Abstract
Invasive weed species exhibit both advantages, such as the potential for allelochemicals in bioherbicide development, and risks, including their threat to crop production. Therefore, this study aims to identify an allelochemical from Solidago altissima, an invasive weed species. The dose-dependent effects of S. altissima shoot and root extracts (SSE, SRE) on the signaling in the forage crop Trifolium repens and germination in various weed species (Echinochloa oryzicola, Cyperus microiria, Alopecurus aequalis, Portulaca oleracea, and Amaranthus retroflexus) were evaluated. The results showed that the T. repens seedlings treated with root extracts exhibited a significant decrease in plant height, dry weight, and chlorophyll content, along with an increase in H2O2 levels. Additionally, antioxidant activities, such as superoxide dismutase, catalase, and peroxidase enzyme activities, were significantly elevated in T. repens treated with SRE. Moreover, SRE treatment significantly inhibited the seed germination of all tested weed species in a concentration-dependent manner. Gas chromatography-mass spectrometry analysis of S. altissima root extract identified a high concentration of methyl kolavenate, a clerodane diterpene predicted to act as a phytotoxic agent. These findings highlight the potential of S. altissima for the development of crop-protective agents while emphasizing its potential risks in agriculture.
Keywords: phytotoxicity, weeds, ROS, antioxidants, phytohormone, methyl kolavenate
1. Introduction
The spread of invasive weed species (WS) is considered a significant threat to sustainable agriculture owing to its potential to cause ecosystem disruption, biodiversity reduction, and lead to economic losses [1]. Among these invasive species, Solidago altissima L., commonly known as goldenrod (Common name; tall goldenrod, family; Asteraceae), of the Asteraceae family, is classified as a perennial weed. Originally native to North America, this species is widely recognized as a significant threat in Europe and Asia [2]. S. altissima can produce 10,000–40,000 seeds, which are easily dispersed by the wind, posing a significant risk of propagation to surrounding areas [3,4]. Additionally, the weed can propagate vegetatively through an underground rhizome that can grow to at least 20 cm in length [5,6]. Hence, exposure to exotic weeds may reduce the productivity and quality of forage crops [7,8].
Trifolium repens L. (Common name; white clover, family; Fabaceae) has a long history of therapeutic applications, owing to its diverse nutrient and mineral composition, alongside its notable nutritional, ecological, and medicinal properties. Furthermore, this forage, demonstrating heightened palatability among herbivorous livestock, is significant for its protein content [9]. The weed is cultivated for grazing, pasture, and hay, contributing directly to the wool, dairy, and meat industries, along with high-value agricultural production [10]. Additionally, it covers the soil surface and develops roots at each node, preventing soil and nutrient loss [11]. T. repens exhibits a strong innate capacity for heavy metal accumulation by enhancing soil enzyme activities and the microbiome, making it a valuable candidate for phytoremediation processes [12]. The invasion of non-native weeds, such as S. altissima, poses a potential threat to the effective cultivation of forage crops, including T. repens. Therefore, identifying the allelopathic effects and allelochemicals in S. altissima may offer novel insights into the interactions between weeds and forage crops.
Allelopathy is defined as the ability of some plant species to release chemicals into their surroundings, which can have beneficial or harmful effects on other plants [13,14]. The allelopathic potential of S. altissima has been shown to be mediated by polyacetylenes [15], diterpenes [16,17], flavonoid glycosides [18], and phenolic compounds [19]. Previous studies have demonstrated that biochemical analyses of S. altissima revealed several compounds, including polyacetylenes, triterpenes, flavonoid glycosides, phenolic compounds, monoterpenes, and sesquiterpenes. Nishidono and Tanaka [20] identified the predominant compounds in S. altissima as the polyacetylene (2Z, 8Z)-10-angeloyloxy matricaria ester and the diterpene 13E-kolavenic acid. Additionally, the study demonstrated that these compounds, derived from the underground parts of S. altissima, exhibit allelopathic effects, with cis-dehydromatricaria ester being the primary active component.
Understanding the signaling mechanisms of crops, involving the interaction of endogenous phytohormones such as abscisic acid (ABA), salicylic acid (SA), and jasmonic acid (JA) with antioxidants, is crucial for enhancing plant immunity against external stressors [21,22]. The allelopathic effects of a crop can significantly influence the cross-talk within the biochemical pathways of the host [23]. Endogenous phytohormones and antioxidants play a crucial role in scavenging toxic radicals, such as reactive oxygen species (ROS), and in maintaining osmotic balance in crops [24]. The invasion of S. altissima in grasslands poses a significant threat to forage production and other crops, along with wetland ecosystems. Additionally, the management of other seasonal weeds in agriculture fields hinders efficient nutrient management and crop production.
Therefore, this study aims to investigate whether allelochemicals extracted from S. altissima can act as bioherbicides to prevent weed growth and identify the compounds responsible for inducing allelopathy and/or phytotoxicity in forage crops. The S. altissima extract was treated to T. repens to observe its allelopathic effects, along with several WS considered potential threats to agricultural production, including Echinochloa oryzicola L., Cyperus microiria L. [25], Alopecurus aequalis L. [26], Portulaca oleracea L. [27], and Amaranthus retroflexus L. [28], to assess its herbicidal potential. The potential cause of this phytotoxic effect was elucidated through the identification of various allelochemicals in the S. altissima extract. The findings of this study could offer insights into both the benefits and risks of S. altissima in relation to crop production and bioherbicide development.
2. Results
2.1. Allelopathic Effects of Solidago altissima on Trifolium repens
2.1.1. Effect of S. altissima Extracts on Seedling Fresh Weight
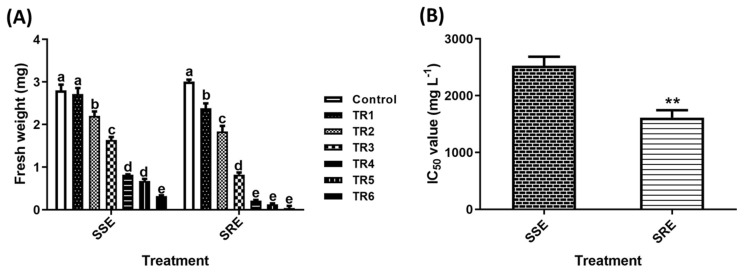
In this study, the S. altissima seedling extract (SSE) and seed root extract (SRE) were treated with different concentrations, as described in the experimental design. The results showed a significant reduction in the fresh weight of T. repens seedlings, with decreases of 20%, 38%, 72%, 92%, 95%, and 98% for SRE and 3%, 26%, 45%, 72%, 77%, and 89%) for SSE at concentrations of 625, 1250, 2500, 5000, 10,000, 20,000 mg/L, respectively, compared to the control (Figure 1A). Based on these results and the IC50 value, the SRE extract was selected for further application in T. repens and other WS (Figure 1B).
Figure 1.
(A) Screening of the effects of SSE and SRE extracts on T. repens seedlings. (B) IC50 values of SSE and SRE extracts. The bars represent the mean ± standard deviation (SD) (n = 3). Significant differences among treatments are indicated by different letters (a–e) based on Duncan’s multiple range test (DMRT, p < 0.05). The IC50 value for each treatment was calculated based on the fresh weight of T. repens seedlings. Statistical significance was determined using a t-test (** p < 0.01).
