Abstract
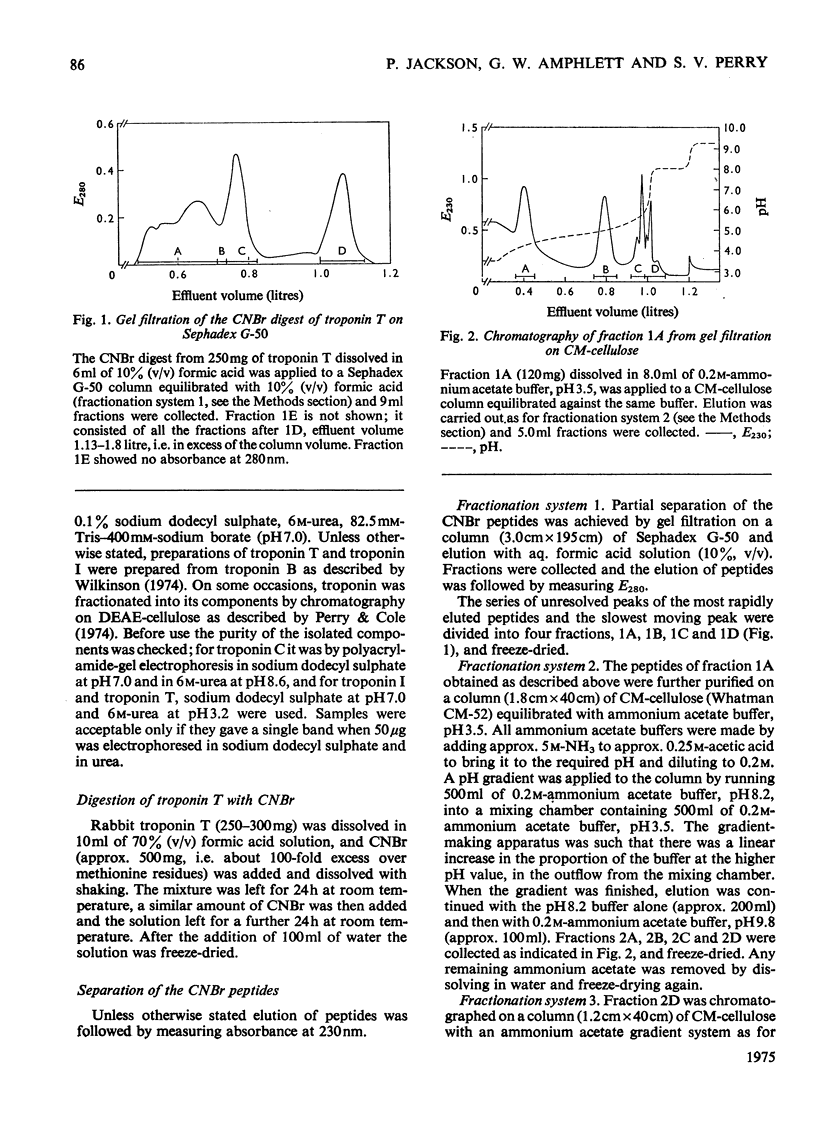
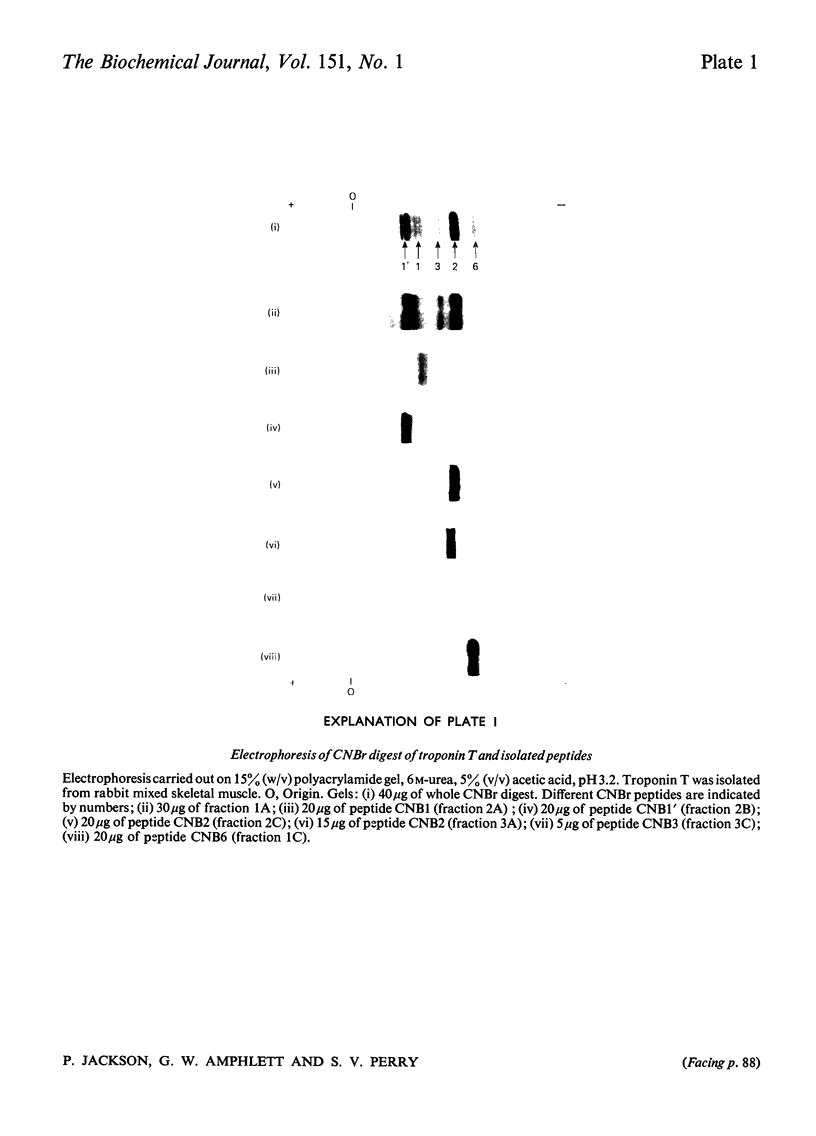
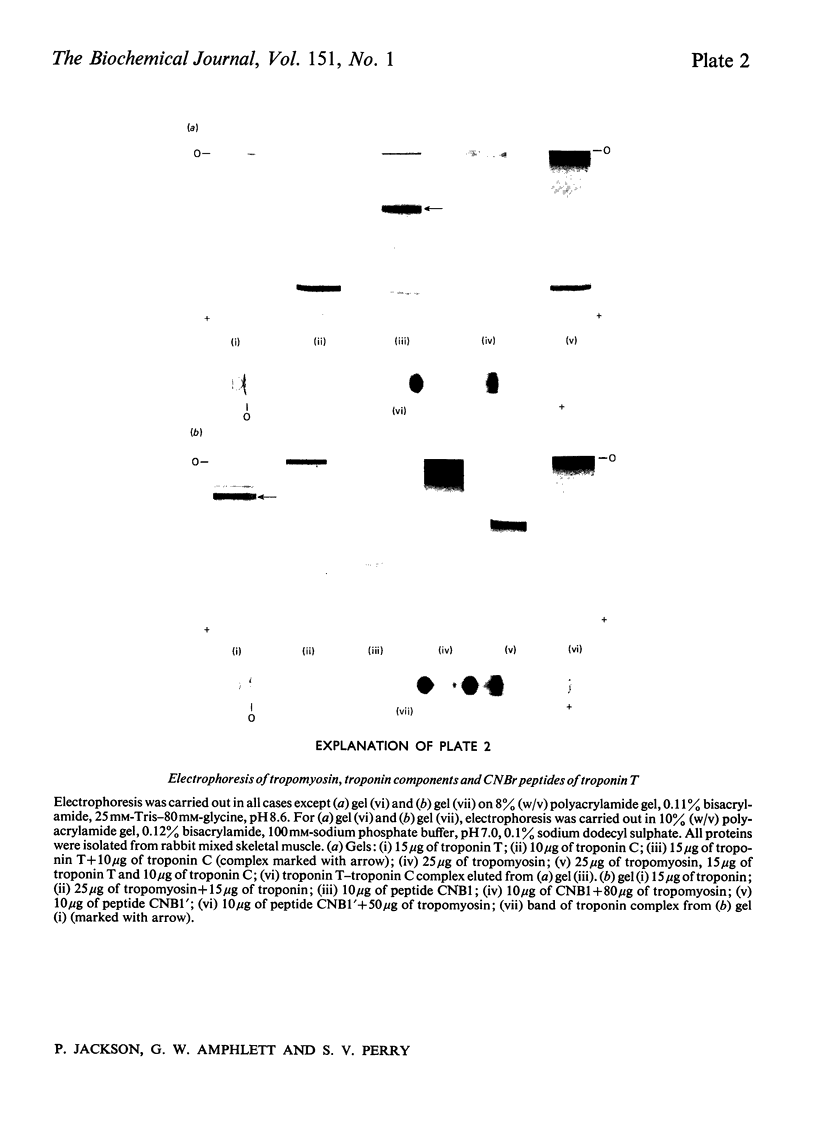
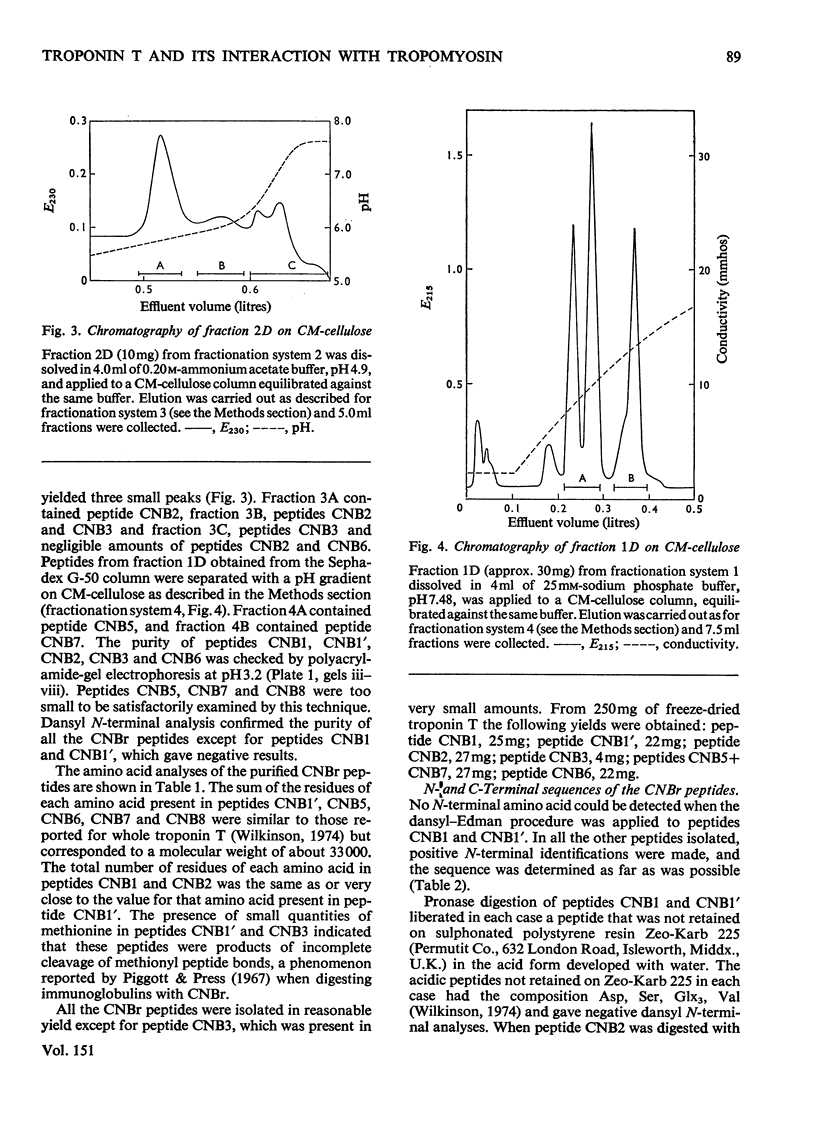
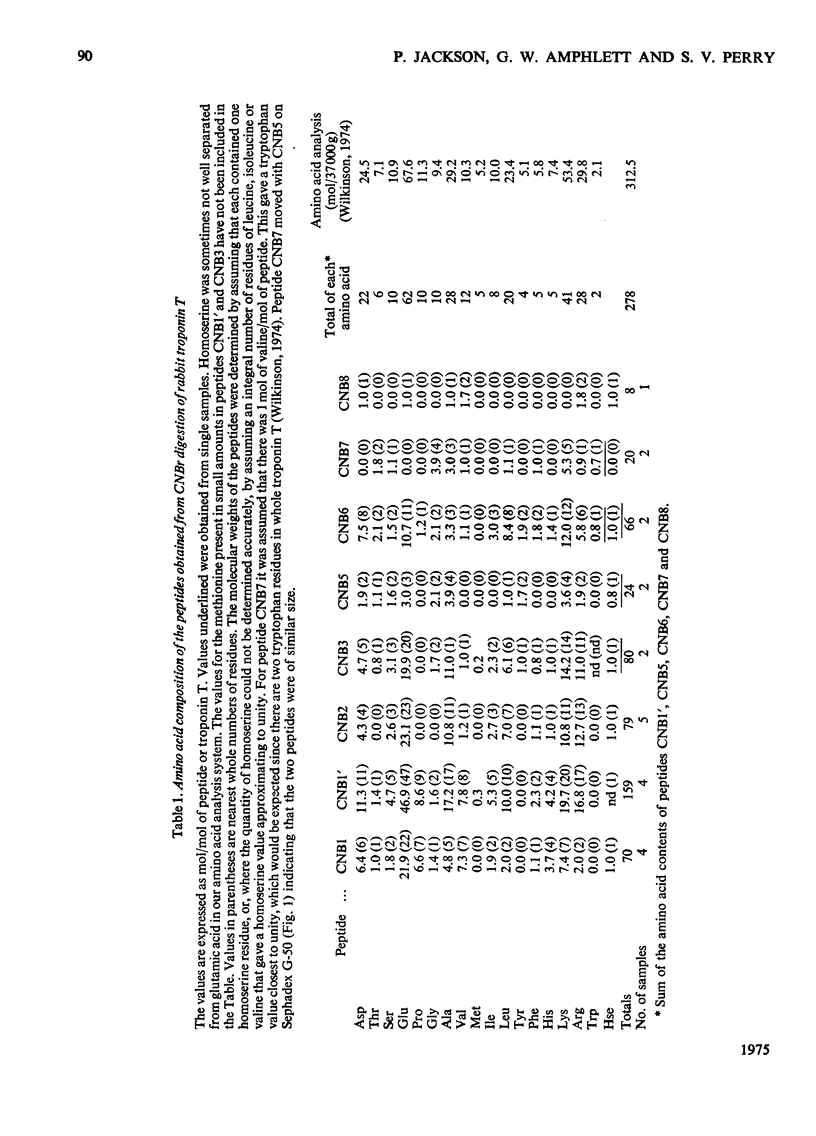
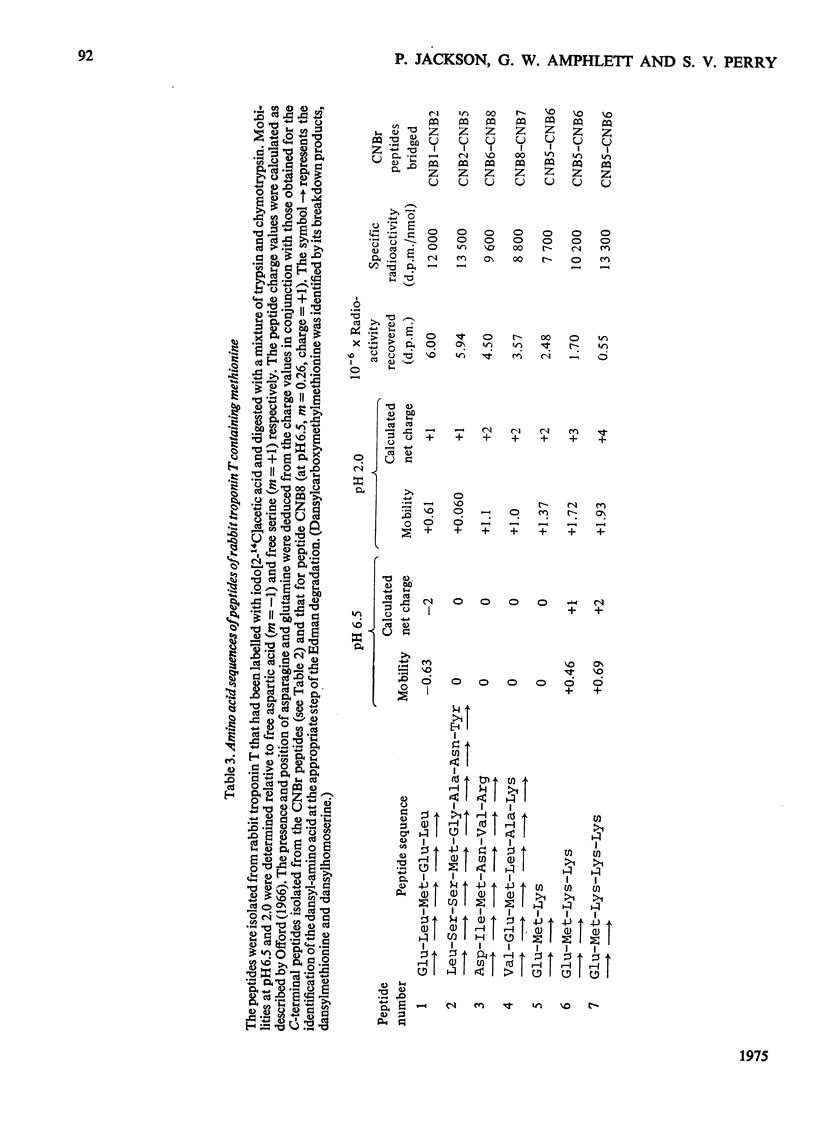
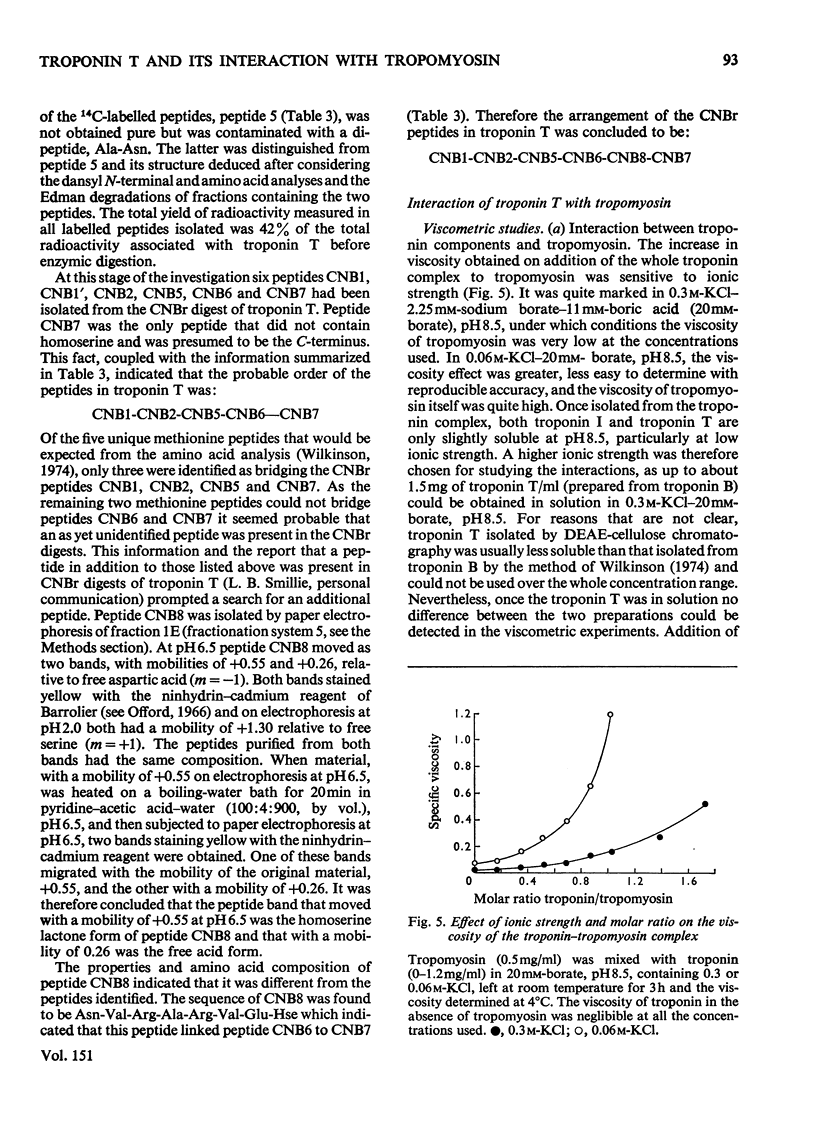
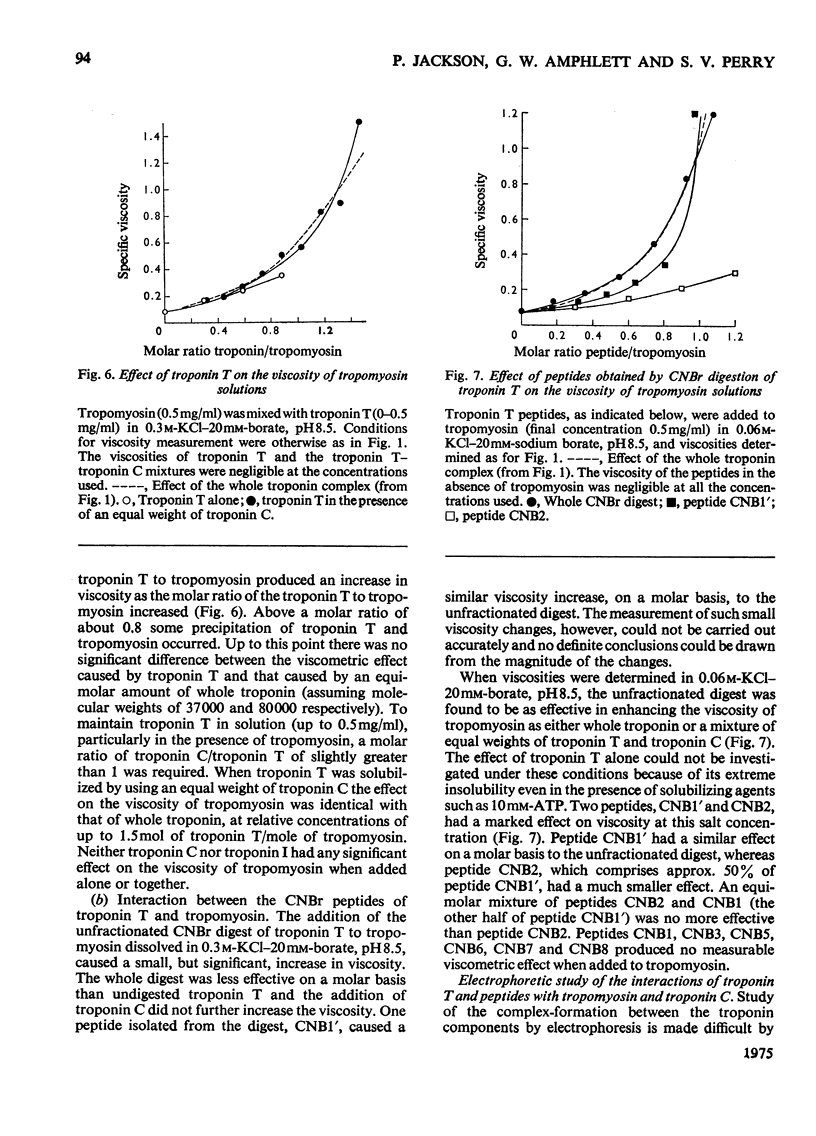
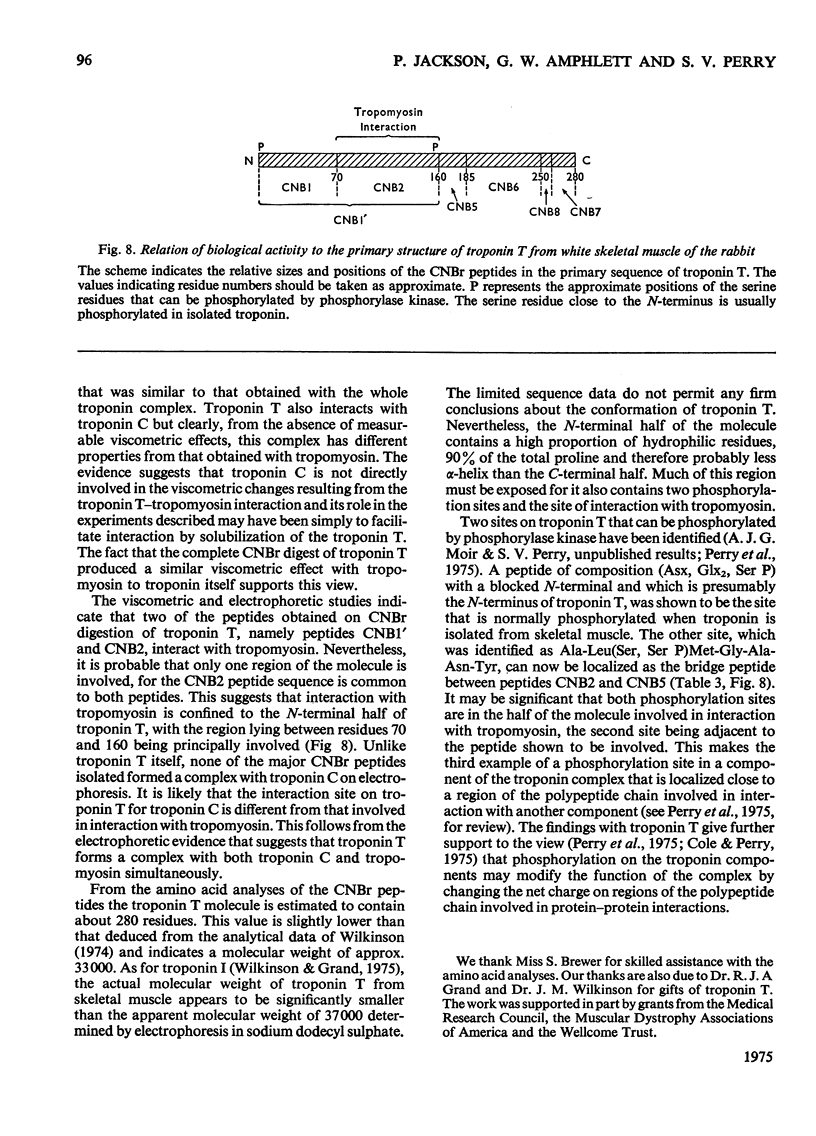
1. Eight peptides were separated from the CNBr digest of troponin T from rabbit white skeletal muscle and characterized. 2. By study of the amino acid sequence of the methionine-containing peptides isolated after chymotryptic and tryptic digestion and of the N- and C-terminals of the CNBr peptides, six of the latter were shown to be arranged in the sequence CNB1-CNB2-CNB5-CNB6-CNB8-CNB7. The other two peptides, CNB1' and CNB3, have been shown to be partial digestion products. 