Abstract
Background:
Optimization of surgical scheduling represents an opportunity to improve resource utilization and increase patient access. Increasing body mass index (BMI) has been associated with increased operating time and may provide an opportunity to more accurately predict operating time.
Objective:
To investigate the relationship between BMI and operative time for benign hysterectomy and develop a predictive model for hysterectomy operating time based on patient BMI.
Methods:
A secondary analysis of women undergoing benign laparoscopic, abdominal, or vaginal hysterectomy between 2014 and 2019 was performed using the American College of Surgeons National Surgical Quality Improvement Program database, N = 117,691. Our primary outcome was log10 transformation of operative time. Multivariable linear regression was used to analyze the relationship between operative time and BMI. A model to predict operating time was created using variables that could be reliably obtained preoperatively.
Results:
From our cohort, 22% of benign hysterectomies were performed abdominally, 16% were vaginal, and 62% were laparoscopic, and mean operative times were 144, 133, and 158 minutes, respectively. For every 10-unit increase in BMI, estimated mean operation time (OT) increased by 12.8%, 8.1%, and 6.5% for abdominal, vaginal, and laparoscopic hysterectomy, respectively. Neither an expanded nor a concise model was able to account for the variability in log10(OT).
Conclusion:
Increasing BMI differentially impacts the operative time in abdominal greater than laparoscopic and vaginal hysterectomy. However, operative time for hysterectomy is highly variable, and its estimation is difficult to reliably predict using common preoperative variables.
Keywords: Body mass index, Hysterectomy, Laparoscopy, Operative time, Surgeons
INTRODUCTION
The operating room (OR) represents one of the largest and most expensive resources within a hospital system, and appropriate utilization of the OR is critical for surgical efficiency and patient access to care.1,2 One key factor in OR utilization is appropriate scheduling of surgeries, which is dependent on accurate prediction of surgical time.2 Overestimation of surgery length may contribute to patient access issues and underutilization of dedicated resources, while underestimation may result in increased resource utilization or strain on available staff. Although this has been an increasing topic of interest and research, to date no universal method has been established by which to predict operating time based on patient variables.2–4
Obesity has been shown to be associated with increased operating time (OT) in several studies.5–10 Specifically, one study demonstrated a greater impact of OT and resource utilization by body mass index (BMI) category for hysterectomy when compared with many other procedures.7 With the prevalence of obesity (BMI > 30 kg/m2) increasing in the United States, from 30.5% to 42.4% from 1999–2000 to 2017–2018, with severe obesity (BMI >40 kg/m2) increasing from 4.7% to 9.2%, this represents a characteristic that needs to be accounted for when predicting OT for surgery scheduling.11
The relationship between obesity and OT represents an opportunity to better predict OT when scheduling hysterectomies. The purpose of our study was to investigate this relationship, and to develop a clinically useful predictive model for OT for benign hysterectomy, stratified by route of surgery (vaginal, laparoscopic, and abdominal) and based on BMI with or without other preoperative clinical factors. Our hypothesis was that increasing BMI is associated with longer OT and that the degree of obesity with other clinical factors could be used to create a predictive model for OT that would determine clinically relevant prolongation in OT. Our overarching goal is to better predict OR time for benign hysterectomy based on degree of obesity, which could improve OR utilization and therefore increase value by decreasing cost.