2.1.2. Effect of Solidago altissima Root Extract Foliar Treatment on the Growth and Chlorophyll Content of Trifolium repens
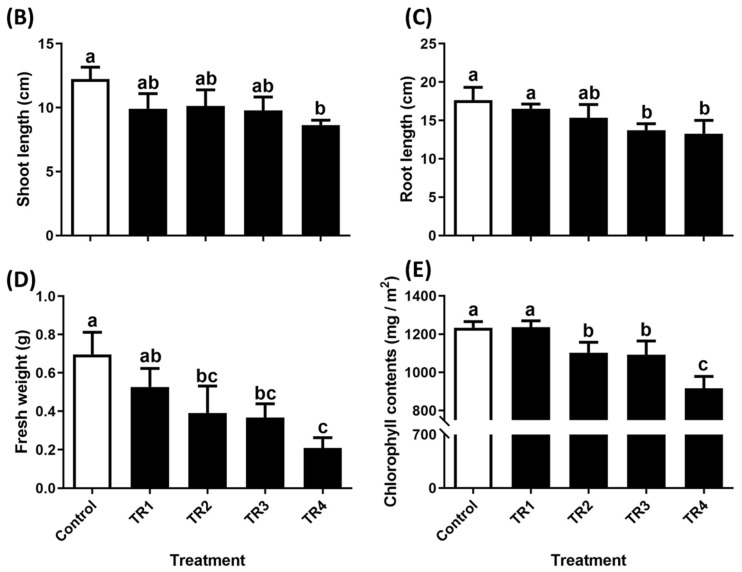
Subsequently, the effects of SRE foliar treatment on T. repens seedlings were evaluated after germination. Figure 2A illustrates the visual observations revealing a significant decline in T. repens appearance, with stunted plant growth as the SRE treatment increased. The SRE treatments TR2, TR3, TR4, and TR5 reduced the fresh weight by 24%, 43%, 47%, and 70%, respectively; the shoot length by 19%, 17%, 20%, and 30%; the root length by 12%, 24%, 31%, and 35%; and the chlorophyll content by 10%, 10%, 11%, and 25%, respectively, compared to that of the control (Figure 2B–E).
Figure 2.
Dose-dependent effects of SRE on morphological variations in T. repens (A) Visual differences. (B) Shoot length. (C) Root length. (D) Fresh weight. (E) Chlorophyll content. Error bars represent the mean ± standard deviation (SD) (n = 5). Letters a–c denote significant differences (p < 0.05) using Duncan’s multiple range test (DMRT).
2.2. Effects of Solidago altissima Extract Foliar Treatment on Reactive Oxygen Species Content and Antioxidant Enzyme Activity
2.2.1. Reactive Oxygen Species Content
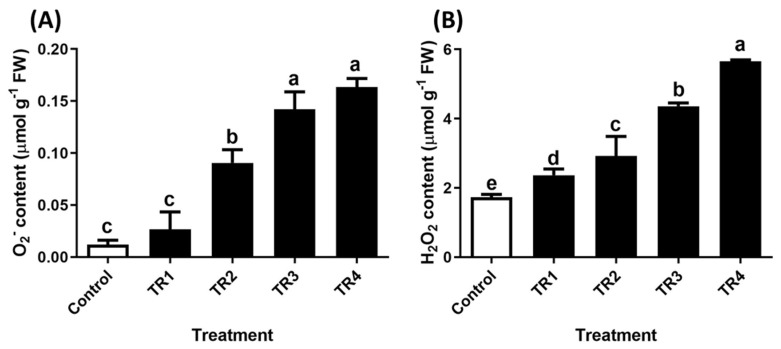
The hydrogen peroxide (H2O2) content in T. repens seedlings was visualized using the 3,3′-Diaminobenzidine (DAB) staining method. Figure 3 and Figure 4 display the quantification results that further validate these findings. The superoxide anion (O2−) content increased by 1.2-fold, 6.6-fold, 11-fold, and 12.7-fold, while H2O2 content increased by 36%, 69%, 152%, and 227% following subsequent treatment with TR2, TR3, TR4, and TR5, respectively, compared to that of the control.
Figure 3.
Effect of SRE treatment on H2O2 accumulation in T. repens seedlings, visualized using DAB staining. H2O2 concentrations in T.repens seedlings increased in the following order: TR5 > TR4 > TR3 > TR2 > TR1.
Figure 4.
Reactive oxygen species (ROS) content in T. repens seedlings after SRE treatment. (A) O2− content. (B) H2O2 content. Bars and error bars represent the mean ± standard deviation (SD), (n = 3). Different letters denote significant differences (p < 0.05), as determined using Duncan’s Multiple Range Test (DMRT).
2.2.2. Effects of Solidago altissima Root Extract Foliar Application on Antioxidant Enzyme Activity
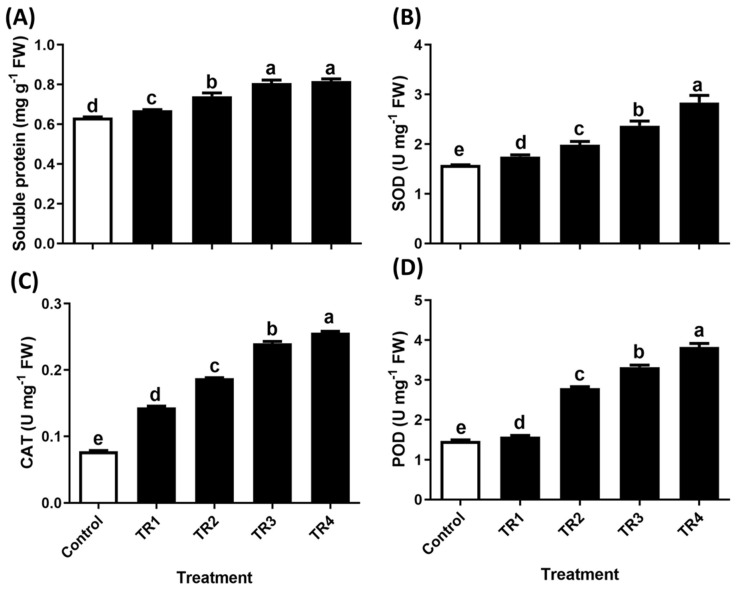
The antioxidant analysis of T. repens seedlings revealed that soluble protein levels increased by 6%, 17%, 27%, and 29%, superoxide dismutase (SOD) activity increased by 10%, 25%, 49%, and 79%, catalase (CAT) activity increased by 85%, 141%, 209%, and 229%, and peroxidase (POD) activity increased by 7%, 89%, 126%, and 160% following subsequent treatments with TR2, TR3, TR4, and TR5, respectively, compared to that of the control (Figure 5A–D).
Figure 5.
Effect of S. altissima extract treatment on soluble protein content and antioxidant activity in T. repens. (A) Soluble protein, (B) superoxide dismutase (SOD), (C) catalase (CAT), and (D) peroxidase (POD) activity. Bars and error bars represent the mean ± standard deviation (SD), (n = 3). Letters above the bars denote significant differences (p < 0.05), determined using Duncan’s Multiple Range Test (DMRT).
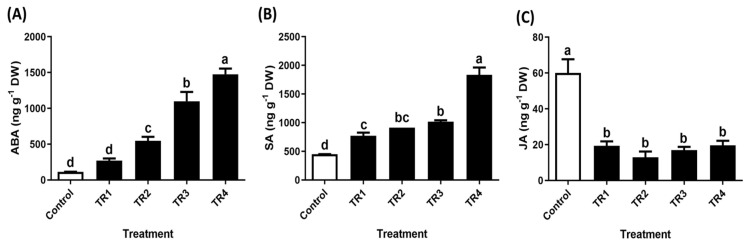
2.3. Quantification of Endogenous Phytohormones in Trifolium repens Leaves
ABA and SA levels significantly increased with higher SRE treatment concentrations, while JA synthesis was significantly inhibited. Briefly, ABA content increased by 1.3-fold, 3.7-fold, 8.6-fold, and 12-fold, while SA content increased by 0.7-fold, 10-fold, 12.6-fold, and 3.06-fold. However, JA levels decreased by 5.7%, 17%, 27%, and 29% with increasing concentrations of TR2, TR3, TR4, and TR5, respectively, compared to that of the control (Figure 6).
Figure 6.
Effect of S. altissima extract treatment on phytohormone content in T. repens. (A) ABA, (B) SA, and (C) JA. Bars and error bars represent the mean ± standard deviation (SD), (n = 3). Letters denote significant differences (p < 0.05) determined using Duncan’s multiple range test (DMRT).