3. The CNBr peptides CNB1' and CNB2 contained a common sequence and were the only peptides in CNBr digests of troponin T that formed a complex with tropomyosin as judged by viscometric and electrophoretic studies. 4. It is concluded that tropomyosin interacts with the N-terminal half of the troponin T molecule approximately in the region lying between residues 70 and 160. 5. Electrophoretic evidence indicates that tropomyosin and troponin C interact with troponin T. 6. None of the major CNBr peptides of troponin T isolated formed a complex with troponin C on electrophoresis at pH 8.6.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAILEY K. End-group assay in some proteins of the keratin-myosin group. Biochem J. 1951 Jun;49(1):23–27. doi: 10.1042/bj0490023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEAVEN G. H., HOLIDAY E. R. Ultraviolet absorption spectra of proteins and amino acids. Adv Protein Chem. 1952;7:319–386. doi: 10.1016/s0065-3233(08)60022-4. [DOI] [PubMed] [Google Scholar]
- Bailey K. Tropomyosin: a new asymmetric protein component of the muscle fibril. Biochem J. 1948;43(2):271–279. doi: 10.1042/bj0430271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole H. A., Perry S. V. The phosphorylation of troponin I from cardiac muscle. Biochem J. 1975 Sep;149(3):525–533. doi: 10.1042/bj1490525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. H. Homology of myosin light chains, troponin-C and parvalbumins deduced from comparison of their amino acid sequences. Biochem Biophys Res Commun. 1974 May 7;58(1):301–308. doi: 10.1016/0006-291x(74)90927-9. [DOI] [PubMed] [Google Scholar]