MATERIALS AND METHODS
Patient Identification
This was a secondary analysis of women undergoing laparoscopic, abdominal, or vaginal hysterectomy for benign indications using the American College of Surgeons National Surgical Quality Improvement Program database (ACS NSQIP). Targeted data files for hysterectomy procedures, including additional perioperative information specific to hysterectomy, became available in a subset of NSQIP participating hospitals in 2014. We therefore collected data between January 1, 2014, and December 31, 2019. Women undergoing abdominal, vaginal, or laparoscopic hysterectomy for benign indications were identified by Current Procedural Terminology (CPT). Women undergoing elective abdominal (CPT 58150, 58152, and 58180), vaginal (CPT 58260, 58262, 58263, 58267, 58270, 58275, 58280, 58285, and 58290–58294), and laparoscopic (CPT 58541–58544, 58550, 58552–58554, and 58570–58573) hysterectomy were eligible for inclusion. Demographic information including patient age at time of surgery, self-identified race/ethnicity, BMI, parity, smoking status (defined as identification as current smoker within the year prior to surgery), history of diabetes, and history of prior pelvic surgery were obtained. Surgical factors including type of hysterectomy, evidence of endometriosis during surgery, uterine weight (greater or less than 250 g), and subspecialty performing the surgery were obtained. Only hysterectomies performed by general Obstetrician-Gynecologists (OB-GYN) and Gynecologic Oncologists were included as the numbers of hysterectomies included in the database performed by other obstetrical and gynecological specialties (eg, maternal-fetal medicine) were too low to analyze separately.
This study was approved under expedited IRB approval by Atrium Health Wake Forest Baptist (IRB #00060681).
Description of Demographic and Surgical Factors
Descriptive statistics were calculated for all variables. Operating time, log10 operation time (OT), BMI, and uterine weight were considered as continuous variables and were reported with mean/standard deviation and with median/interquartile range. Age in quintiles, route of hysterectomy, year of surgery, parity, history of diabetes, smoking status, history of abdominal surgery, history of pelvic surgery, evidence of endometriosis at time of surgery, uterine weight, and subspecialty performing the surgery were considered categorical variables and were reported as frequencies. To allow for differential effects of age as a predictor across its spectrum, models were fitted with that variable in both continuous form and in quintiles.
Outcome Selection
The primary outcome of this analysis was OT. Initial evaluation of its distribution revealed significant heteroscedasticity; therefore, the log10 transformation of OT (log10OT) was modeled as the dependent variable.
Development of a Prediction Model
The relationship between BMI and OT was analyzed using a multivariable linear regression model that included age (analyzed both as a continuous variable as well as categorical by quintiles), race/ethnicity (categorical), year of operation (categorical), smoking status (dichotomous), history of diabetes (dichotomous), parity (categorical), route of hysterectomy (categorical), final uterine weight (dichotomous; less than or greater than/equal to 250 g), history of pelvic surgery (dichotomous), history of abdominal surgery (dichotomous), evidence of endometriosis at time of surgery (dichotomous), and subspecialty performing the surgery (dichotomous, general OB-GYN vs gynecology oncology), and all two-way interactions between covariates. Backwards variable elimination was used to select a “best” predictive model for log10OT. All models considered included both BMI and age as predictors. The large number of observations resulted in very small standard errors for regression parameters; therefore, backward elimination was performed using a cut-off P value of P < 10−9 (P < .00000001) for inclusion in the final model. To provide a model that might be more easily utilized with factors obtained easily from the medical record, a more restrictive selection was performed by evaluating partial η2 statistics and including only terms that accounted for 1% or more of the total variability after rounding. The predictive ability of the resulting model was expressed by the model’s coefficient of determination (R2). After model selection was performed, predicted OT and 95% confidence limits (CLs) were found via back-transformation.
RESULTS
Study Sample Demographics
Within the NSQIP database, we identified 117,691 patients who underwent hysterectomy for benign indications (Tables 1 and 2). Of these, 29,325 (24.9%) underwent abdominal hysterectomy (AH), 14,496 (12.3%) underwent vaginal hysterectomy (VH), and 73,870 (62.8%) underwent laparoscopic hysterectomy (LH) (Table 2). Most subjects were Non-Hispanic White (55.2%), nonsmokers (84.7%), and had a history of prior pelvic surgery (57.0%). The mean age of subjects was 47.4 years old and mean BMI was 31.2 (Tables 1 and 2). Most hysterectomies in our study sample were performed by general OB-GYN (79.8%) (Table 2).
Table 1.
