Abstract
In higher plant chloroplasts, transthylakoid proton motive force serves both to drive the synthesis of ATP and to regulate light capture by the photosynthetic antenna to prevent photodamage. In vivo probes of the proton circuit in wild-type and a mutant strain of Arabidopsis thaliana show that regulation of light capture is modulated primarily by altering the resistance of proton efflux from the thylakoid lumen, whereas modulation of proton influx through cyclic electron flow around photosystem I is suggested to play a role in regulating the ATP/NADPH output ratio of the light reactions.
Keywords: ATP synthase proton conductivity, cyclic electron flow, linear electron flow, energy-dependent nonphotochemical quenching, protein motive force
Photosynthesis converts light energy into chemical energy, ultimately powering the vast majority of our ecosystem (1). Higher plant photosynthesis is initiated through absorption of light by antennae complexes that funnel the energy to photosystem (PS) II and I. The photosystems operate in sequence with the plastoquinone pool, the cytochrome b6f complex, and plastocyanin to oxidize H2O and reduce NADP+ to NADPH in what is termed linear electron flow (LEF). LEF is coupled to proton translocation, establishing a transthylakoid electrochemical gradient of protons, termed the proton motive force (pmf) (2), comprised of electric field (ΔΨ) and pH (ΔpH) gradients (3).
Dual Role of the pmf
The pmf plays two central roles in higher plant photosynthesis (4). First, pmf drives the normally endergonic synthesis of ATP through the CF1-CF0 ATP synthase (ATP synthase) (5). Both the ΔpH and ΔΨ components of pmf contribute to ATP synthesis in a thermodynamically, and probably kinetically, equivalent fashion (6). Second, pmf is a key signal for initiating photoprotection of the photosynthetic reaction centers through energy-dependent nonphotochemical quenching (qE), a process that harmlessly dissipates excessively absorbed light energy as heat (7–10). Only the ΔpH component of pmf, through acidification of the lumen, is effective in initiating qE by activating violaxanthin de-epoxidase, a lumen-localized enzyme that converts violaxanthin to antheraxanthin and zeaxanthin, and by protonating lumen-exposed residues of PsbS, a pigment-binding protein of the PS II antenna complex (11).
A Need for Flexibility in the Light Reactions
A major open question concerns how the light reactions achieve the flexibility required to meet regulatory needs and match downstream biochemical demands (12). In LEF to NADP+, the synthesis of ATP and the production of NADPH are coupled, producing a fixed ATP/NADPH output ratio. LEF alone is probably unable to satisfy the variable ATP/NADPH output ratios required to power the sum of the Calvin–Benson cycle (13, 14) and other metabolic processes (alternate electron and ATP sinks) that are variably engaged under different physiological conditions (12, 15, 16). Failure to match ATP/NADPH output with demand will lead to buildup of products and depletion of substrates for the light reactions, leading to inhibition of the entire process.
The generation of pmf is likewise coupled to LEF, so it is clear that the sensitivity of antenna regulation (or qE) must also be modulated in some way to avoid catastrophic failure of photoprotection (12, 15, 17–19). Longer-term acclimation of the qE response can involve altering the sensitivity of the regulatory machinery to lumen pH by changing the xanthophyll pigment and/or PsbS levels (12, 20). However, dramatic changes in light intensity and/or CO2 availability can occur over the seconds-to-hours time scale (8), requiring short-term adjustments. Indeed, it has been demonstrated that short-term alteration of CO2 or O2 levels can strongly modulate (by up to 6-fold) the sensitivity of qE with respect to LEF (17, 18).
Two Types of Flexibility Mechanisms
Two general types of models have been proposed to account for the flexibility required to meet these changing demands (12). In “Type I” mechanisms, proton flux into the lumen is increased through alternate electron transfer pathways, especially cyclic electron flow associated with PSI (CEF1), a mechanism that returns electrons from PSI to the plastoquinone pool, thereby increasing the magnitude of the pmf relative to that generated by LEF alone (12). Other processes are also possible, for example, turnover of a plastid terminal oxidase (21, 22), but these processes would have to run at relatively high rates to significantly impact the overall ATP/NADPH balance. For C3 vascular plants, CEF1 has been suggested to supply the relatively small fluxes (10–15% of that supplied by LEF) of protons required to balance ATP/NADPH output for the Calvin–Benson cycle and nitrogen assimilation (13, 14). It is a matter of intense debate (23, 24) as to whether CEF1 can run at sufficiently high rates to alter qE responses by up to 6-fold, especially given the expected large ATP/NADPH imbalances such large fluxes would likely incur (12, 16).