2.4. Identification of Allelochemicals in Solidago altissima Roots
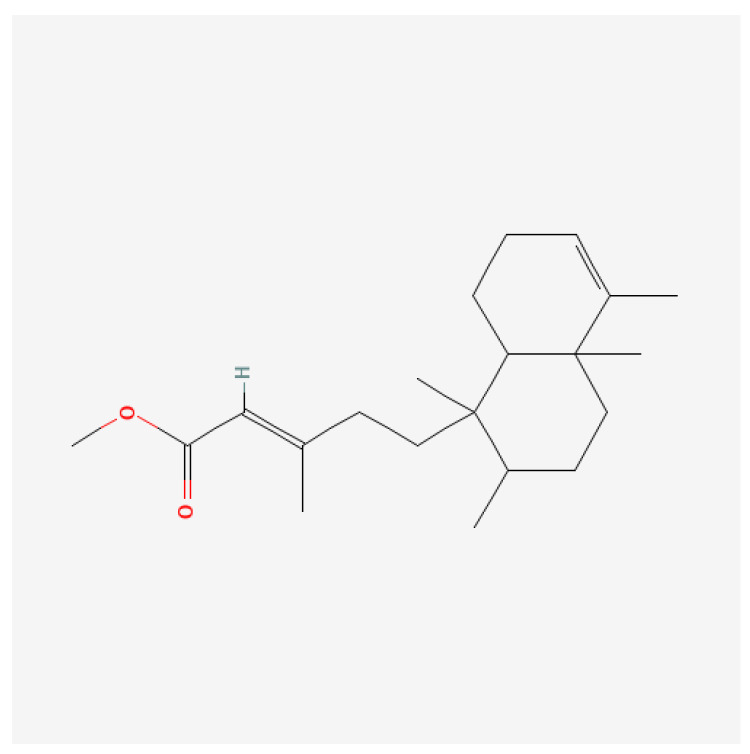
Gas chromatography-mass spectrometry (GC-MS) analysis of fraction AE from the SRE extract revealed several allelochemicals. Among these, methyl kolavenate (kolavenic acid) emerged as the most abundant (35.68%) compound (Figure 7). Other compounds identified in significant quantities included n-hexadecanoic acid (4.21%), chondrillasterol (3.49%), 1,6-dibromohexane (2.76%), kolavenol (2.30%), and (E)-4,4-dimethyl-2-pentene (2.19%) (Table 1).
Figure 7.
Chemical structure of the predominant compound, methyl kolavenate, identified in the extract.
Table 1.
Major compounds identified in fraction AE extracted from S. altissima.
| No. | Compound Name | Peak Area (%) |
|---|---|---|
| 1 | Methyl kolavenate | 35.68 |
| 2 | n-Hexadecanoic acid | 4.21 |
| 3 | Chondrillasterol | 3.49 |
| 4 | Carvyl angelate | 2.76 |
| 5 | Kolavenol | 2.30 |
| 6 | (E)-4,4-Dimethyl-2-pentene | 2.19 |
| 7 | 10(E),12(Z)-Conjugated linoleic acid | 1.57 |
| 8 | (E)-Longipinane | 1.44 |
| 9 | (Z,Z)-9,12-Octadecadienoic acid | 1.21 |
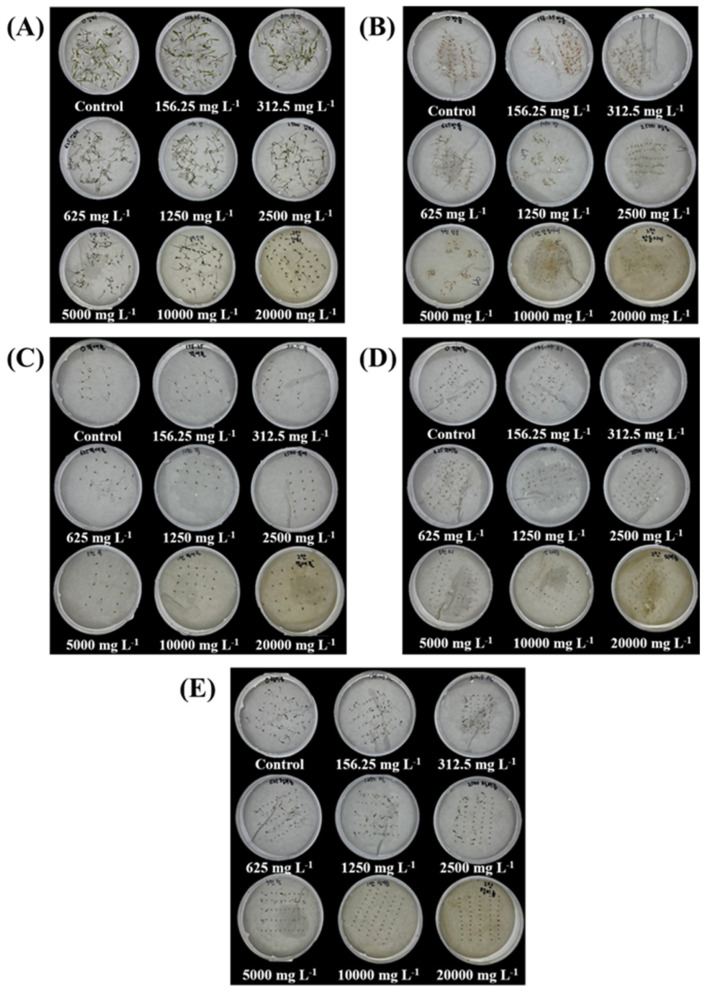
2.5. Effect of SRE Treatment on Germination Inhibition of Weed Species
Figure 8 illustrates the effect of different SRE concentrations on the germination of E. oryzicola, C. microiria, A. aequalis, P. oleracea, and A. retroflexus, with germination completely inhibited as the SRE concentration increased.
Figure 8.
Effect of SRE treatment on the germination of different weed species: (A) E. oryzicola, (B) C. microiria, (C) A. aequalis, (D) P. oleracea, and (E) A. retroflexus.
3. Discussion
The development of bioherbicides using extracts from invasive WS offers a promising strategy for controlling these species in agricultural settings [29]. In this study, SRE was identified as a potential bioherbicide owing to its inhibitory effects on the germination and development of E. oryzicola, C. microiria, A. aequalis, P. oleracea, and A. retroflexus. The phytotoxic effects of SRE treatment on T. repens seedlings were evaluated based on morphological levels, antioxidant activity, endogenous phytohormone levels, ROS accumulation, and allelochemical identification.
This study demonstrated that SRE treatment induced severe toxicity in T. repens seedlings, resulting in stunted growth, high ROS levels, and mortality at higher concentrations, indicating its potential threat to forage crop production. Furthermore, the growth of T. repens seedlings was significantly reduced following SRE treatment, primarily due to oxidative stress induced by ROS. The most commonly produced ROS include O2−, H2O2, hydroxyl radical (·OH), and singlet oxygen (1O2) [30]. To assess H2O2 accumulation in plants treated with SRE foliar DAB staining was conducted, revealing a significant increase, particularly at the highest SRE concentration (Figure 5). These findings are consistent with those of Kim, et al. [31], which demonstrate an extract from Solanum carolinense containing solanidan-3-ol (3 β,5 α) induced significant phytotoxicity in the forage crop Festuca arundinacea by increasing O2− and H2O2 levels.
The identification of allelochemicals in the extract offers insights into the causative agents responsible for inducing phytotoxicity. The SRE extract was found to contain the highest proportion of the compound methyl kolavenate (35.6%). A previous study by Morimoto [32] reports that methyl kolavenate, a clerodane diterpene, exhibits antifeedant properties. Clerodane diterpenes exhibit antifeedant [33], antifungal [34], and antibacterial [35] activities. Zhao, et al. [36] suggest that methyl kolavenate and its analogs exhibit cytotoxicity, which is due to hydroxy and carboxyl functional groups. Siddiqui, et al. [37] report that the carboxyl group specifically contributes to the toxicity observed in crops. Based on these findings, methyl kolavenate present in SRE is predicted to be the primary causal agent responsible for the phytotoxic effects observed in T. repens and other weeds. Similar findings were reported by Barbosa, et al. [38] and Faizi, et al. [39], who demonstrates the antifeedant, antibacterial, and antifungal activities of methyl kolavenate. However, other compounds present in lower proportions in the SRE extract may contribute to its phytotoxic effects.
Allelochemicals can induce excessive ROS production, leading to the activation of free radicals and oxidizing enzymes in host plants [40]. In response, plants initiate immune defense mechanisms to detoxify ROS and mitigate damage under diverse abiotic stress conditions [41]. Our results demonstrated that SRE treatment significantly increased O2− and H2O2 levels. Antioxidant enzymes, such as SOD, CAT, and POD, play a critical role in scavenging ROS and preventing cell death. This finding is consistent with that of Farooq, et al. [42] and Sharma, et al. [43]. The interaction among SOD, POD, and CAT is essential for the conversion of O2− into H2O2 and subsequently into H2O [44,45]. In this study, CAT and POD activities were significantly increased in T. repens following SRE treatment. POD catalyzes the decomposition of H2O2 into H2O and O2 by oxidizing phenolic compounds or other antioxidants. In contrast, CAT removes H2O2 directly without the participation of other cofactors [46]. This finding is consistent with that of previous studies demonstrating the role of SOD, CAT, and POD in scavenging toxic radicals and enhancing stress tolerance [47,48].