Continuous Demographic Variables Obtained from 117,691 Subjects Undergoing Benign Hysterectomy from January 1, 2014 to December 31, 2019
| Characteristic N = 117,691 |
Mean (SD) | Median (IQR) |
|---|---|---|
| Operation time (minutes) | 135.2 (64.4) | 122 (90, 165) |
| Log10 (operation time) | 2.09 (0.19) | 2.09 (1.95, 2.22) |
| BMI (kg/m2) | 31.2 (7.8) | 29.9 (25.5, 35.5) |
| Uterine Weight* (grams) | 283.5 (425.8) | 145 (90, 282) |
| Age (years) | 47.4 (10.6) | 46 (41, 52) |
*Uterine weight only available for 112,667 subjects.
SD, standard deviation; IQR, interquartile range; BMI, body mass index.
Table 2.
Categorical Demographic Variables Obtained from 117,691 Subjects Undergoing Benign Hysterectomy from January 1, 2014 to December 31, 2019
| Characteristic N = 117,691 |
N (%) |
|---|---|
| Age in quintiles | |
| Minimum to 20th percentile (18–40 years) | 28,878 (24.5) |
| 20th–40th percentiles (41–45 years) | 25,306 (21.5) |
| 40th–60th percentiles (46–51 years) | 29,840 (25.4) |
| 60th–80th percentiles (52–61 years) | 18,874 (16.0) |
| 80th percentile to maximum (62–90 years) | 13,053 (11.1) |
| Year of hysterectomy | |
| 2014 | 12,157 (10.3) |
| 2015 | 14,570 (12.4) |
| 2016 | 18,523 (15.7) |
| 2017 | 20,373 (17.3) |
| 2018 | 24,910 (21.2) |
| 2019 | 27,158 (23.1) |
| Hysterectomy type | |
| Abdominal | 29,325 (24.9) |
| Vaginal | 14,496 (12.3) |
| Laparoscopic | 73,870 (62.8) |
| Race/ethnicity | |
| Hispanic | 10,749 (9.1) |
| Non-Hispanic African American | 19,670 (16.7) |
| Non-Hispanic Asian | 3,685 (3.1) |
| Non-Hispanic White | 18,635 (15.8) |
| Other (Non-Hispanic) or Unknown | 64,952 (55.2) |
| History of diabetes | 9,451 (8.0) |
| Current smoker within last year | 18,004 (15.3) |
| Parity (number of births of viable gestational age) | |
| 0 | 24,846 (21.1) |
| 1 | 18,739 (15.9) |
| 2 | 38,435 (32.7) |
| 3 | 22,325 (19.0) |
| 4 | 8,741 (7.4) |
| 5 | 2,785 (2.4) |
| 6 or more | 1,820 (1.6) |
| History of prior abdominal surgery | 32,039 (27.2) |
| History of prior pelvic surgery | 67,107 (57.0) |
| Endometriosis present | 16,502 (14.0) |
| Uterine weight > 250 grams* | 32,228 (27.8) |
| Surgical subspecialty | |
| General OB-GYN | 93,917 (79.8) |
| Gynecologic oncology | 23,774 (20.2) |
*Uterine weight > 250 grams only available for 115,981 subjects.
Primary Outcome
The total mean OT for all subjects was 135.2 minutes, with mean OTs of 144, 133, and 158 minutes for AH, VH, and LH, respectively (data not shown). Given significant heteroscedasticity of OT, log10 transformation of OT was performed to reduce skewness, with a mean log10 OT of 2.09. When stratified by BMI, the only variable noted to modify OT was route of surgery; for every 10-unit increase in BMI, mean estimated OT increased by approximately 12.8%, 8.1%, and 6.5% for AH, VH, and LH, respectively (Figure 1). In regression analysis, uterine weight >250 g, history of abdominal surgery, and presence of endometriosis noted at time of surgery were all independently statistically associated with increasing operative time (data not shown). However, interactions between these variables and BMI were not significant, and the relationship between BMI and operative time was therefore not modified by these factors.
Figure 1.