In Type II mechanisms, lumen acidification with respect to LEF is adjusted without changing the relative flux of protons into the lumen, thus modulating qE sensitivity without impacting ATP/NADPH output. This phenomenon is thought to be achieved by varying either the conductivity of the CF1-CF0 ATP synthase to proton efflux as measured by electrochromic shift (ECS) decay (gH+), i.e., the inverse of the resistance to proton efflux from the lumen or the relative fraction of pmf stored as ΔpH (12, 16–18, 24).
Probing the pmf to Gain Insight into the Flexibility Mechanisms
Recently, a series of in vivo probes of the pmf have been introduced (2, 3, 16, 25–28), allowing contributions from Types I and II flexibility mechanisms to be directly assessed. These techniques are based on kinetic analyses of the ECS (26) of photosynthetic pigments, which yields absorbance changes proportional to changes in transthylakoid ΔΨ (29). Several useful parameters can be obtained from analysis of ECS decay kinetics during brief dark perturbations of the steady state, including estimates of the relative flux of protons through the ATP synthase (νH+, which at steady state equals flux of protons into the lumen), the magnitude of the light-induced pmf, the fraction of pmf stored as ΔpH and ΔΨ, and gH+ (3, 16–18, 25, 26, 28). Combined with standard chlorophyll a fluorescence assays, from which estimates of LEF can be obtained (30), one can calculate the pmf generated by LEF alone (i.e., pmfLEF = LEF/gH+), a key parameter for estimating fractional changes in CEF1 turnover (17, 18).
Using these probes of the proton circuit, it was shown that in intact Nicotiana tabacum (tobacco) leaves, lowering atmospheric CO2 from 372 to 0 ppm led to a ≈5-fold increase in the dependence of qE on LEF (17). The effect could be entirely accounted for by a proportional (i.e., 5-fold) decrease in gH+, so that even modest rates of LEF generated a substantial pmf and a robust qE response (17, 18). A similar (≈6-fold) change in qE sensitivity was observed when both O2 and CO2 were lowered (to 1% and 50 ppm, respectively), but in this case, both changes in gH+ and increased partitioning of pmf into ΔpH were invoked to explain the effect (18). In both cases, the ratio of vH+/LEF remained essentially constant (within noise levels), indicating that contributions from CEF1 to proton flux were either small or remained a relatively constant fraction of those from LEF, as previously found for tobacco (25). On the whole, these results support a large role for Type II mechanisms in modulating qE sensitivity upon short term changes in CO2/O2 levels, but they do not rule out smaller contributions from Type I mechanisms in balancing ATP/NADPH output (12, 16, 28).
On the other hand, Munekage et al. (31, 32) recently presented partial characterization of a mutant strain of Arabidopsis thaliana, termed pgr5 for proton gradient regulation, which showed two provocative phenotypes. First, nonphotochemical reduction of the plastoquinone pool, attributed to the key step in CEF1, was inhibited in pgr5. Second, qE was severely diminished. It is reasonable to hypothesize that the loss of PGR5 blocks CEF1 and, thereby, abolishes a significant flux of protons needed to activate qE (31, 32). Evidence for such a hypothesis would support a large role for Type I mechanisms in modulating qE sensitivity (33) while arguing against Type II models (12, 17, 18). On the other hand, mutation of pgr5 could indirectly affect qE by disrupting downstream processes and modulating metabolic pool sizes (31, 32). Here, we present an experimental test for causal links between the loss of PGR5, steady-state proton flux, and the qE response, allowing us to determine the relative roles of Type I and II flexibility responses.
Materials and Methods
Plant Strains and Growth Conditions. Wild-type A. thaliana (Wt-background strain gl1) (31) and pgr5 plants were grown in tightly controlled chambers under a 16:8 photoperiod at an average of ≈70 μmol photons m-2s-1 photosynthetically active radiation and at 23°C. These growth conditions stably reproduced the phenotypes seen previously over the entire experimental period (31). Wt (gl1) and pgr5 seeds were a gift from T. Shikanai (Nara Institute of Science and Technology, Ikoma, Nara, Japan).
