Abstract
1. An enzyme that can be induced in rat uteri by oestrogens and that catalyses the oxidation of guaiacol and the metabolism and binding of [4-14C]oestradiol to protein in the presence of H2O2 was partially purified by (NH4)2SO4 fractionation and polyacrylamide-gel chromatography. 2. The molecular weight of this uterine peroxidase was estimated to be about 40 000 and thus shown to differ from that of eosinophil peroxidase. 3. Cycloheximide, which blocks the increase in peroxidase activity brought about by oestrogen, was used to determine the half-life (about 4h) of the induced uterine enzyme.
Full text
PDF

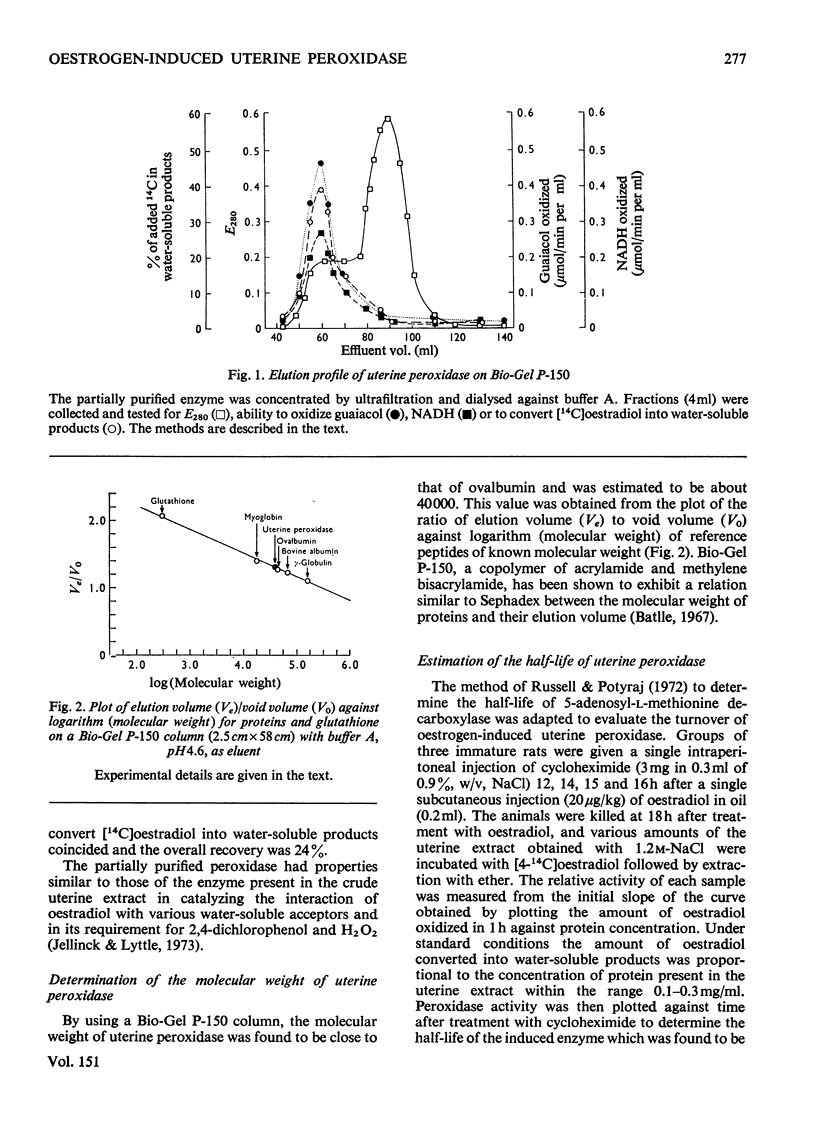
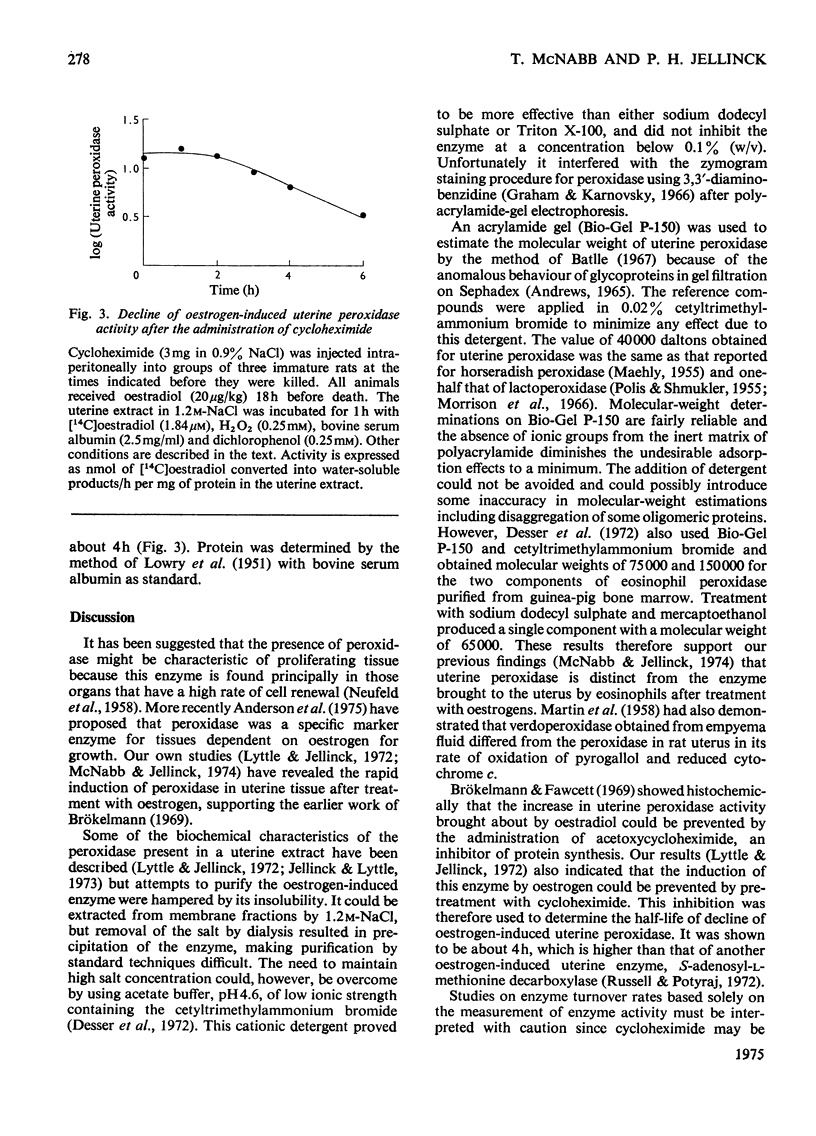

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. A., Kang Y. H., DeSombre E. R. Endogenous peroxidase: specific marker enzyme for tissues displaying growth dependency on estrogen. J Cell Biol. 1975 Mar;64(3):668–681. doi: 10.1083/jcb.64.3.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle A. M. Estimation of molecular weights of proteins by Bio-Gel P gel filtration. J Chromatogr. 1967 May;28(1):82–88. [PubMed] [Google Scholar]
- Brökelmann J., Fawcett D. W. The localization of endogenous peroxidase in the rat uterus and its induction by estradiol. Biol Reprod. 1969 Apr;1(1):59–71. doi: 10.1095/biolreprod1.1.59. [DOI] [PubMed] [Google Scholar]
- Brökelmann J. Peroxidase-associated binding of estradiol by the rat uterus. J Histochem Cytochem. 1969 Jun;17(6):394–397. doi: 10.1177/17.6.394. [DOI] [PubMed] [Google Scholar]