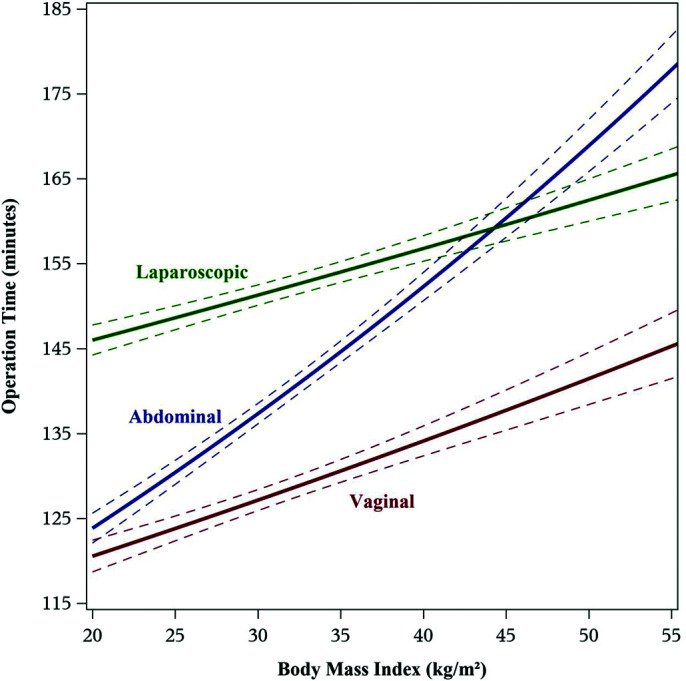
Mean operating times and 95% confidence intervals versus body mass index (BMI), stratified by route of hysterectomy. Predicted means from Model 1 (“best” predictive model from backwards elimination variable selection) include the following predictor variables: BMI, age, smoking status, parity, history of pelvic surgery, surgeon subspecialty, presence of endometriosis at time of surgery, and all 2-way interactions.
Predictive Modeling of Operating Time
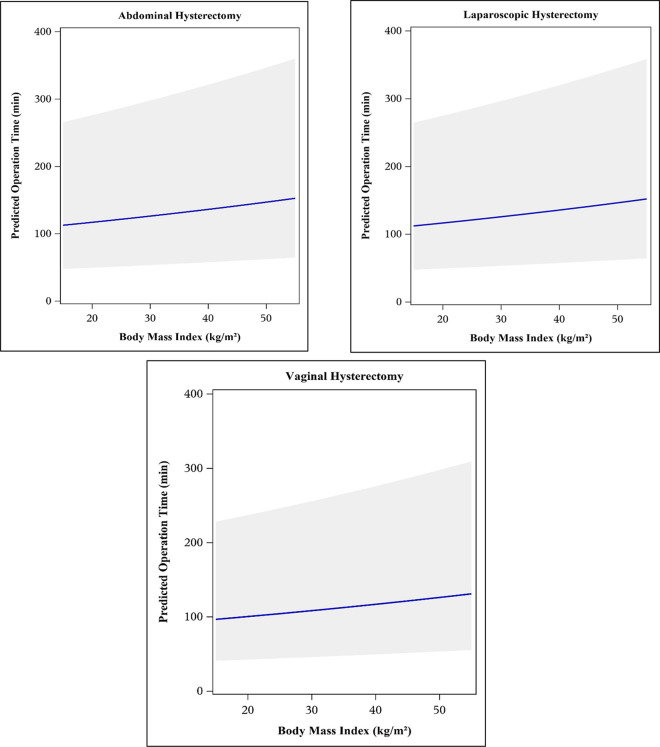
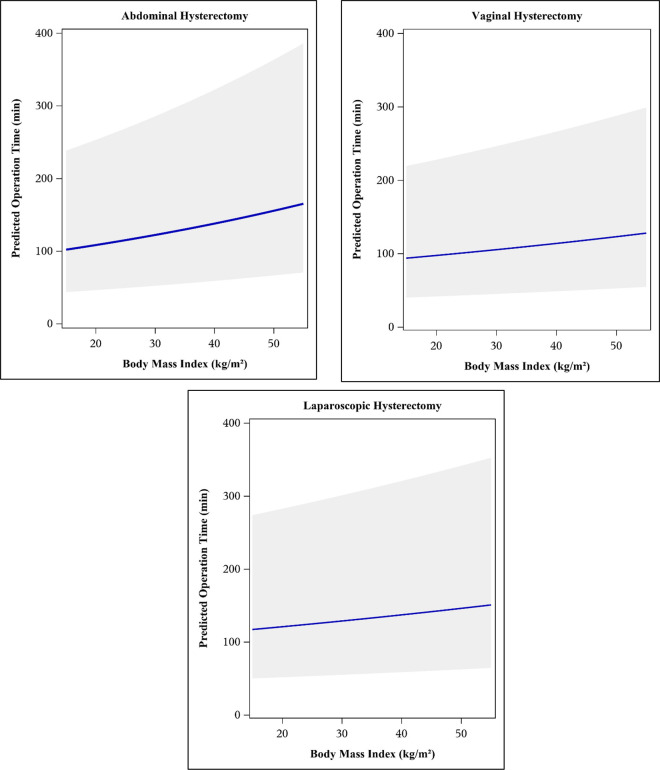
A “best” predictive model for log10OT, built using backwards elimination, included the following variables: BMI, age, smoking status, parity, history of pelvic surgery, subspecialty performing the surgery, and presence of endometriosis at time of surgery, and all two-way interactions between covariates (Model 1, Figure 2). The second, more restrictive model was built using ANOVA to determine η2 and included BMI, age, and route of hysterectomy (Model 2, Figure 3). Neither model accounted for the large percentage of the total variability in log10(OT) with an R2 value of 6.2% for Model 1 and 3.9% for Model 2.
Figure 2.
Predicted surgical operating time with 95% confidence bands using Model 1 (“best” predictive model from backwards elimination variable selection). Model includes the following predictor variables: body mass index (BMI), age, smoking status, parity, history of pelvic surgery, surgeon subspecialty, presence of endometriosis at time of surgery, and two-way interactions for BMI × (type of hysterectomy), (age [quintiles]) × (type of hysterectomy), (age [quintiles]) × subspecialty, (type of hysterectomy) × parity, (type of hysterectomy) × (prior pelvic surgery), (type of hysterectomy) × subspecialty, and (type of hysterectomy) × (presence of endometriosis).
Figure 3.
Predicted surgical operating time (new observation) with 95% confidence bands using Model 2 (reduced model, variables with partial η2 ≥ 10% of total). Model includes following predictor variables: body mass index (BMI), age (in quintiles), and hysterectomy type.