Spectroscopic Assays. Fully expanded leaves from ≈23- to 26-day-old plants were used in spectroscopic assays. Room air (372 ppm CO2/21% O2) or premixed gases from cylinders (i.e., 50 ppm CO2/21% O2) were bubbled through water (for humidification) before entering the measuring chamber of the spectrophotometer. Leaves were clamped into the measuring chamber of a nonfocusing optics spectrophotometer/chlorophyll fluorometer, specifically designed for use on leaves (17, 18, 34). Leaves were first exposed to 26–216 μmol photons m-2s-1 photosynthetically active radiation from a series of red light emitting diodes (maximum emission wavelength of 637 nm) to reach steady-state conditions (10 min). Further preillumination had little additional effect. After this actinic period, the steady-state (Fs) and light saturated (FM′) levels of chlorophyll a fluorescence yield were obtained (17, 18), from which estimates of the efficiency of PSII photochemistry (ΦII) were calculated (30). Estimates of LEF were obtained by using ΦII as in ref. 35. Analyses of the ECS decay kinetics upon perturbation of the steady state with an ≈300-ms dark period were performed as described in refs. 17, 18, and 26. Previous assays showed linear correlations between estimates of LEF taken from fluorescence and absorbance measurements with our instruments (25–27, 34), suggesting that the spectroscopic techniques probed similarly responding populations of chloroplasts. Absorbance changes at 505, 520, and 535 nm were recorded in series, and those attributable to changes in ECS were deconvoluted from background signals according to the following equation (25, 26):
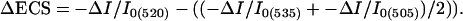 |
[1] |
An estimate of steady-state, light-induced pmf, termed ECSt, was taken as the total amplitude of ECS decay from its steady-state level to its minimum quasi-stable level after ≈300 ms dark period (16–18). Relative estimates of the conductivity of the thylakoid membrane to protons (gH+), primarily attributable to the turnover of the ATP synthase, were obtained by taking the inverse of the time constant for ECS decay (τECS) (16–18, 28). Relative estimates of the pmf attributable to proton flux from LEF, termed pmfLEF, were calculated by using the following equation (16, 18, 28):
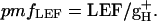 |
[2] |
This parameter estimates the light-driven proton flux through the ATP synthase based on the extent of LEF and the kinetic properties of the ATP synthase turnover, as reviewed in ref. 28.
Western Blot Analyses. Crude leaf extracts from Wt and pgr5 were prepared as described in ref. 36. Flash-frozen tissue was ground in a mortar and pestle before resuspension in SDS/PAGE sample buffer. Ten micrograms of protein, as estimated by using the BCA Protein Assay Kit (Pierce), from each preparation was loaded onto an SDS/PAGE gel. Protein was transferred to poly(vinyl difluoride) membranes and probed with antibody directed against the β-subunit of the ATP synthase (a gift from Alice Barkan, University of Oregon, Eugene, OR). Immunoreactive bands were detected on radiographic film by using the SuperSignal West Pico Chemiluminescent Substrate kit (Pierce). Similar conclusions were reached when the gel was loaded with 10, 30, or 90 μg of protein, indicating that the assay was within the linear range of detection (data not shown).
Results and Discussion
Effects of Lowering CO2 Levels and Loss of PGR5 on LEF and qE Sensitivity. Fig. 1A shows plots of qE as a function of LEF from 26–216 μmol photons m-2s-1 for the wild type (Wt, gl1) (31) under ambient air (372 ppm CO2/21% O2) and two different treatments that lowered light saturated LEF by about the same extent. Low CO2 air (LC; 50 ppm CO2/21% O2) reduced light-saturated LEF in Wt by ≈30%, a typical response for A. thaliana (35). A similar lowering of light-saturated LEF was obtained by using pgr5 under ambient air. These conditions were chosen to avoid significant photoinhibition, which appeared in pgr5 at >216 μmol photons m-2s-1 as a decrease in LEF, as well as large changes in the partitioning of the pmf into ΔΨ and ΔpH, a phenomenon that has been previously observed in N. tabacum under severe stress (18). Under more extreme conditions (higher light intensities or lower CO2 levels), results were qualitatively consistent with those presented here (data not shown) as long as partitioning of pmf into ΔΨ and ΔpH was considered (18).
