZ; TL, transgenic UAS-luc+.
References
- 1.Migeon, B. R., Brown, T. R., Axelman, J. & Migeon, C. J. (1981) Proc. Natl. Acad. Sci. USA 78, 6339-6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sack, J. S., Kish, K. F., Wang, C., Attar, R. M., Kiefer, S. E., An, Y., Wu, G. Y., Scheffler, J. E., Salvati, M. E., Krystek, S. R., Jr., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 4904-4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gottlieb, B., Beitel, L. K., Wu, J. H. & Trifiro, M. (2004) Hum. Mutat. 23, 527-533. [DOI] [PubMed] [Google Scholar]
- 4.Yeh, S., Tsai, M. Y., Xu, Q., Mu, X. M., Lardy, H., Huang, K. E., Lin, H., Yeh, S. D., Altuwaijri, S., Zhou, X., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 13498-13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsumoto, T., Takeyama, K., Sato, T. & Kato, S. (2003) J. Steroid Biochem. Mol. Biol. 85, 95-99. [DOI] [PubMed] [Google Scholar]
- 6.Lyon, M. F. & Hawkes, S. G. (1970) Nature 227, 1217-1219. [DOI] [PubMed] [Google Scholar]
- 7.Chang, C., Chen, Y. T., Yeh, S. D., Xu, Q., Wang, R. S., Guillou, F., Lardy, H. & Yeh, S. (2004) Proc. Natl. Acad. Sci. USA 101, 6876-6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Gendt, K., Swinnen, J. V., Saunders, P. T., Schoonjans, L., Dewerchin, M., Devos, A., Tan, K., Atanassova, N., Claessens, F., Lecureuil, C., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 1327-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holdcraft, R. W. & Braun, R. E. (2004) Development (Cambridge, U.K.) 131, 459-467. [DOI] [PubMed] [Google Scholar]
- 10.Heinlein, C. A. & Chang, C. (2002) Endocr. Rev. 23, 175-200. [DOI] [PubMed] [Google Scholar]
- 11.McKenna, N. J., Lanz, R. B. & O'Malley, B. W. (1999) Endocr. Rev. 20, 321-344. [DOI] [PubMed] [Google Scholar]
- 12.Heery, D. M., Kalkhoven, E., Hoare, S. & Parker, M. G. (1997) Nature 387, 733-736. [DOI] [PubMed] [Google Scholar]
- 13.Ding, X. F., Anderson, C. M., Ma, H., Hong, H., Uht, R. M., Kushner, P. J. & Stallcup, M. R. (1998) Mol. Endocrinol. 12, 302-313. [DOI] [PubMed] [Google Scholar]
- 14.Bevan, C. L., Hoare, S., Claessens, F., Heery, D. M. & Parker, M. G. (1999) Mol. Cell. Biol. 19, 8383-8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alen, P., Claessens, F., Verhoeven, G., Rombauts, W. & Peeters, B. (1999) Mol. Cell. Biol. 19, 6085-6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikonen, T., Palvimo, J. J. & Janne, O. A. (1997) J. Biol. Chem. 272, 29821-29828. [DOI] [PubMed] [Google Scholar]
- 17.He, B., Kemppainen, J. A. & Wilson, E. M. (2000) J. Biol. Chem. 275, 22986-22994. [DOI] [PubMed] [Google Scholar]
- 18.Xu, J., Qiu, Y., DeMayo, F. J., Tsai, S. Y., Tsai, M. J. & O'Malley, B. W. (1998) Science 279, 1922-1925. [DOI] [PubMed] [Google Scholar]
- 19.Gehin, M., Mark, M., Dennefeld, C., Dierich, A., Gronemeyer, H. & Chambon, P. (2002) Mol. Cell. Biol. 22, 5923-5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mark, M., Yoshida-Komiya, H., Gehin, M., Liao, L., Tsai, M. J., O'Malley, B. W., Chambon, P. & Xu, J. (2004) Proc. Natl. Acad. Sci. USA 101, 4453-4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu, J., Liao, L., Ning, G., Yoshida-Komiya, H., Deng, C. & O'Malley, B. W. (2000) Proc. Natl. Acad. Sci. USA 97, 6379-6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu, D., Ellis, H. M., Lee, E. C., Jenkins, N. A., Copeland, N. G. & Court, D. L. (2000) Proc. Natl. Acad. Sci. USA 97, 5978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nemoz-Gaillard, E., Tsai, M.-J. & Tsai, S. Y. (2003) NURSA e-Journal 1, ID 4.06142003.1, www.nursa.org.