- Desser R. K., Himmelhoch S. R., Evans W. H., Januska M., Mage M., Shelton E. Guinea pig heterophil and eosinophil peroxidase. Arch Biochem Biophys. 1972 Feb;148(2):452–465. doi: 10.1016/0003-9861(72)90164-6. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Himmelhoch S. R., Evans W. H., Mage M. G., Peterson E. A. Purification of myeloperoxidases from the bone marrow of the guinea pig. Biochemistry. 1969 Mar;8(3):914–921. doi: 10.1021/bi00831a022. [DOI] [PubMed] [Google Scholar]
- Jellinck P. H., Lyttle C. R. Estrogen-induced uterine enzymes in the control of estradiol action. Adv Enzyme Regul. 1973;11:17–33. doi: 10.1016/0065-2571(73)90006-x. [DOI] [PubMed] [Google Scholar]
- Jellinck P. H., Woo J. Changes in oestrogen metabolism induced in rat liver microsomes by subcutaneous pellets of oestrone and testosterone. J Endocrinol. 1967 Sep;39(1):99–104. doi: 10.1677/joe.0.0390099. [DOI] [PubMed] [Google Scholar]
- KLEBANOFF S. J. INACTIVATION OF ESTROGEN BY RAT UTERINE PREPARATIONS. Endocrinology. 1965 Feb;76:301–311. doi: 10.1210/endo-76-2-301. [DOI] [PubMed] [Google Scholar]
- Kenney F. T. Turnover of rat liver tyrosine transaminase: stabilization after inhibition of protein synthesis. Science. 1967 Apr 28;156(3774):525–528. doi: 10.1126/science.156.3774.525. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lyttle C. R., Jellinck P. H. Metabolism of (4- 14 C)oestradiol by oestrogen-induced uterine peroxidase. Biochem J. 1972 Apr;127(3):481–487. doi: 10.1042/bj1270481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAEHLY A. C., CHANCE B. The assay of catalases and peroxidases. Methods Biochem Anal. 1954;1:357–424. doi: 10.1002/9780470110171.ch14. [DOI] [PubMed] [Google Scholar]