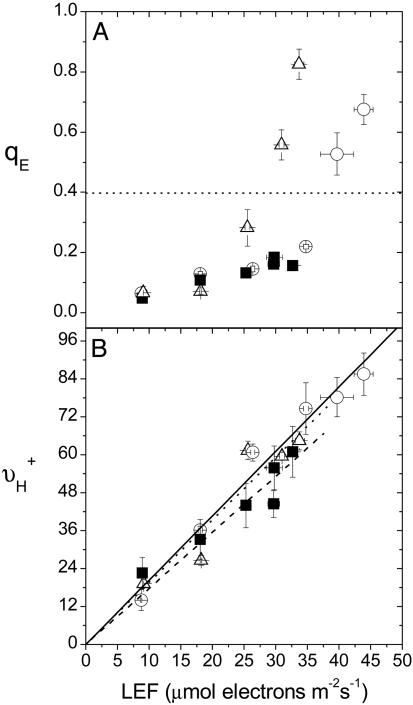
Fig. 1.
LEF dependencies of antenna regulation and light-driven proton flux across the thylakoid membrane. Chlorophyll a fluorescence yield and ECS analyses were used to obtain estimates of energy-dependent exciton quenching (qE)(A) and steady-state proton flux into the lumen (νH+) (B), respectively, from 26 to 216 μmol photons m-2s-1 on leaves from A. thaliana Wt under ambient (372 ppm CO2/21% O2) (○) and low CO2 (LC; 50 ppm CO2/21% O2) (▵) air, as well as pgr5 under ambient air (▪) and plotted as a function of estimated LEF (18). Linear regressions of LEF versus νH+ are shown in B, the regression slopes of which are 2.035 (solid line), 2.038 (dotted line), and 1.774 (dashed line) for Wt ambient air, Wt/LC air, and pgr5 ambient air, respectively. Slopes for Wt/atmospheric and pgr5/atmospheric were judged by analysis of covariance to be statistically different (P < 0.05). Error bars represent SE for n = 3–6.
In Wt under ambient air, a flux of ≈40 μmol electrons m-2s-1 generated a qE of 0.4, whereas the same level of qE was achieved at a flux of ≈27 μmol electrons m-2s-1 under LC air (Fig. 1 A). At saturating light, qE was ≈35% larger under LC than ambient air, despite having a slower LEF. Thus, similar to previous observations in N. tabacum (17, 18), lowering CO2 in Wt increased the sensitivity of qE with respect to LEF. In contrast, the ≈30% decrease in LEF that occurred in the absence of PGR5 was not accompanied by a corresponding increase in the light saturated qE response, but was rather 4- to 6-fold lower in comparison with that in the Wt.
Effects of Lowering CO2 Levels and Loss of PGR5 on Contributions of CEF1 to the Proton Budget. In Wt, varying the CO2 levels had no observable effects on the relationship between νH+ and LEF (Fig. 1B), arguing against large CO2-dependent changes in contributions from Type I modulation (12, 16–18). On the other hand, the slope of νH+ vs. LEF was ≈13% smaller (P < 0.05) in pgr5 than in Wt (Fig. 1B). The results were not significantly altered by forcing the linear fits through the origin. Although these small differences could be the result of small systematic errors, e.g., in LEF measurements (37), they are also consistent with results from Munekage et al. (31, 32) that PGR5 is important for steady-state CEF1, and we thus adopt this view as our working model.
