Abstract
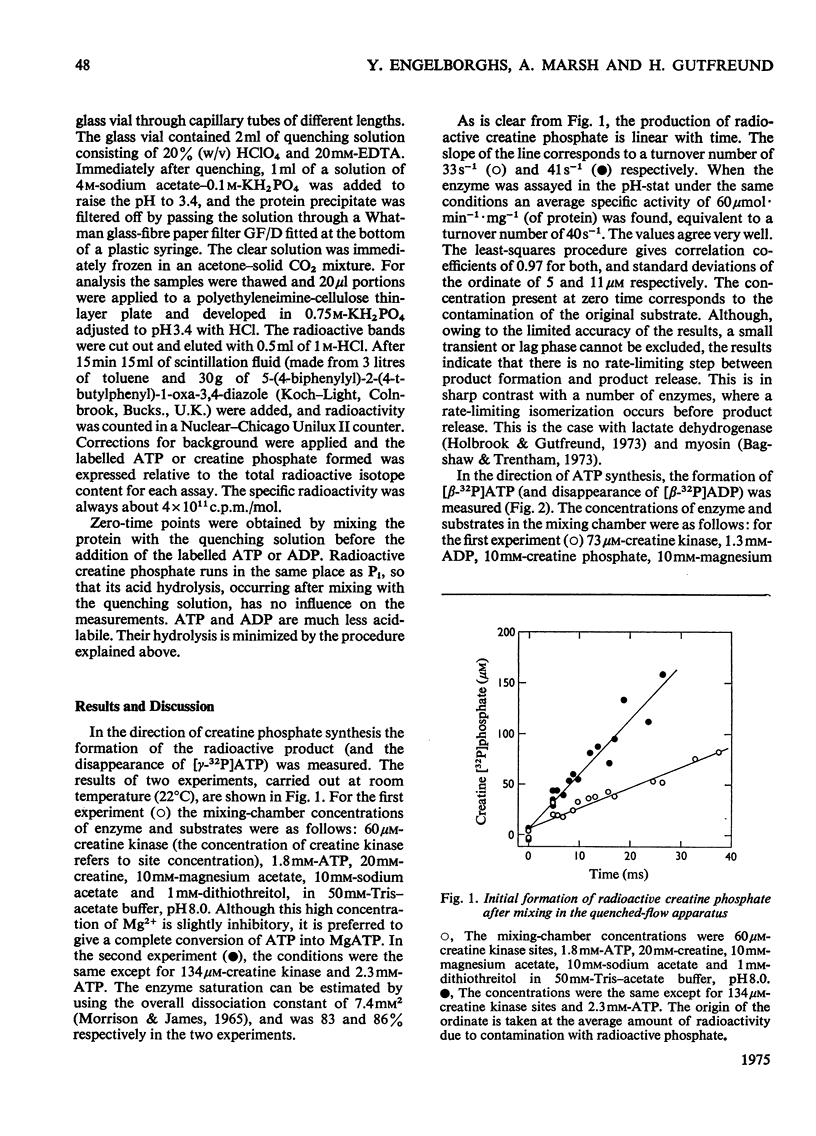
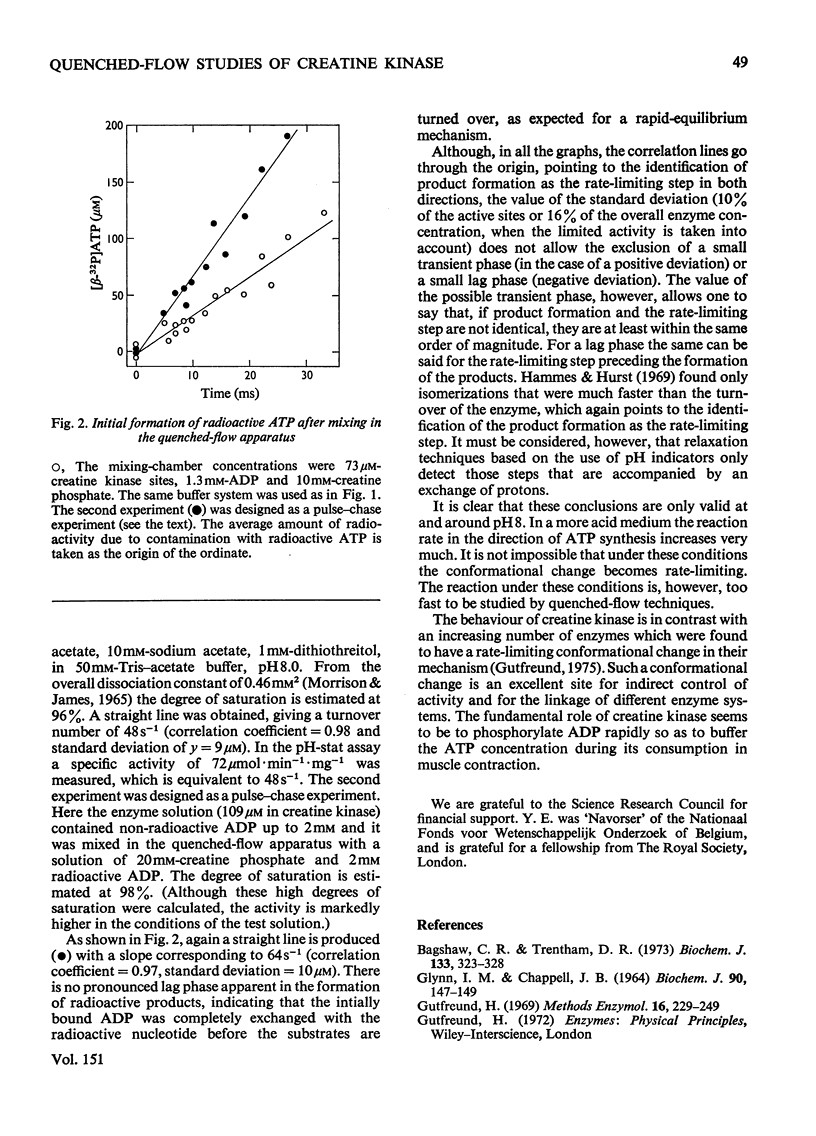
The reaction catalysed by creatine kinase was studied in both directions by quenched-flow techniques to follow the initial product formation in the millisecond range. In both directions the amount of product formed increases linearly with time, and the turnover number corresponds to the steady-state value. Extrapolation to zero time indicates the absence of either a large transient phase or a large lag phase in both directions. This indicates that the actual chemical reaction is rate-limiting, and that all possible isomerizations before or after the chemical step must be much more rapid.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagshaw C. R., Trentham D. R. The reversibility of adenosine triphosphate cleavage by myosin. Biochem J. 1973 Jun;133(2):323–328. doi: 10.1042/bj1330323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes G. G., Hurst J. K. Relaxation spectra of adenosine triphosphate-creatine phosphotransferase. Biochemistry. 1969 Mar;8(3):1083–1094. doi: 10.1021/bi00831a040. [DOI] [PubMed] [Google Scholar]
- Holbrook J. J., Gutfreund H. Approaches to the study of enzyme mechanisms lactate dehydrogenase. FEBS Lett. 1973 Apr 15;31(2):157–169. doi: 10.1016/0014-5793(73)80095-x. [DOI] [PubMed] [Google Scholar]
- KUBY S. A., NODA L., LARDY H. A. Adenosinetriphosphate-creatine transphosphorylase. I. Isolation of the crystalline enzyme from rabbit muscle. J Biol Chem. 1954 Jul;209(1):191–201. [PubMed] [Google Scholar]
- Morrison J. F., Cleland W. W. Isotope exchange studies of the mechanism of the reaction catalyzed by adenosine triphosphate: creatine phosphotransferase. J Biol Chem. 1966 Feb 10;241(3):673–683. [PubMed] [Google Scholar]
- Morrison J. F., James E. The mechanism of the reaction catalysed by adenosine triphosphate-creatine phosphotransferase. Biochem J. 1965 Oct;97(1):37–52. doi: 10.1042/bj0970037. [DOI] [PMC free article] [PubMed] [Google Scholar]