The leaves of plants serve as the primary site for metabolism, where various secondary metabolites are produced and stored [49,50]. SRE treatment significantly reduced chlorophyll content, as visually observed. This was further confirmed by the quantification results, which showed a significant increase in toxic radical ions, such as O2− and H2O2. The oxygen produced during photosynthesis accepts electrons, creating O2– and H2O2 in photosystems I and II [51]. Considering that chlorophyll plays a crucial role in photosynthesis, particularly in the transfer and assimilation of light energy [52,53], its degradation contributes to oxidative stress.
Endogenous phytohormone modulation is a key co-factor in combating oxidative stress and maintaining crop vitality [54]. The proper synthesis of these hormones is crucial for regulating cross-signaling and modulating the biosynthetic pathways of various metabolites [55,56]. The increase in ABA and SA levels is counterbalanced by JA under external stress conditions to enhance crop growth [57,58]. Our results indicated a significant increase in ABA and SA content, while JA levels decreased in T. repens following foliar SRE treatment. This shift in hormone balance contributed to a significant reduction in crop growth and development, indicating higher stress levels in the plants. Overall, exogenous treatments with phytohormones, such as ABA, JA, and SA, have beneficial effects on plants under stress. However, a few studies report that these treatments can induce toxic effects. For example, Guan, et al. [59] and Refs. [58,60] demonstrate that ABA treatments in corn plants increased H2O2 and O2− content, subsequently enhancing antioxidant enzyme synthesis.
WS management is a significant challenge in agriculture and several ecological sites. Despite the observed phytotoxic effects of SRE on T. repens seedlings, its application inhibited the germination and growth of all five WS, highlighting its potential as a bioherbicide.
4. Materials and Methods
4.1. Plant Material
Weed species (WS: S. altissima, E. oryzicola, C. microiria, A. aequalis, P. oleracea, A. retroflexus) were collected from different locations in Panmun-dong (Jinju-si, Republic of Korea). White clover seeds were purchased from Ubiqsolution (Guri, Republic of Korea).
4.2. Plant Experiment
4.2.1. Preparation of Solidago altissima Extract
The shoots and roots of S. altissima were separately shade-dried and ground into fine powder. The preparation of SSE and root SRE followed the method described by Cho, et al. [61] with slight modifications. Briefly, 200 g of S. altissima shoot and root powder was divided into four beakers (50 g per breaker) and suspended in 500 mL of methanol (MeOH). The mixture was stirred at 500 rpm for 24 h. The resulting extracts were combined, concentrated using a rotary evaporator (Rotary Evaporator N-1100VW, Eyela, Tokyo, Japan), and reconstituted in 100 mL distilled water (d-H2O). The extracts were freeze-dried, yielding dried SSE and SRE, which were subsequently stored at −80 °C.
4.2.2. In Vitro Seed Bioassay for T. repens and Weed Species
A stock solution of SSE and/or SRE at 20,000 mg/L was serially diluted with d-H2O to prepare different concentrations: (control; only d-H2O, T1; 625 mg/L), T2 1250 mg/L, T3; 2500 mg/L, T4; 5000 mg/L, T5; 10,000 mg/L, and T6; 20,000 mg/L. Two milliliters of solution from each concentration were dispensed onto a filter paper (Advantec no. 2, Toyo Roshi Kaisha Ltd., Tokyo, Japan) and placed in a Petri dish (60 × 15 mm). T. repens seeds were sterilized with 3% sodium hypochlorite (NaOCl) and rinsed three times with autoclaved d-H2O. Subsequently, 20 seeds were placed in each Petri dish and allowed to germinate for 7 days in a plant growth system (JSPC-420C, JSR Corporation, Gongju, Republic of Korea) under controlled conditions: 20 °C temperature, 60% relative humidity, 6850 lux light intensity, and 16/8 h day/night photoperiod, as described by Liu, et al. [62]. A dose-response curve was constructed based on the fresh weight of the forage crop seedlings, and the half-maximal inhibitory concentration (IC50) was calculated. The root extract demonstrated significant efficiency in exerting crop dominance and inducing phytotoxic effects in T. repens. Consequently, the root extract was selected for further analysis. Based on the results of the T. repens seed bioassay, the SRE was applied to WS seeds, with the stock solution further diluted to concentrations of 312.5 mg/L and 156.25 mg/L.
4.2.3. Solidago altissima Root Treatment on Trifolium repens Seedlings
Overall, 20 T. repens seeds were sown in each pot (9 cm × 10 cm) containing a 5 cm layer of horticultural soil (Hanareum, Shinsung Mineral, Goesan, Republic of Korea) and covered with an additional 2 cm layer of soil. Several doses of SRE were prepared from a stock solution, and the treatments were designated as follows: TR1 (control, d-H2O only), TR2 (12,500 mg/L SRE), TR3 (25,000 mg/L SRE), TR4 (50,000 mg/L SRE), and TR5 (100,000 mgL SRE). Three weeks after sowing, 0.1% TWEEN-20 was added as a surfactant to 5 mL of each treatment solution. The experiment was conducted with three replicates, with each pot containing 20 plants. Foliar applications (spraying 5 mL/pot) were administered three times at 1-week intervals. Following the third foliar application, morphological attributes such as shoot length, root length (focused on the main root), and fresh weight were measured. Five plants exhibiting similar growth patterns were selected from each pot to assess morphological attributes. To measure fresh weight, the plants were carefully removed from the soil without drying. The chlorophyll content of T. repens was quantified using a portable chlorophyll content meter (CCM-300, ADC BioScientific Ltd., Herts, UK). Plant growth conditions were maintained as described in Section 4.2.2.
4.3. Reactive Oxygen Species Analysis in Trifolium repens
DAB Test and Analysis of O2− and H2O2 Levels
For the DAB test, the leaves were excised from the plants and stained with a DAB reagent (1 mg/mL, pH 3.8) for 12 h in the dark. Subsequently, decolorization was performed using absolute ethanol (EtOH) at 85 °C. The leaves were incubated in a 60% glycerol (C3H8O3) solution and visualized. The methodology for quantifying O2− was based on the approach outlined by Navari-Izzo, et al. [63]. A spectrophotometer (Multiskan GO, Thermo Scientific, Waltham, MA, USA) was used to detect O2− and H2O2. H2O2 analysis was conducted using the OxiTec Hydrogen Peroxide/Peroxidase Assay Kit (Biomax Co., Ltd., Nowon-gul, Seoul, Republic of Korea), following the manufacturer’s instructions. Absorbance readings were taken at 580 nm and 570 nm for O2− and H2O2, respectively.
4.4. Antioxidant Analysis
4.4.1. Soluble Protein Content
The soluble protein content was quantified using the method described by Bradford [64] and Park, et al. [65]. Protein samples were extracted with 50 mM sodium phosphate (Na3PO4) buffer (pH 7.0) and treated with Bradford reagent (Coomassie G-250, Thermo Fisher Scientific Korea Ltd., Seoul, Republic of Korea). Absorbance reading was recorded at 595 nm using a spectrophotometer, as described in Section 4.3. A protein standard curve was generated using bovine serum albumin.
4.4.2. Measurement of Superoxide Dismutase, Catalase, and Peroxidase Activities
The extraction was performed using a 50 mM phosphate buffer containing 1 mM ethylenediaminetetraacetic acid (EDTA), and 1% polyvinylpyrrolidone (PVP), and the samples were incubated at 4 °C for 10 min, followed by centrifugation (26,452× g, 30 min, 4 °C) to separate the supernatant. Antioxidant enzyme activity in forage was measured using commercial assay kits. The activities of SOD, CAT, and POD were analyzed using the OxiTec SOD Assay Kit, OxiTec Catalase Assay Kit, and OxiTec Hydrogen Peroxide/Peroxidase Assay Kit (Biomax Co., Ltd., Nowon-gu, Seoul, Republic of Korea), respectively, following the instructions of the manufacturer.