DISCUSSION
We demonstrated a positive correlation between BMI and OT across all types of hysterectomy, with OT for AH disproportionately increased with increasing BMI when compared with VH and LH. Additionally, increasing BMI, uterine weight >250 g, a history of abdominal surgery, and presence of endometriosis at time of surgery were all independently associated with increased OT. However, although we created two models to predict OT for benign hysterectomy, neither model was able to account for the large percentage of variability in OT. Therefore, a simple model to predict OT for benign hysterectomy using the NSQIP database is unlikely to be feasible.
Our findings are consistent with prior studies that demonstrate that increasing BMI is positively associated with OT in several procedures, including hysterectomy, especially when BMI ≥ 40.6,9,10,12,13 One prior study in which a “BMI impact factor” was created to estimate the effect that increasing BMI has on degree of resource utilization showed that BMI had a greater impact on hysterectomy than many other procedures, including, but not limited to, inguinal hernia, thyroidectomy, cholecystectomy, colorectal resection, prostatectomy, appendectomy, and mastectomy.7 Many of these studies incorporate BMI as a categorical variable defined by the World Health Organization (WHO) obesity classification. While analysis of OT by category of BMI has been valuable, it does not provide the ability to distinguish outcomes based on degrees of obesity, particularly above a BMI of 40, which is the cut-off value for severe obesity. From 1999 to 2000 through 2017–2020, the prevalence of adult obesity in the United States increased from 30.5% to 42.4% and the prevalence of severe obesity (BMI ≥40) increased from 4.7% to 9.2%.11 With a larger number of patients falling into these categories, a need to stratify outcomes by degree of obesity is growing increasingly critical for appropriate counseling.
