Abstract
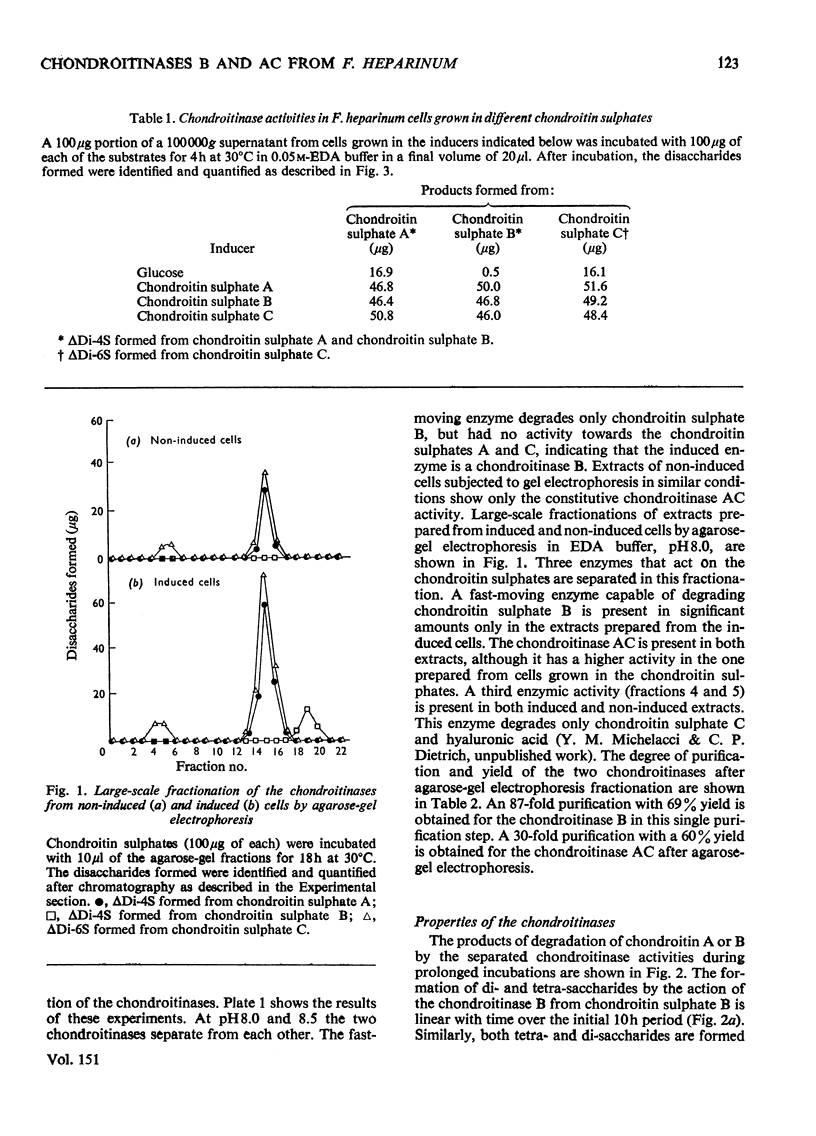
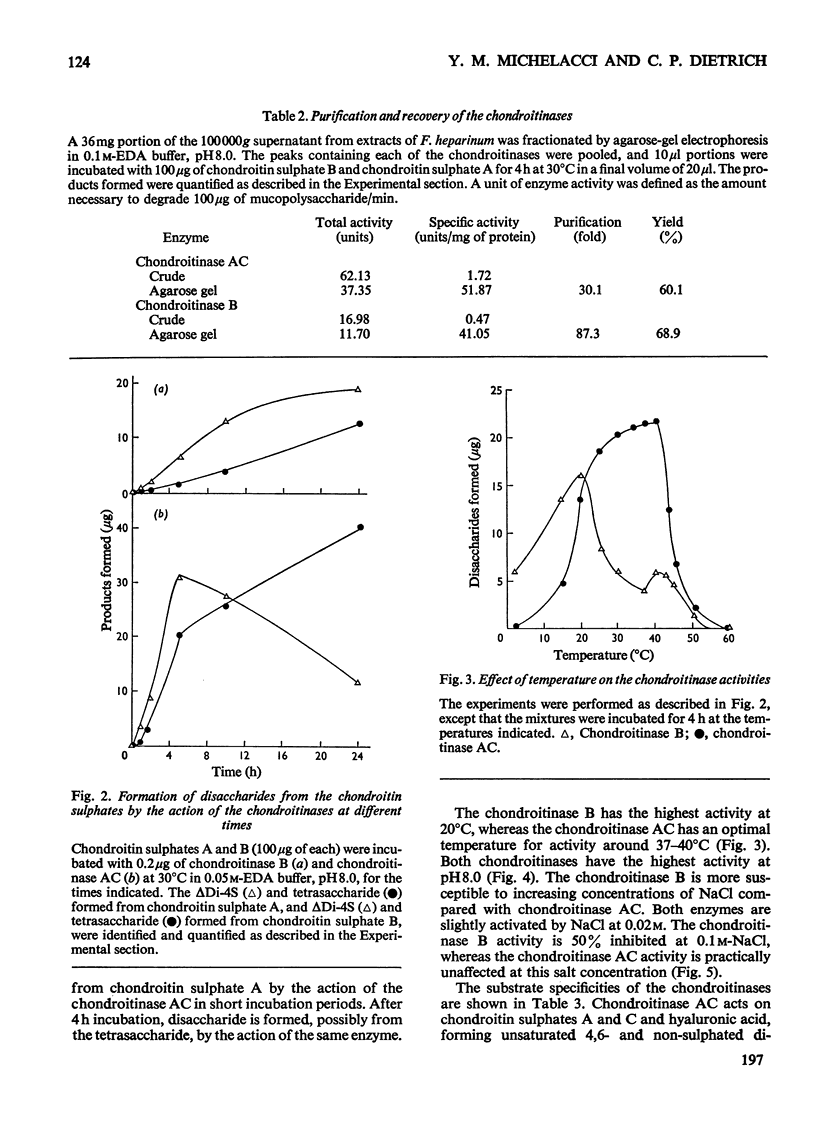
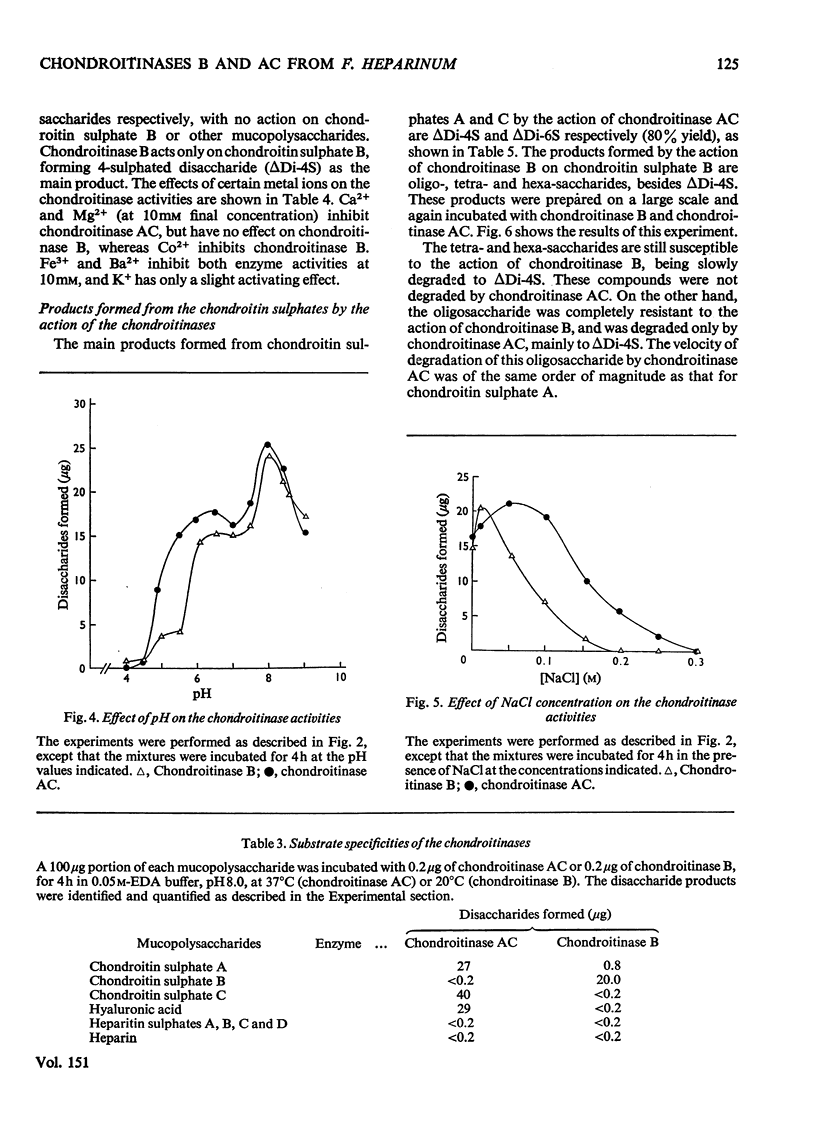
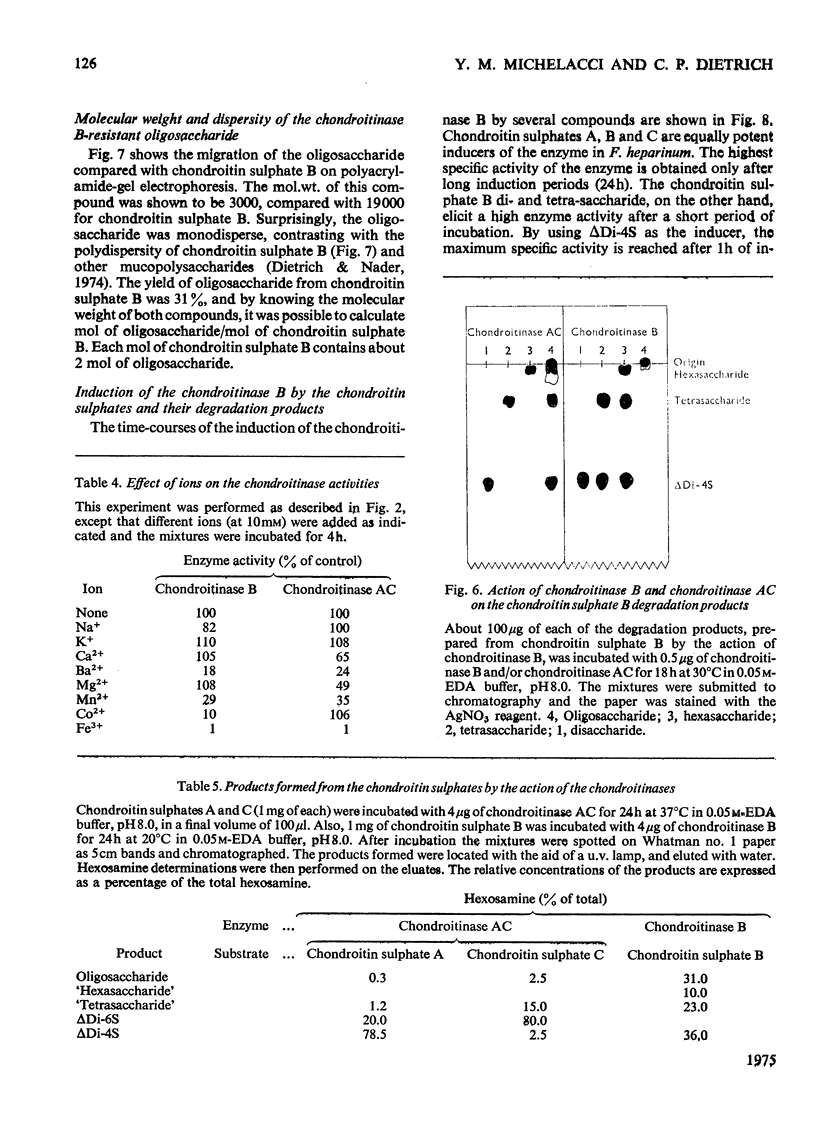
A chondroitinase that degrades only chondroitin sulphate B was isolated from Flavobacterium heparinum, and separated from a constitutive chondroitinase AC also present in extracts of F. heparinum. The enzyme acts only on chondroitin sulphate B, producing oligo- and tetra-saccharides, plus an unsaturated 4-sulphated disaccharide (deltaDi-4S). The oligosaccharide fraction (mol. wt. 3000) is susceptible to chondroitinase AC, producing mainly deltaDi-4S. The chondroitinase B is distinguished from chondroitinase AC by several properties, such as the effect of certain metal ions, temperature for optimal activity, and susceptibility to increasing salt concentrations. The enzyme is induced in F. heparinum by all the chondroitin sulphates, as well as by the disaccharides prepared from the chondroitins. The mechanism of induction of the enzyme and the structure of chondroitin sulphate B are discussed in relation to these results.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dietrich C. P., Dietrich S. M. Simple micro method for identification of heparin and other acidic mucopolysaccharides from mammalian tissues. Anal Biochem. 1972 Mar;46(1):209–215. doi: 10.1016/0003-2697(72)90413-7. [DOI] [PubMed] [Google Scholar]