4.5. Phytohormone Analysis
4.5.1. Quantification of Abscisic Acid and Jasmonic Acid
Quantification of ABA in plants was conducted following the method described by Gam, et al. [66]. A mixture of isopropanol and acetic acid (95:5) with d6-ABA as the internal standard was used for ABA extraction and calibration. Similarly, the quantification of JA in plants was performed based on the protocol described by Shahzad et al. (2016) [67]. JA was extracted using an extraction solution of acetone and citric acid (70:30), with [9,10-2H2]-9,10-dihydro-JA serving as the internal standard. Methylation was performed using ethereal diazomethane (CH2N2) followed by dichloromethane (CH2Cl2). The samples were analyzed using GC-MS. Quantification was determined by comparing the peak areas of ions 190 and 194 for ABA and ion 88 for JA.
4.5.2. Quantification of Salicylic Acid
Endogenous SA was quantified following the method described by Seskar et al. [68]. Samples were extracted with 90% MeOH, before being dried using a Savant Speedvac concentrator (Thermo Fisher Scientific, Waltham, MA, USA). The supernatant was treated with 5% trichloroacetic acid, followed by the addition of an SA extraction solution composed of ethyl acetate (EtOAc), cyclopentane (C5H10), and–isopropanol (C3H8O) in a 49.5:49.5:1 ratio. The organic layer containing SA was dried under nitrogen (N2), reconstituted with 100% MeOH, and analyzed using a high-performance liquid chromatography (HPLC) system Shimadzu LC-10) equipped with an RF-10AXL detector (excitation at 305 nm, emission at 365 nm).
4.6. Identification of Allelochemicals in Solidago altissima
4.6.1. Liquid–Liquid Extraction
To perform liquid–liquid extraction (LLE), the root powder of S. altissima was dissolved in d-H2O and transferred into a separatory funnel. Equal volumes of n-hexane (C6H14), chloroform (CHCl3), EtOAc, and n-butanol (BuOH) were added, each in a 1:1 ratio to d-H2O, to separate the solvent fractions. The solvent fractions obtained were dehydrated using anhydrous sodium sulfate (Na2SO4) and subsequently concentrated. Each concentrated organic solvent layer was freeze-dried and used in a seed bioassay, as described in Section 4.2.2.
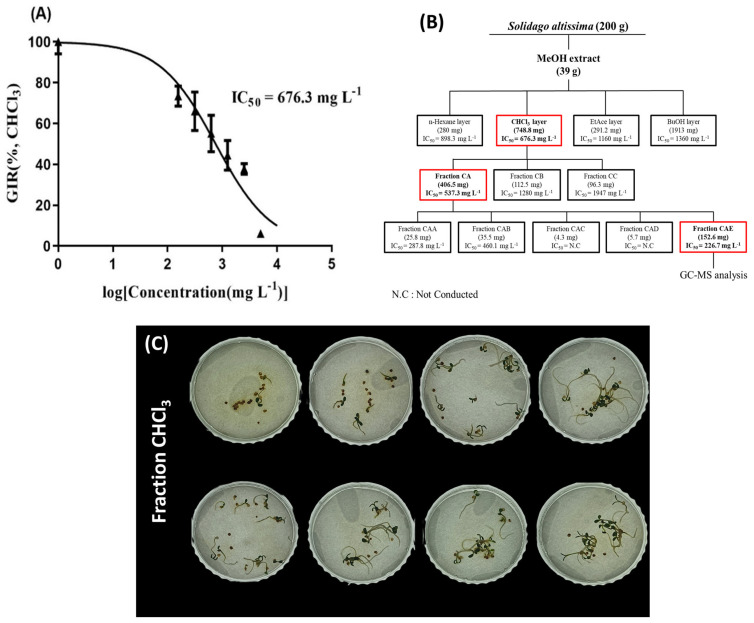
Each fraction demonstrated a different IC50 value: C6H14 (898.3 mg/L), CHCl3 (676.3 mg/L), EtOAc (1160 mg/L), and BuOH (1360 mg/L). The CHCl3 fraction (dry weight, 748.8 mg), which exhibited the lowest IC50 value based on fresh weight, was selected for further separation through column chromatography. Figure 9 illustrates the LLE process diagram.
Figure 9.
(A) Curve illustrating the germination inhibition rate (GIR) of T. repens after treatment with the CHCl3 fraction, with an IC50 of 676.3 mg/L. Error bars represent the standard deviation (SD) (n = 3). (B) Schematic diagram of the liquid–liquid extraction process and fraction distribution of SRE. (C) Visual observation of the dose-dependent effect of CHCl3 fraction treatment on SRE in T. repens germination.
4.6.2. Column Chromatography and Instrumental Analysis
The first column chromatography was performed using silica gel 60 (0.040–0.063 mm, Merck, Kenilworth, NJ, USA) with a mixed solvent system of C6H14-CHCl3-MeOH (5:8:1.3) as the mobile phase. Fractions CA, CB, and CC were separated, concentrated in a rotary evaporator, and subsequently used in a seed bioassay as described in Section 2.2.2. Fraction A (406.5 mg), exhibiting the lowest IC50 value based on fresh weight, was subjected to second-column chromatography using a mixed solvent system of C6H14-CHCl3 (1:10) as the mobile phase. This process yielded five fractions: CAA, CAB, CAC, CAD, and CAE. Among these, the CAE fraction, which had the lowest IC50, was diluted in CHCl3 and prepared for GC-MS analysis. The CAE fraction was analyzed in scan mode using an Agilent 7890B Network GC System combined with a 5977B Network Mass Selective Detector (Agilent Technologies, Palo Alto, CA, USA) (Table S1).
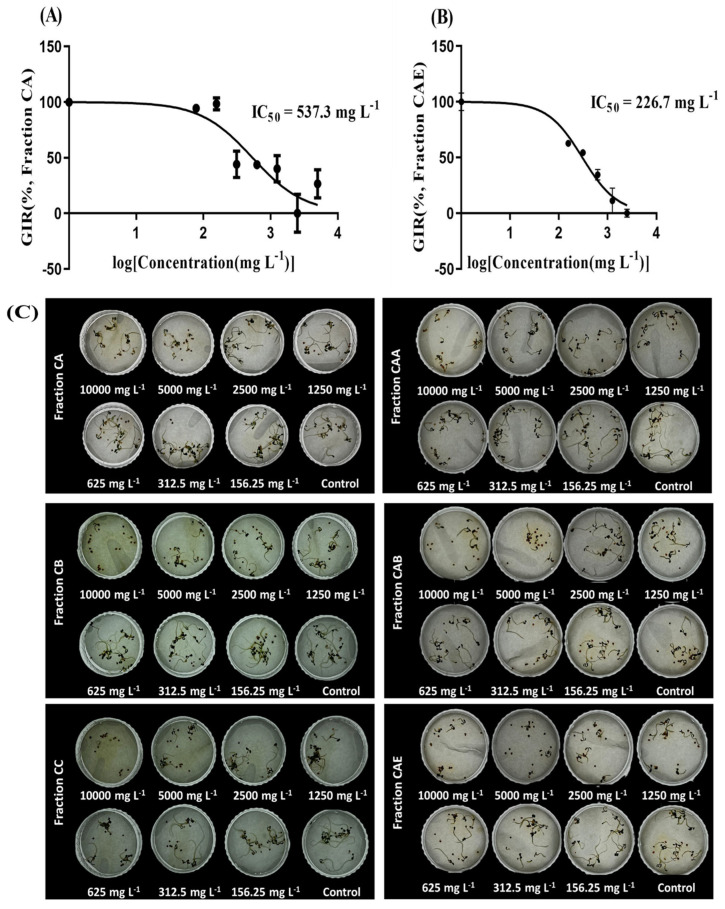
After primary column chromatography, three fractions—CA, CB, and CC—were collected. Each fraction was concentrated under reduced pressure, and a seed bioassay was conducted to determine their individual IC50 values: fraction CA (537.3 mg/L), fraction CB (1280 mg/L), and fraction CC (1947 mg/L). The second column chromatography was performed using fraction CA, which exhibited the lowest IC50 value, resulting in the separation of five additional fractions: CAA, CAB, CAC, CAD, and CAE. The IC50 values for the fractions collected in sufficient quantities for the seed bioassay were as follows: fraction CAA (287.8 mg/L), fraction CAB (460.1 mg/L), and fraction CAE (226.7 mg/L). The CAE fraction, which demonstrated the lowest IC50 value, was selected for GC-MS analysis. Figure 10 illustrates the germination inhibition curves for T. repens treated with fractions CA and AE.