- 24.Buchholz, F., Angrand, P. O. & Stewart, A. F. (1996) Nucleic Acids Res. 24, 3118-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faber, P. W., King, A., van Rooij, H. C., Brinkmann, A. O., de Both, N. J. & Trapman, J. (1991) Biochem. J. 278, 269-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grossmann, M. E., Lindzey, J., Blok, L., Perry, J. E., Kumar, M. V. & Tindall, D. J. (1994) Biochemistry 33, 14594-14600. [DOI] [PubMed] [Google Scholar]
- 27.Marmorstein, R., Carey, M., Ptashne, M. & Harrison, S. C. (1992) Nature 356, 408-414. [DOI] [PubMed] [Google Scholar]
- 28.Zouboulis, C. C. & Degitz, K. (2004) Exp. Dermatol. 13, 5-10. [DOI] [PubMed] [Google Scholar]
- 29.Ketelslegers, J. M., Hetzel, W. D., Sherins, R. J. & Catt, K. J. (1978) Endocrinology 103, 212-222. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto, A. M. & Bremner, W. J. (1984) J. Clin. Endocrinol. Metab. 58, 609-614. [DOI] [PubMed] [Google Scholar]
- 31.Stricker, H. J. (2001) Urology 58, 24-27. [DOI] [PubMed] [Google Scholar]
- 32.Verhelst, J., Denis, L., Van Vliet, P., Van Poppel, H., Braeckman, J., Van Cangh, P., Mattelaer, J., D'Hulster, D. & Mahler, C. (1994) Clin. Endocrinol. (Oxford) 41, 525-530. [DOI] [PubMed] [Google Scholar]
- 33.Veldhuis, J. D., Urban, R. J. & Dufau, M. L. (1992) J. Clin. Endocrinol. Metab. 74, 1227-1235. [DOI] [PubMed] [Google Scholar]
- 34.Zhou, Q., Nie, R., Prins, G. S., Saunders, P. T., Katzenellenbogen, B. S. & Hess, R. A. (2002) J. Androl. 23, 870-881. [PubMed] [Google Scholar]
- 35.Claessens, F., Celis, L., Peeters, B., Heyns, W., Verhoeven, G. & Rombauts, W. (1989) Biochem. Biophys. Res. Commun. 164, 833-840. [DOI] [PubMed] [Google Scholar]
- 36.Heemers, H., Verrijdt, G., Organe, S., Claessens, F., Heyns, W., Verhoeven, G. & Swinnen, J. V. (2004) J. Biol. Chem. 279, 30880-30887. [DOI] [PubMed] [Google Scholar]
- 37.Verrijdt, G., Schoenmakers, E., Alen, P., Haelens, A., Peeters, B., Rombauts, W. & Claessens, F. (1999) Mol. Endocrinol. 13, 1558-1570. [DOI] [PubMed] [Google Scholar]
- 38.Devos, A., Claessens, F., Alen, P., Winderickx, J., Heyns, W., Rombauts, W. & Peeters, B. (1997) Mol. Endocrinol. 11, 1033-1043. [DOI] [PubMed] [Google Scholar]
- 39.Shen, R., Sumitomo, M., Dai, J., Hardy, D. O., Navarro, D., Usmani, B., Papandreou, C. N., Hersh, L. B., Shipp, M. A., Freedman, L. P., et al. (2000) Mol. Cell Endocrinol. 170, 131-142. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, J., Thomas, T. Z., Kasper, S. & Matusik, R. J. (2000) Endocrinology 141, 4698-4710. [DOI] [PubMed] [Google Scholar]
- 41.Grossmann, M. E., Lindzey, J., Kumar, M. V. & Tindall, D. J. (1994) Mol. Endocrinol. 8, 448-455. [DOI] [PubMed] [Google Scholar]
- 42.Lindzey, J., Grossmann, M., Kumar, M. V. & Tindall, D. J. (1993) Mol. Endocrinol. 7, 1530-1540. [DOI] [PubMed] [Google Scholar]
- 43.Grad, J. M., Dai, J. L., Wu, S. & Burnstein, K. L. (1999) Mol. Endocrinol. 13, 1896-1911. [DOI] [PubMed] [Google Scholar]
- 44.Hill, C. M., Anway, M. D., Zirkin, B. R. & Brown, T. R. (2004) Biol. Reprod. 71, 1348-1358. [DOI] [PubMed] [Google Scholar]
- 45.Ma, H., Hong, H., Huang, S. M., Irvine, R. A., Webb, P., Kushner, P. J., Coetzee, G. A. & Stallcup, M. R. (1999) Mol. Cell. Biol. 19, 6164-6173. [DOI] [PMC free article] [PubMed] [Google Scholar]