- Collins J. H., Potter J. D., Horn M. J., Wilshire G., Jackman N. The amino acid sequence of rabbit skeletal muscle troponin C: gene replication and homology with calcium-binding proteins from carp and hake muscle. FEBS Lett. 1973 Nov 1;36(3):268–272. doi: 10.1016/0014-5793(73)80388-6. [DOI] [PubMed] [Google Scholar]
- Cummins P., Perry S. V. The subunits and biological activity of polymorphic forms of tropomyosin. Biochem J. 1973 Aug;133(4):765–777. doi: 10.1042/bj1330765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Kodama A. A new protein factor promoting aggregation of tropomyosin. J Biochem. 1965 Jul;58(1):107–108. doi: 10.1093/oxfordjournals.jbchem.a128157. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Wakabayashi T., Ebashi F. Troponin and its components. J Biochem. 1971 Feb;69(2):441–445. doi: 10.1093/oxfordjournals.jbchem.a129486. [DOI] [PubMed] [Google Scholar]
- Greaser M. L., Gergely J. Reconstitution of troponin activity from three protein components. J Biol Chem. 1971 Jul 10;246(13):4226–4233. [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Cohen C. Letter: Troponin subunit interactions. J Mol Biol. 1973 Dec 15;81(3):409–413. doi: 10.1016/0022-2836(73)90150-2. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Perrie W. T., Perry S. V. An electrophoretic study of the low-molecular-weight components of myosin. Biochem J. 1970 Aug;119(1):31–38. doi: 10.1042/bj1190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrie W. T., Smillie L. B., Perry S. B. A phosphorylated light-chain component of myosin from skeletal muscle. Biochem J. 1973 Sep;135(1):151–164. doi: 10.1042/bj1350151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. V., Cole