Figure 10.
Dose-response inhibition curves illustrating germination inhibition rate (GIR) of T. repens for chromatography fractions with the highest IC50 values. (A) Dose-response inhibition curve of fraction CA. (B) Dose-response inhibition curve of fraction CAE. Error bars represent the standard deviation (SD) (n = 3). Visual observation of the dose-dependent effects of different SRE fractions on T. repens germination (C). Figure 1 details the fractionation methodology.
4.7. Statistical Analysis
The in vitro seed bioassay results were analyzed using Duncan’s multiple range test (DMRT, p < 0.05). IC50 data were compared using Student’s t-tests (*** p < 0.001, ** p < 0.01, * p < 0.05). Other experimental data were analyzed using analysis of variance (ANOVA) followed by DMRT (p < 0.05) to identify differences among mean values. Statistical analyses were conducted using GraphPad Prism (Version 8; GraphPad Software, San Diego, CA, USA) and SAS statistical software (Version 9.4; SAS Institute, Cary, NC, USA).
5. Conclusions
Overall, our findings demonstrate that S. altissima extract exhibits allelopathic potential and contains clerodane diterpene compounds, with methyl kolavenate constituting a significant portion. This compound potentially contributes to the observed phytotoxic effects on T. repens by inducing oxidative stress through ROS production and germination inhibition in other weed species. To validate the specific phytotoxic effects, further experiments are required to test methyl kolavenate and other identified compounds individually across several plant species. The findings of this study offer valuable insights for developing effective strategies to manage alien invasive species and enhance forage crop production.
Acknowledgments
We express our sincere gratitude to the team members of the crop physiology lab and the Department of Applied Biosciences of Kyungpook National University for providing us with a necessary equipped platform for experimenting Also we greatly acknowledge the contribution and resources provided by KNU NGS Center (Daegu, South Korea) for centrifugation and sample preparation.
Abbreviations
| SSE | S. altissima shoot extract |
| SRE | S. altissima root extract |
| WS | Weed species |
| ABA | Abscisic acid |
| JA | Jasmonic acid |
| SA | Salicylic acid |
| ROS | Reactive oxygen species |
Supplementary Materials
The following supporting information is available for download at https://www.mdpi.com/article/10.3390/plants14010096/s1. Figure S1: Effect of SRE organic solvent fraction treatment on the fresh weight of T. repens (A) C6H14, (B) CHCl3, (C) EtOAc, (D) BuOH. Figure S2: Effect of SRE-CHCl3 layer first-column chromatography fractions on the fresh weight of T. repens (A) fraction CA, (B) fraction CB, (C) fraction CC. Figure S3: Effect of SRE-CHCl3-fraction CA second-column chromatography fractions on the fresh weight of T. repens (A) fraction CAA, (B) fraction CAB, (C) fraction CAE. Figure S4: Effect of SRE treatment on the fresh weight of different weed species (A) Echinochloa oryzicola, (B) Cyperus microiria, (C) Alopecurus aequalis, (D) Portulaca oleracea, (E) Amaranthus retroflexus. Figure S5: Effect of SRE treatment on the plumule length of different weed species (A) Echinochloa oryzicola, (B) Cyperus microiria, (C) Alopecurus aequalis, (D) Portulaca oleracea, (E) Amaranthus retroflexus. Figure S6: Effect of SRE treatment on the radicle length of different weed species (A) Echinochloa oryzicola, (B) Cyperus microiria, (C) Alopecurus aequalis, (D) Portulaca oleracea, (E) Amaranthus retroflexus. Table S1: GC-MS condition for CAE fraction used in this study.
Author Contributions
Conceptualization, I.-J.L. and H.-J.G.; methodology, H.-J.G. and Y.K.; software, J.-I.W. and M.Y.B.; validation, I.-J.L., M.I.-U.-H., S.-M.K., A.A. and S.S.; formal analysis, H.-J.G., Y.K. and J.R.J.; investigation, H.-J.G. and I.-J.L.; resources, I.-J.L.; data curation, A.A.; writing—original draft preparation, A.A. and H.-J.G.; writing—review and editing, I.-J.L., M.I.-U.-H., S.S. and K.-Y.K.; visualization, H.-J.G. and B.-K.A.; supervision, I.-J.L.; funding acquisition, I.-J.L. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was financially supported by the Rural Development Administration, Republic of Korea, Agenda Program (Project No. PJ 015026022022), Biological materials Specialized Graduate Program through the Korea Environmental Industry & Technology Institute (KEITI) funded by the Ministry of Environment (MOE), and Korea Basic Science Institute (National Research Facilities and Equipment Center) grant funded by the Ministry of Education (2021R1A6C101A416).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hussain M.I., Abideen Z., Danish S., Asghar M.A., Iqbal K. Integrated weed management for sustainable agriculture. Sustain. Agric. Rev. 2021;52:367–393. [Google Scholar]
- 2.Etterson J.R., Delf D.E., Craig T.P., Ando Y., Ohgushi T. Parallel patterns of clinal variation in Solidago altissima in its native range in central USA and its invasive range in Japan. Botany. 2008;86:91–97. doi: 10.1139/B07-115. [DOI] [Google Scholar]
- 3.Maddox G.D., Cook R.E., Wimberger P.H., Gardescu S. Clone structure in four Solidago altissima (Asteraceae) populations: Rhizome connections within genotypes. Am. J. Bot. 1989;76:318–326. doi: 10.1002/j.1537-2197.1989.tb11315.x. [DOI] [Google Scholar]
- 4.Meyer A.H., Schmid B. Seed dynamics and seedling establishment in the invading perennial Solidago altissima under different experimental treatments. J. Ecol. 1999;87:28–41. doi: 10.1046/j.1365-2745.1999.00316.x. [DOI] [Google Scholar]
- 5.Cain M.L. Models of clonal growth in Solidago altissima. J. Ecol. 1990;78:27–46. doi: 10.2307/2261034. [DOI] [Google Scholar]
- 6.Weber E. Biological flora of central Europe: Solidago altissima L. Flora. 2000;195:123–134. doi: 10.1016/S0367-2530(17)30960-X. [DOI] [Google Scholar]
- 7.Jung J.S., Kim J.G., Park H.S., Lee S.H., Kim H.S., Kim W.H., Kim Y.J., Choi G.J. The effects of improvement of botanical composition technology application on botanical composition and dry matter productivity in Rumex acetosella dominated hilly pasture. J. Korean Soc. Grassl. Forage Sci. 2016;36:81–88. doi: 10.5333/KGFS.2016.36.2.81. [DOI] [Google Scholar]
- 8.Kim Y., Chung C., Choi Y., Lim Y., Han S., Na K. Effect of herbicide application on weed control and forage production in alpine grassland predominated with red sorrel (Rumex acetosella L.) J. Anim. Sci. Technol. 2003;45:865–874. [Google Scholar]
- 9.Wang H., Wu Y., He Y., Li G., Ma L., Li S., Huang J., Yang G. High-quality chromosome-level de novo assembly of the Trifolium repens. BMC Genom. 2023;24:326. doi: 10.1186/s12864-023-09437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmad S., Zeb A. Phytochemical profile and pharmacological properties of Trifolium repens. J. Basic Clin. Physiol. Pharmacol. 2021;32:20200015. doi: 10.1515/jbcpp-2020-0015. [DOI] [PubMed] [Google Scholar]
- 11.Cho H.-S., Seo M.-C., Kim J.-H., Sang W.-G., Shin P., Lee G.H. Effects of spring seeding on growth and carbon uptake of clover species in upland soil. Korean J. Soil Sci. Fertil. 2017;50:644–652. doi: 10.7745/KJSSF.2017.50.6.644. [DOI] [Google Scholar]