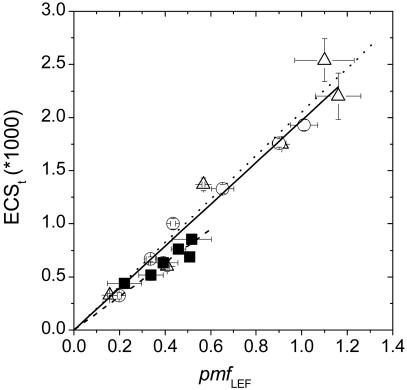
This model is supported in separate estimates of proton flux and pmf. The data in Fig. 2 shows the relationships between estimates of the pmf attributable solely to proton translocation by LEF (pmfLEF) and the total pmf (ECSt), driven by the sum of LEF and other process (i.e., CEF1). Within the noise level, the relationships for Wt under the two CO2 levels overlapped (analysis of covariance indicated no significant differences in slopes, P = 0.6), implying that either LEF accounted for the vast majority of estimated pmf, or that contributions from other processes (see above), most notably CEF1, were a constant fraction of LEF. Again, the slope of pmfLEF versus ECSt was ≈14% smaller in pgr5 in comparison to Wt under ambient conditions, a difference that was statistically significant (analysis of covariance, P < 0.05).
Fig. 2.
The relationship between light-induced pmf and the pmf generated by LEF alone. ECS and chlorophyll a fluorescence yield analyses were performed on leaves from A. thaliana Wt plants and pgr5 to estimate light-induced pmf (ECSt) and LEF, respectively, from which estimates of the pmf generated by LEF alone (pmfLEF) were obtained (i.e., pmfLEF = LEF/gH+). Linear regressions of pmfLEF versus ECSt are shown, the slopes of which are 1.972 (solid line), 2.053 (dotted line), and 1.701 (dashed line) for Wt/ambient air, Wt/LC air, and pgr5/ambient air, respectively. Slopes for Wt/atmospheric and pgr5/atmospheric were ≈14% different and judged by analysis of covariance to be statistically different (P < 0.05). The small difference (≈4%) between the slopes of Wt/atmospheric versus Wt/LC was not statistically significant (P = 0.6). Conditions and symbols are as in Fig. 1. Error bars represent SE for n = 3–6.
It is important to note that the ECSt estimate of pmf is based on the light-dark difference in the amplitude of the ECS signal (17, 18), whereas the pmfLEF estimate of pmf is based on ECS decay kinetics (18), i.e., the latter is not sensitive to changes in the absolute ECS response. The leaf contents of photosynthetic complexes were equivalent in Wt and pgr5 (31), and the amplitudes of the rapid (<1 ms) ECS responses after saturating, single turnover flashes, which reflect charge separation in PSII and PSI centers (38), were indistinguishable, with Wt and pgr5 giving 3.5 ± 0.35 and 3.5 ± 0.24 (ΔI/I0 × 1,000) respectively, indicating essentially identical responses to ΔΨ. Overall, the constancy of these results supports the validity of comparisons of the ECS-derived parameters between the two strains.
Differences in qE Sensitivity Between Wt and pgr5 Can Be Largely Attributed to Changes in gH+. The above flux estimates suggest differences in contributions to light-induced pmf from processes other than LEF, consistent with a difference in CEF1 engagement between Wt and pgr5 (31, 32). However, the modest (≈13%) decrease in νH+ in the absence of PGR5 was far too small to directly account for the corresponding 4- to 6-fold decrease in the qE response at light-saturated LEF (Fig. 1 A). In this regard, it was striking that the pgr5 mutant exhibited lowered LEF without a corresponding increase in qE sensitivity, in contrast to what was observed in the Wt upon lowering CO2 (Fig. 1 A).
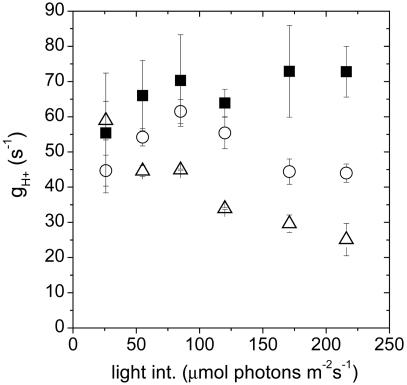
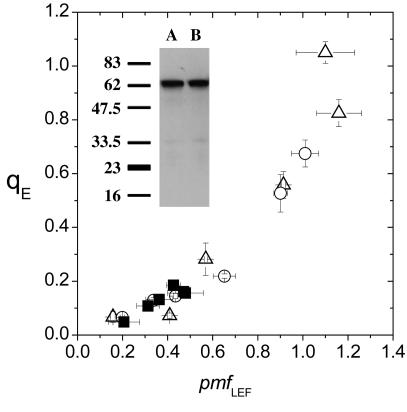
Fig. 3 shows that gH+ decreased in the Wt upon lowering CO2 but remained similar or was substantially increased in pgr5, as is especially evident at the higher light intensities. Within the noise level, plots of qE against pmfLEF for Wt under the two CO2 levels and pgr5 overlapped (Fig. 4), indicating that, as was reported in refs. 17 and 18, changes in gH+ could predominantly account for the differences in the qE response. We thus conclude that in pgr5 more facile proton efflux from the lumen through the ATP synthase, accompanied by decreases in LEF and probably CEF1, prevented the buildup of steady-state pmf and, thus, inhibited the qE response.