H. A. Phosphorylation of troponin and the effects of interactions between the components of the complex. Biochem J. 1974 Sep;141(3):733–743. doi: 10.1042/bj1410733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot P. J., Press E. M. Cyanogen bromide cleavage and partial sequence of the heavy chain of a pathological immunoglobulin G. Biochem J. 1967 Aug;104(2):616–626. doi: 10.1042/bj1040616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porath J., Axen R., Ernback S. Chemical coupling of proteins to agarose. Nature. 1967 Sep 30;215(5109):1491–1492. doi: 10.1038/2151491a0. [DOI] [PubMed] [Google Scholar]
- SANGER F., THOMPSON E. O. Halogenation of tyrosine during acid hydrolysis. Biochim Biophys Acta. 1963 May 14;71:468–471. doi: 10.1016/0006-3002(63)91108-9. [DOI] [PubMed] [Google Scholar]
- Sender P. M. Muscle fibrils: Solubilization and gel electrophoresis. FEBS Lett. 1971 Sep 15;17(1):106–110. doi: 10.1016/0014-5793(71)80575-6. [DOI] [PubMed] [Google Scholar]
- Thomas D. B., Winzler R. J. Structure of glycoproteins of human erythrocytes. Alkali-stable oligosaccharides. Biochem J. 1971 Aug;124(1):55–59. doi: 10.1042/bj1240055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eerd J. P., Kawasaki Y. Effect of calcium(II) on the interaction between the subunits of troponin and tropomyosin. Biochemistry. 1973 Nov 20;12(24):4972–4980. doi: 10.1021/bi00748a024. [DOI] [PubMed] [Google Scholar]
- Wilkinson J. M. A method for purifying methionine-containing peptides by radioactive labelling. FEBS Lett. 1969 Aug;4(3):170–172. doi: 10.1016/0014-5793(69)80226-7. [DOI] [PubMed] [Google Scholar]
- Wilkinson J. M., Perry S. V., Cole H. A., Trayer I. P. The regulatory proteins of the myofibril. Separation and biological activity of the components of inhibitory-factor preparations. Biochem J. 1972 Mar;127(1):215–228. doi: 10.1042/bj1270215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson J. M. The preparation and properties of the components of troponin B. Biochim Biophys Acta. 1974 Aug 8;359(2):379–388. doi: 10.1016/0005-2795(74)90238-4. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Greaser M. L., Cassens R. G. Interactions of troponin subunits with different forms of tropomyosin. J Ultrastruct Res. 1974 Jul;48(1):33–58. doi: 10.1016/s0022-5320(74)80043-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Maruyama K. Interaction of troponin I and tropomyosin. J Biochem. 1973 May;73(5):1111–1114. doi: 10.1093/oxfordjournals.jbchem.a130166. [DOI] [PubMed] [Google Scholar]