- Dietrich C. P. Enzymic degradation of heparin. A glucosaminidase and a glycuronidase from Flavobacterium heparinum. Biochemistry. 1969 May;8(5):2089–2094. doi: 10.1021/bi00833a046. [DOI] [PubMed] [Google Scholar]
- Dietrich C. P. Enzymic degradation of heparin. A sulphamidase and a sulphoesterase from Flavobacterium heparinum. Biochem J. 1969 Jan;111(1):91–95. doi: 10.1042/bj1110091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich C. P., Nader H. B. Fractionation and properties of four heparitin sulfates from beef lung tissue. Isolation and partial characterization of a hemogeneous species of heparitin sulfate. Biochim Biophys Acta. 1974 Mar 20;343(1):34–44. doi: 10.1016/0304-4165(74)90237-2. [DOI] [PubMed] [Google Scholar]
- Dietrich C. P. Novel heparin degradation products. Isolation and characterization of novel disaccharides and oligosaccharides produced from heparin by bacterial degradation. Biochem J. 1968 Jul;108(4):647–654. doi: 10.1042/bj1080647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich C. P., Silva M. E., Michelacci Y. M. Sequential degradation of heparin in Flavobacterium heparinum. Purification and properties of five enzymes involved in heparin degradation. J Biol Chem. 1973 Sep 25;248(18):6408–6415. [PubMed] [Google Scholar]