Fig. 3.
The light intensity dependence of the proton conductivity of the ATP synthase (gH+). Estimates of gH+ in Wt and pgr5 from 26 to 216 μmol photons m-2s-1 were obtained by taking the inverse of the time constant for ECS decay during a 300-ms dark perturbation of steady-state conditions. Conditions and symbols are as in Fig. 1. Error bars represent SE for n = 3–6.
Fig. 4.
The relationship between energy-dependent exciton quenching and the pmf generated solely by LEF. Estimates of energy-dependent quenching (qE) and the pmf generated solely by LEF (i.e., pmfLEF) were obtained as in Figs. 1 and 2, respectively. ATP synthase content in Wt (A) and pgr5 (B) was estimated by Western blot analyses by using polyclonal serum directed against the β-subunit of the ATP synthase (Inset). Conditions and symbols are as in Fig. 1. Error bars represent SE for n = 3–6.
In principle, gH+ could be modulated by changing the specific activity of ATP synthase or its content in the thylakoids. Hence, a ≈2-fold increase in the size of the ATP synthase pool could give rise to the observed ≈2-fold increase (i.e., at higher light intensities) in gH+ in pgr5 (Fig. 3). However, ATP synthase content in Wt and pgr5 was estimated by Western analyses and found to be essentially identical (Fig. 4 Inset). In addition, low light-induced activation of the ATP synthase by thioredoxin and leakage of the thylakoid membrane to protons were indistinguishable between Wt and pgr5 (data not shown), essentially as seen for other C3 plants (38). These data, taken together with the observed similarities in gH+ at low light, lead us to conclude that the differences in gH+ between Wt and pgr5 were caused by alterations in steady-state substrate or affecter concentrations (17).
The decrease in maximal LEF in pgr5 is probably due to loss of PSI electron acceptors and a buildup of reduced intermediates (31, 32). A similar decrease in LEF was seen when CO2 was lowered, but in contrast to the enhanced gH+ that occurred in the absence of PGR5, such a decrease in LEF was accompanied by substantial decreases in gH+ (Fig. 3), resulting in a net increase in both pmf and qE. These results demonstrate an important role for `tuning' the activity of the ATP synthase in the signal pathway that regulates light capture (39). Excessive turnover rates (i.e., large gH+ values) will result in facile proton efflux, preventing buildup of pmf and diminishing the qE response. On the other hand, inappropriate decreases in ATP synthase turnover rates can result in excessive buildup of pmf, over-acidifying the lumen and causing subsequent pH-induced degradation of the photosynthetic apparatus (4, 40).
From the above, we conclude that changes in CEF1 upon loss of PGR5 constitute a flux of protons approximately <13% of that from LEF, resulting in a commensurate decrease in ATP output. Because consumption of ATP and NADPH by the Calvin–Benson cycle is coupled, even a small ATP/NADPH imbalance could conceivably give rise to not only a buildup of ADP and [Pi], but also a substantial reduction of NADP+, restricting the availability of PSI electron acceptors and, thereby, lowering LEF, as was observed in pgr5 both here and in ref. 31.
Possible Causal Relation Between Pgr5- and gH+. We proposed in ref. 17 that lowering CO2 will lead to the buildup of phosphorylated metabolites in the stroma, depleting stromal [Pi] below its KM (≈1 mM) at the ATP synthase. This reaction will result in lowering of the effective gH+ and subsequent increases in steady-state pmf and qE. A small ATP/NADPH imbalance is expected to result from the absence of the PGR5-mediated CEF1. The deficit is obviously satisfied but only by substantially slower processes, e.g., alternative cyclic electron transfer processes or export of NADPH (12, 16). We thus expect in pgr5 a buildup of stromal [Pi] above its KM at the ATP synthase, maintaining high gH+ even when LEF is restricted. Thus, in this model, the loss of CEF1 in pgr5 indirectly attenuates both steady-state pmf and qE.