- 12.Lin H., Liu C., Li B., Dong Y. Trifolium repens L. regulated phytoremediation of heavy metal contaminated soil by promoting soil enzyme activities and beneficial rhizosphere associated microorganisms. J. Hazard. Mater. 2021;402:123829. doi: 10.1016/j.jhazmat.2020.123829. [DOI] [PubMed] [Google Scholar]
- 13.Vivanco J.M., Bais H.P., Stermitz F.R., Thelen G.C., Callaway R.M. Biogeographical variation in community response to root allelochemistry: Novel weapons and exotic invasion. Ecol. Lett. 2004;7:285–292. doi: 10.1111/j.1461-0248.2004.00576.x. [DOI] [Google Scholar]
- 14.Chengxu W., Mingxing Z., Xuhui C., Bo Q. Review on allelopathy of exotic invasive plants. Procedia Eng. 2011;18:240–246. doi: 10.1016/j.proeng.2011.11.038. [DOI] [Google Scholar]
- 15.Kobayashi A., Morimoto S., Shibata Y., Yamashita K., Numata M. C10-polyacetylenes as allelopathic substances in dominants in early stages of secondary succession. J. Chem. Ecol. 1980;6:119–131. doi: 10.1007/BF00987532. [DOI] [Google Scholar]
- 16.Nishino C., Manabe S., Kazui M., Matsuzaki T. Piscicidal cis-clerodane diterpenes from Solidago altissima L: Absolute configurations of 5α, 10α-cis-clerodanes. Tetrahedron Lett. 1984;25:2809–2812. doi: 10.1016/S0040-4039(01)81296-X. [DOI] [Google Scholar]
- 17.Tori M., Katto A., Sono M. Nine new clerodane diterpenoids from rhizomes of Solidago altissima. Phytochemistry. 1999;52:487–493. doi: 10.1016/S0031-9422(99)00273-3. [DOI] [Google Scholar]
- 18.Wu B., Takahashi T., Kashiwagi T., Tebayashi S.-i., Kim C.-S. New flavonoid glycosides from the leaves of Solidago altissima. Chem. Pharm. Bull. 2007;55:815–816. doi: 10.1248/cpb.55.815. [DOI] [PubMed] [Google Scholar]
- 19.Jin H., Tanaka T., Kouno I., Ishimaru K. A new kaempferol trioside from Solidago altissima L. J. Nat. Med. 2007;61:351–354. doi: 10.1007/s11418-007-0139-6. [DOI] [Google Scholar]
- 20.Nishidono Y., Tanaka K. Comprehensive characterization of polyacetylenes and diterpenes from the underground parts of Solidago altissima L. and their contribution to the overall allelopathic activity. Phytochemistry. 2022;193:112986. doi: 10.1016/j.phytochem.2021.112986. [DOI] [PubMed] [Google Scholar]
- 21.Souza L.A., Monteiro C.C., Carvalho R.F., Gratão P.L., Azevedo R.A. Dealing with abiotic stresses: An integrative view of how phytohormones control abiotic stress-induced oxidative stress. Theor. Exp. Plant Physiol. 2017;29:109–127. doi: 10.1007/s40626-017-0088-8. [DOI] [Google Scholar]
- 22.Złotek U., Szymanowska U., Jakubczyk A., Sikora M., Świeca M. Effect of arachidonic and jasmonic acid elicitation on the content of phenolic compounds and antioxidant and anti-inflammatory properties of wheatgrass (Triticum aestivum L.) Food Chem. 2019;288:256–261. doi: 10.1016/j.foodchem.2019.02.124. [DOI] [PubMed] [Google Scholar]
- 23.Latif S., Chiapusio G., Weston L. Advances in Botanical Research. Volume 82. Elsevier; Amsterdam, The Netherlands: 2017. Allelopathy and the role of allelochemicals in plant defence; pp. 19–54. [Google Scholar]
- 24.Torun H., Novák O., Mikulík J., Pěnčík A., Strnad M., Ayaz F.A. Timing-dependent effects of salicylic acid treatment on phytohormonal changes, ROS regulation, and antioxidant defense in salinized barley (Hordeum vulgare L.) Sci. Rep. 2020;10:13886. doi: 10.1038/s41598-020-70807-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pardo G., Marí A.I., Aibar J., Cirujeda A. Do crop rotations in rice reduce weed and Echinochloa spp. infestations? Recomm. Integr. Weed Control. Agron. 2021;11:454. [Google Scholar]
- 26.Zhao N., Li Q., Guo W., Zhang L., Wang J. Effect of environmental factors on germination and emergence of shortawn foxtail (Alopecurus aequalis) Weed Sci. 2018;66:47–56. doi: 10.1017/wsc.2017.42. [DOI] [Google Scholar]
- 27.Elbalola A.A. Evidence for increased competitive ability (EICA) in Prosopis juliflora (Sw.) Dc (mesquite) under P. juliflora-Portulaca oleracea L. (purslane) field competition. Afr. J. Ecol. 2021;59:1075–1079. doi: 10.1111/aje.12885. [DOI] [Google Scholar]
- 28.Khan A.M., Mobli A., Werth J.A., Chauhan B.S. Germination and seed persistence of Amaranthus retroflexus and Amaranthus viridis: Two emerging weeds in Australian cotton and other summer crops. PLoS ONE. 2022;17:e0263798. doi: 10.1371/journal.pone.0263798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts J., Florentine S., Fernando W.D., Tennakoon K.U. Achievements, developments and future challenges in the field of bioherbicides for weed control: A global review. Plants. 2022;11:2242. doi: 10.3390/plants11172242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gill S.S., Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Kim L.-R., Adhikari A., Kang Y., Gam H.-J., Kang S.-M., Kim K.-Y., Lee I.-J. Investigation of Solanum carolinense Dominance and Phytotoxic Effect in Festuca arundinacea with Special Reference to Allelochemical Identification, Analysis of Phytohormones and Antioxidant Mechanisms. Agronomy. 2022;12:1954. doi: 10.3390/agronomy12081954. [DOI] [Google Scholar]
- 32.Morimoto M. Chemical defense against insects in Heterotheca subaxillaris and three Orobanchaceae species using exudates from trichomes. Pest Manag. Sci. 2019;75:2474–2481. doi: 10.1002/ps.5395. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y., Liu Y.-C., Li W.-Y., Guo K., Liu Y., Li S.-H. Antifeedant, cytotoxic, and anti-inflammatory neo-clerodane diterpenoids in the peltate glandular trichomes and fresh leaves of Ajuga forrestii. Phytochemistry. 2021;186:112731. doi: 10.1016/j.phytochem.2021.112731. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen M.V., Han J.W., Le Dang Q., Ryu S.M., Lee D., Kim H., Choi G.J. Clerodane Diterpenoids Identified from Polyalthia longifolia Showing Antifungal Activity against Plant Pathogens. J. Agric. Food Chem. 2021;69:10527–10535. doi: 10.1021/acs.jafc.1c02200. [DOI] [PubMed] [Google Scholar]
- 35.Murthy M.M., Subramanyam M., Bindu M.H., Annapurna J. Antimicrobial activity of clerodane diterpenoids from Polyalthia longifolia seeds. Fitoterapia. 2005;76:336–339. doi: 10.1016/j.fitote.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Zhao G.X., Jung J.H., Smith D.L., Wood K.V., McLaughlin J.L. Cytotoxic clerodane diterpenes from Polyalthia longifolia. Planta Med. 1991;57:380–383. doi: 10.1055/s-2006-960122. [DOI] [PubMed] [Google Scholar]
- 37.Siddiqui R., Akbar N., Khatoon B., Kawish M., Ali M.S., Shah M.R., Khan N.A. Novel Plant-Based Metabolites as Disinfectants against Acanthamoeba castellanii. Antibiotics. 2022;11:248. doi: 10.3390/antibiotics11020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barbosa A.L.P., Wenzel-Storjohann A., Barbosa J.D., Zidorn C., Peifer C., Tasdemir D., Çiçek S.S. Antimicrobial and cytotoxic effects of the Copaifera reticulata oleoresin and its main diterpene acids. J. Ethnopharmacol. 2019;233:94–100. doi: 10.1016/j.jep.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 39.Faizi S., Khan R.A., Mughal N.R., Malik M.S., Sajjadi K.e.S., Ahmad A. Antimicrobial activity of various parts of Polyalthia longifolia var. pendula: Isolation of active principles from the leaves and the berries. Phytother. Res. 2008;22:907–912. doi: 10.1002/ptr.2414. [DOI] [PubMed] [Google Scholar]
- 40.Rudrappa T., Bonsall J., Gallagher J.L., Seliskar D.M., Bais H.P. Root-secreted allelochemical in the noxious weed Phragmites australis deploys a reactive oxygen species response and microtubule assembly disruption to execute rhizotoxicity. J. Chem. Ecol. 2007;33:1898–1918. doi: 10.1007/s10886-007-9353-7. [DOI] [PubMed] [Google Scholar]