- Dietrich C. P. Studies on the induction of heparin-degrading enzymes in Flavobacterium heparinum. Biochemistry. 1969 Aug;8(8):3342–3347. doi: 10.1021/bi00836a031. [DOI] [PubMed] [Google Scholar]
- HOFFMAN P., LINKER A., LIPPMAN V., MEYER K. The structure of chondroitin sulfate B from studies with Flavobacterium enzymes. J Biol Chem. 1960 Nov;235:3066–3069. [PubMed] [Google Scholar]
- Hilborn J. C., Anastassiadis P. A. Estimation of the molecular weights of acidic mucopolysaccharides by polyacrylamide gel electrophoresis. Anal Biochem. 1971 Jan;39(1):88–92. doi: 10.1016/0003-2697(71)90465-9. [DOI] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Michelacci Y. M., Dietrich C. P. Isolation and partial characterization of an induced chondroitinase B from Flavobacterium heparinum. Biochem Biophys Res Commun. 1974 Feb 27;56(4):973–980. doi: 10.1016/s0006-291x(74)80284-6. [DOI] [PubMed] [Google Scholar]
- Michelacci Y. M., Dietrich C. P. Studies on the induction of a chondroitinase in Flavobacterium heparinum. Biochimie. 1973;55(8):893–898. doi: 10.1016/s0300-9084(73)80166-x. [DOI] [PubMed] [Google Scholar]
- RONDLE C. J., MORGAN W. T. The determination of glucosamine and galactosamine. Biochem J. 1955 Dec;61(4):586–589. doi: 10.1042/bj0610586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese E. T., Lola J. E., Parrish F. W. Modified substrates and modified products as inducers of carbohydrases. J Bacteriol. 1969 Dec;100(3):1151–1154. doi: 10.1128/jb.100.3.1151-1154.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILLER S., SLOVER G. A., DORFMAN A. A method for the separation of acid mucopolysaccharides: its application to the isolation of heparin from the skin of rats. J Biol Chem. 1961 Apr;236:983–987. [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Silva M. E., Dietrich C. P. Isolation and partial characterization of three induced enzymes from Flavobacterium heparinum involved in the degradation of heparin and heparitin sulfates. Biochem Biophys Res Commun. 1974 Feb 27;56(4):965–972. doi: 10.1016/s0006-291x(74)80283-4. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Saito H., Yamagata T., Anno K., Seno N., Kawai Y., Furuhashi T. Formation of three types of disulfated disaccharides from chondroitin sulfates by chondroitinase digestion. J Biol Chem. 1968 Apr 10;243(7):1543–1550. [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]