These results support a “division of labor” model for pmf modulation, whereby Type I mechanisms act mainly to adjust ATP/NADPH output, whereas Type II mechanisms alter the sensitivity of antenna regulatory pathways while maintaining pmf in an optimal range for energy transduction. Finally, it is clear from these results that a further understanding of the interaction of the photosynthetic apparatus within the plant will require an integrated, yet quantitative, “systems” approach on the intact plant under true steady-state conditions. Spectroscopic tools, such as we have applied here, will be essential for this progress.
Acknowledgments
This work was supported by U.S. Department of Energy Grant DE-FG03-98ER20299 and U.S. National Science Foundation Grant IBN-0084329.
Author contributions: T.J.A., J.A.C., A.K., and D.M.K. designed research; T.J.A., J.A.C., and D.M.K. performed research; T.J.A., A.K., and D.M.K. analyzed data; A.K. contributed new reagents/analytic tools; and T.J.A., J.A.C., and D.M.K. wrote the paper.
Abbreviations: CEF1, cyclic electron flow associated with PSI; ECS, electrochromic shift; LC, low CO2 (50 ppm CO2, 21% O2); LEF, linear electron flow; PS, photosystem; pmf, proton motive force; pmfLEF, pmf generated by LEF; qE, energy-dependent nonphotochemical quenching.
References
- 1.Ort, D. R. & Yocum, C. F. (1996) in Oxygenic Photosynthesis: The Light Reactions, ed. Yocum, C. F. (Kluwe, Dordrecht, The Netherlands), pp. 1-9.
- 2.Kramer, D. M., Cruz, J. A. & Kanazawa, A. (2003) Trends Plant Sci. 8, 27-32. [DOI] [PubMed] [Google Scholar]
- 3.Cruz, J. A., Sacksteder, C. A., Kanazawa, A. & Kramer, D. M. (2001) Biochemistry 40, 1226-1237. [DOI] [PubMed] [Google Scholar]
- 4.Kramer, D., Sacksteder, C. & Cruz, J. (1999) Photosynth. Res. 60, 151-163. [Google Scholar]
- 5.Capaldi, R. A. & Aggeler, R. (2002) Trends in Biochem. Sci. 27, 154-160. [DOI] [PubMed] [Google Scholar]
- 6.Fischer, S. & Gräber, P. (1999) FEBS Lett. 457, 327-332. [DOI] [PubMed] [Google Scholar]
- 7.Asada, K. (2000) Philos. Trans. R. Soc. London B 355, 1419-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller, P., Li, X. & Niyogi, K. K. (2001) Plant Physiol. 125, 1558-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niyogi, K. K. (1999) Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 333-359. [DOI] [PubMed] [Google Scholar]
- 10.Niyogi, K. K. (2000) Curr. Opin. Plant Biol. 3, 455-460. [DOI] [PubMed] [Google Scholar]
- 11.Li, X., Bjorkman, O., Shih, C., Grossman, A. R., Rosenquist, M., Jansson, S. & Niyogi, K. K. (2000) Nature 403, 391-395. [DOI] [PubMed] [Google Scholar]
- 12.Kramer, D. M., Avenson, T. J. & Edwards, G. E. (2004) Trends Plant Sci. 9, 349-357. [DOI] [PubMed] [Google Scholar]
- 13.Allen, J. F. (2002) Cell 110, 273-276. [DOI] [PubMed] [Google Scholar]
- 14.Allen, J. F. (2003) Trends Plant Sci. 8, 15-19. [DOI] [PubMed] [Google Scholar]
- 15.Nixon, P. J. & Mullineaux, C. W. (2001) in Advances in Photosynthesis and Respiration: Regulation of Photosynthesis, eds. Aro, E. & Anderson, B. (Kluwer, Dordrecht, The Netherlands), Vol. 11.