- 41.Hasanuzzaman M., Bhuyan M.B., Zulfiqar F., Raza A., Mohsin S.M., Mahmud J.A., Fujita M., Fotopoulos V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants. 2020;9:681. doi: 10.3390/antiox9080681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farooq M.A., Niazi A.K., Akhtar J., Farooq M., Souri Z., Karimi N., Rengel Z. Acquiring control: The evolution of ROS-Induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol. Biochem. 2019;141:353–369. doi: 10.1016/j.plaphy.2019.04.039. [DOI] [PubMed] [Google Scholar]
- 43.Sharma P., Jha A.B., Rama Shanker D., Pessarakli M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012;2012:217037. doi: 10.1155/2012/217037. [DOI] [Google Scholar]
- 44.Szőllősi R. Oxidative Damage Plants. Academic Press; Cambridge, MA, USA: 2014. Superoxide dismutase (SOD) and abiotic stress tolerance in plants: An overview; pp. 89–129. [DOI] [Google Scholar]
- 45.Corpas F.J., González-Gordo S., Palma J.M. Plant Peroxisomes: A Factory of Reactive Species. Front. Plant Sci. 2020;11:853. doi: 10.3389/fpls.2020.00853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaitanya K.V., Sundar D., Masilamani S., Ramachandra Reddy A. Variation in heat stress-induced antioxidant enzyme activities among three mulberry cultivars. Plant Growth Regul. 2002;36:175–180. doi: 10.1023/A:1015092628374. [DOI] [Google Scholar]
- 47.Rajput V.D., Singh R.K., Verma K.K., Sharma L., Quiroz-Figueroa F.R., Meena M., Gour V.S., Minkina T., Sushkova S., Mandzhieva S. Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology. 2021;10:267. doi: 10.3390/biology10040267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu J., Yang J., Duan X., Jiang Y., Zhang P. Increased expression of native cytosolic Cu/Zn superoxide dismutase and ascorbate peroxidase improves tolerance to oxidative and chilling stresses in cassava (Manihot esculenta Crantz) BMC Plant Biol. 2014;14:208. doi: 10.1186/s12870-014-0208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krumsri R., Kato-Noguchi H., Poonpaiboonpipat T. Allelopathic effect of sphenoclea zeylanica gaertn. On rice (‘Oryza sativa’ L.) germination and seedling growth. Aust. J. Crop Sci. 2020;14:1450–1455. doi: 10.21475/ajcs.20.14.09.p2494. [DOI] [Google Scholar]
- 50.Poonpaiboonpipat T., Krumsri R., Kato-Noguchi H. Allelopathic and Herbicidal Effects of Crude Extract from Chromolaena odorata (L.) RM King and H. Rob. on Echinochloa crus-galli and Amaranthus viridis. Plants. 2021;10:1609. doi: 10.3390/plants10081609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pospíšil P. Production of reactive oxygen species by photosystem II. Biochim. Biophys. Acta (BBA)-Bioenerg. 2009;1787:1151–1160. doi: 10.1016/j.bbabio.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 52.Arnao M., Hernández-Ruiz J. Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J. Pineal Res. 2009;46:58–63. doi: 10.1111/j.1600-079X.2008.00625.x. [DOI] [PubMed] [Google Scholar]
- 53.Imran M., Latif Khan A., Shahzad R., Aaqil Khan M., Bilal S., Khan A., Kang S.-M., Lee I.-J. Exogenous melatonin induces drought stress tolerance by promoting plant growth and antioxidant defence system of soybean plants. AoB Plants. 2021;13:plab026. doi: 10.1093/aobpla/plab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ciura J., Kruk J. Phytohormones as targets for improving plant productivity and stress tolerance. J. Plant Physiol. 2018;229:32–40. doi: 10.1016/j.jplph.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 55.Brunetti C., Fini A., Sebastiani F., Gori A., Tattini M. Modulation of phytohormone signaling: A primary function of flavonoids in plant–environment interactions. Front. Plant Sci. 2018;9:1042. doi: 10.3389/fpls.2018.01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kapoor B., Kumar P., Sharma R., Kumar A. Regulatory interactions in phytohormone stress signaling implying plants resistance and resilience mechanisms. J. Plant Biochem. Biotechnol. 2021;30:813–828. doi: 10.1007/s13562-021-00739-0. [DOI] [Google Scholar]
- 57.Lackman P., González-Guzmán M., Tilleman S., Carqueijeiro I., Pérez A.C., Moses T., Seo M., Kanno Y., Häkkinen S.T., Van Montagu M.C. Jasmonate signaling involves the abscisic acid receptor PYL4 to regulate metabolic reprogramming in Arabidopsis and tobacco. Proc. Natl. Acad. Sci. USA. 2011;108:5891–5896. doi: 10.1073/pnas.1103010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thaler J.S., Humphrey P.T., Whiteman N.K. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 2012;17:260–270. doi: 10.1016/j.tplants.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 59.Guan L.M., Zhao J., Scandalios J.G. Cis-elements and trans-factors that regulate expression of the maize Cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signaling molecule for the response. Plant J. 2000;22:87–95. doi: 10.1046/j.1365-313x.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- 60.Jiang M., Zhang J. Effect of Abscisic Acid on Active Oxygen Species, Antioxidative Defence System and Oxidative Damage in Leaves of Maize Seedlings. Plant Cell Physiol. 2001;42:1265–1273. doi: 10.1093/pcp/pce162. [DOI] [PubMed] [Google Scholar]
- 61.Cho N.-K., Lee S.-E., Choi J.-S., Hwang K.-H., Koo S.-J., Wang H.-Y., Kim S.-M. Isolation of a New Herbicidal Compound Angelicin from Curly Dock (Rumex crispus L.) Korean J. Weed Sci. 2010;30:183–190. doi: 10.5660/KJWS.2010.30.3.183. [DOI] [Google Scholar]
- 62.Liu H.-L., Lee Z.-X., Chuang T.-W., Wu H.-C. Effect of heat stress on oxidative damage and antioxidant defense system in white clover (Trifolium repens L.) Planta. 2021;254:103. doi: 10.1007/s00425-021-03751-9. [DOI] [PubMed] [Google Scholar]
- 63.Navari-Izzo F., Pinzino C., Quartacci M.F., Sgherri C.L.M. Superoxide and Hydroxyl Radicai Generation, and Superoxide Dismutase in PII Membrane Fragments from Wheat. Free Radic. Res. 1999;31:3–9. doi: 10.1080/10715769900301251. [DOI] [PubMed] [Google Scholar]
- 64.Bradford M.M. A dye binding assay for protein. Anal. Biochem. 1976;72:e54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 65.Park H.-S., Kazerooni E.A., Kang S.-M., Al-Sadi A.M., Lee I.-J. Melatonin enhances the tolerance and recovery mechanisms in Brassica juncea (L.) Czern. under saline conditions. Front. Plant Sci. 2021;12:593717. doi: 10.3389/fpls.2021.593717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gam H.-J., Injamum-Ul-Hoque M., Kang Y., Ahsan S., Hasan M.M., Shaffique S., Kang S.-M., Lee I.-J. Allelopathic effect of the methanol extract of the weed species-red sorrel (Rumex acetosella L.) on the growth, phytohormone content and antioxidant activity of the cover crop-white clover (Trifolium repens L.) BMC Plant Biol. 2024;24:523. doi: 10.1186/s12870-024-05240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shahzad R., Waqas M., Khan A.L., Asaf S., Khan M.A., Kang S.-M., Yun B.-W., Lee I.-J. Seed-borne endophytic Bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa. Plant Physiol. Biochem. 2016;106:236–243. doi: 10.1016/j.plaphy.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 68.Seskar M., Shulaev V., Raskin I. Endogenous Methyl Salicylate in Pathogen-Inoculated Tobacco Plants1. Plant Physiol. 1998;116:387–392. doi: 10.1104/pp.116.1.387. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article and Supplementary Materials.