- MARTIN A. P., NEUFELD H. A., LUCAS F. V., STOTZ E. Characterization of uterine peroxidase. J Biol Chem. 1958 Jul;233(1):206–208. [PubMed] [Google Scholar]
- McNabb T., Jellinck P. H. Origin of oestrogen-induced uterine peroxidase. J Endocrinol. 1974 Aug;62(2):415–416. doi: 10.1677/joe.0.0620415. [DOI] [PubMed] [Google Scholar]
- NEUFELD H. A., LEVAY A. N., LUCAS F. V., MARTIN A. P., STOTZ E. Peroxidase and cytochrome oxidase in rat tissues. J Biol Chem. 1958 Jul;233(1):209–211. [PubMed] [Google Scholar]
- Russell D. H., Potyraj J. J. Spermine synthesis in the uterus of the ovariectomized rat in response to oestradiol-17 . Biochem J. 1972 Aug;128(5):1109–1115. doi: 10.1042/bj1281109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIMKE R. T., SWEENEY E. W., BERLIN C. M. THE ROLES OF SYNTHESIS AND DEGRADATION IN THE CONTROL OF RAT LIVER TRYPTOPHAN PYRROLASE. J Biol Chem. 1965 Jan;240:322–331. [PubMed] [Google Scholar]
- SCHIMKE R. T. THE IMPORTANCE OF BOTH SYNTHESIS AND DEGRADATION IN THE CONTROL OF ARGINASE LEVELS IN RAT LIVER. J Biol Chem. 1964 Nov;239:3808–3817. [PubMed] [Google Scholar]
- Schimke R. T., Ganschow R., Doyle D., Arias I. M. Regulation of protein turnover in mammalian tissues. Fed Proc. 1968 Sep-Oct;27(5):1223–1230. [PubMed] [Google Scholar]
- Smith D. C., Klebanoff S. J. A uterine fluid-mediated sperm-inhibitory system. Biol Reprod. 1970 Oct;3(2):229–235. doi: 10.1093/biolreprod/3.2.229. [DOI] [PubMed] [Google Scholar]
- TEMPLE S., HOLLANDER V. P., HOLLANDER N., STEPHENS M. L. Estradiol activation of uterine reduced diphosphopyridine nucleotide oxidase. J Biol Chem. 1960 May;235:1504–1509. [PubMed] [Google Scholar]