- 16.Cruz, J. A., Avenson, T. J., Kanazawa, A., Takizawa, K., Edwards, G. E. & Kramer, D. M. (2004) J. Exp. Bot. 56, 395-406. [DOI] [PubMed] [Google Scholar]
- 17.Kanazawa, A. & Kramer, D. M. (2002) Proc. Natl. Acad. Sci. USA 99, 12789-12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avenson, T. J., Cruz, J. A. & Kramer, D. M. (2004) Proc. Natl. Acad. Sci. USA 101, 5530-5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heber, U. & Walker, D. (1992) Plant Physiol. 100, 1621-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demmig-Adams, B. & Adams, W. W. I. (1992) Annu. Rev. Plant Physiol. Plant Mol. Biol. 43, 599-626. [Google Scholar]
- 21.Joët, T., Genty, B., Josse, E. M., Kuntz, M., Cournac, L. & Peltier, G. (2002) J. Biol. Chem. 277, 31623-31630. [DOI] [PubMed] [Google Scholar]
- 22.Aluru, M. R. & Rodermel, S. R. (2004) Physiol. Plant. 120, 4-11. [DOI] [PubMed] [Google Scholar]
- 23.Johnson, G. N. (2004) Trends Plant Sci. 9, 570-571. [DOI] [PubMed] [Google Scholar]
- 24.Kramer, D. M., Avenson, T. J. & Edwards, G. E. (2004) Trends Plant Sci. 9, 571-572. [DOI] [PubMed] [Google Scholar]
- 25.Sacksteder, C., Kanazawa, A., Jacoby, M. E. & Kramer, D. M. (2000) Proc. Natl. Acad. Sci. USA 97, 14283-14288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sacksteder, C. & Kramer, D. M. (2000) Photosynth. Res. 66, 145-158. [DOI] [PubMed] [Google Scholar]
- 27.Kramer, D. & Sacksteder, C. A. (1998) Photosynth. Res. 56, 103-112. [Google Scholar]
- 28.Avenson, T. J., Kanazawa, A., Cruz, J. A., Takizawa, K., Ettinger, W. E. & Kramer, D. M. (2005) Plant Cell Environ. 28, 97-109. [Google Scholar]
- 29.Witt, H. T. (1979) Biochim. Biophys. Acta 505, 355-427. [DOI] [PubMed] [Google Scholar]
- 30.Genty, B., Briantais, J.-M. & Baker, N. R. (1989) Biochim. Biophys. Acta 990, 87-92. [Google Scholar]
- 31.Munekage, Y., Hojo, M., Meurer, J., Endo, T., Tasaka, M. & Shikanai, T. (2002) Cell 110, 361-371. [DOI] [PubMed] [Google Scholar]
- 32.Munekage, Y., Hashimoto, M., Miyake, C., Tomizawa, K., Endo, T., Tasaka, M. & Shikanai, T. (2004) Nature 429, 579-582. [DOI] [PubMed] [Google Scholar]
- 33.Golding, A. J. & Johnson, G. N. (2003) Planta 218, 107-114. [DOI] [PubMed] [Google Scholar]
- 34.Sacksteder, C. A., Jacoby, M. E. & Kramer, D. M. (2001) Photosynth. Res. 70, 231-240. [DOI] [PubMed] [Google Scholar]
- 35.Donahue, R. A., Poulson, M. E. & Edwards, G. E. (1997) Photosynth. Res. 52, 263-269. [Google Scholar]
- 36.Jauh, G. Y., Phillips, T. E. & Rogers, J. C. (1999) Plant Cell 11, 1867-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kramer, D. M. & Crofts, A. R. (1996) in Photosynthesis and the Environment. Advances in Photosynthesis, ed. Baker, N. (Kluwer, Dordrecht, The Netherlands), pp. 25-66.
- 38.Kramer, D. & Crofts, A. (1989) Biochim. Biophys. Acta 976, 28-41. [Google Scholar]
- 39.Herbert, S. K. (2002) Proc. Natl. Acad. Sci. USA 99, 12518-12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Majeran, W., Olive, J., Drapier, D., Vallon, O. & Wollman, F. A. (2001) Plant Physiol. 126, 421-433. [DOI] [PMC free article] [PubMed] [Google Scholar]