Previous work evaluating the clinical impact of obesity in minimally invasive hysterectomy found that OT increased by class of obesity, with the longest OT noted in patients with class III obesity.10 Similarly, a study evaluating OT of postpartum tubal ligation demonstrated increased OT per each 1-point increase in BMI8 The results of both of these studies are consistent with our results, however no prior studies of which we are aware have examined the association between BMI as a continuous variable and OT across all routes of benign hysterectomy. Our study was able to demonstrate that increasing BMI has a differential effect on OT regardless of route. The increase in OT per unit increase in BMI for minimally invasive hysterectomy (LH and VH) is similar (0.6% increase and 0.8% increase, respectively), however the increase in OT for AH per unit increase in BMI is 1.2%.
Using data provided by the NSQIP dataset, our analysis shows that mean OT increases with increasing BMI. This increase persists across all three types of hysterectomy but is more notable among those undergoing AH. In addition to BMI, uterine weight, presence of endometriosis and prior abdominal surgery were also independently associated with longer OT, although these did not significantly affect the relationship between BMI and OT. Although we constructed both robust and simple predictive models using variables that can be extracted from the electronic health record, we were unable to identify a model that could accurately predict OT due to wide variation in OT not accounted for by the available variables. Our sample includes a subset of surgeries categorized by CPT codes that primarily indicate the type of hysterectomy performed but include concomitant procedures (CPT codes 58152, 58267, 58275, 58280, 58285, and 58293). These codes were included in our analysis as they include procedures often performed at time of uncomplicated hysterectomy, and as hospital coding administration often assign codes based on description within the operative dictation, in an effort to ensure inclusion of all benign hysterectomies for consideration. We did not include targeted data files for gyne(cology) reconstruction from the ACS-NSQIP data set in order to avoid inclusion of procedures with the primary purpose of reconstruction to avoid inflation of surgical times during hysterectomy. To evaluate whether inclusion of the above-listed CPT codes may have contributed to bias in our analysis, the above codes were excluded and the analysis was repeated (data not shown). The above CPT codes comprised 0.4% of our sample, and their exclusion minimally impacted the results with no significant change in mean OTs or model prediction, suggesting our analysis is not skewed by incorporation of codes that may include these combined procedures.
Obese patients are at higher risk of intraoperative and postoperative complications compared to normal weight or overweight peers.6,10 While an abdominal approach to hysterectomy in patients with increasing BMI may be preferentially considered based on factors such as surgeon preference, difficulty of procedure, increasing uterine size, anticipated adhesive disease, or endometriosis, our study demonstrates that this would disproportionately result in longer OT, which is associated with increased costs and adverse surgical outcomes.14–16 Therefore, especially in patients with increasing BMI, a minimally invasive approach should be pursued whenever possible. We suggest surgeons consider all the above factors when scheduling hysterectomies to better utilize OR and staff resources.
A predictive model for hysterectomy OT based on patient characteristics has yet to be created. Based on the large variability of OT in our models not explained by variables available in the NSQIP data set, we can conclude that other confounding variables are present and influencing OT. Such variables may be specific to surgical training, surgeon technique or preference, or hospital culture, and therefore may be unable to be captured in a large national dataset. To account for some of these variables, similar analyses could potentially be performed within high-volume hospitals, with a smaller number of surgeons but an increased level of granularity in the available perioperative data. Additionally, future research should continue to focus on minimizing costs and improving clinical outcomes, particularly in surgical environments, in the setting of a population being affected by increasing rates of obesity.
A strength of this study is the use of a national data set including a large number of patients undergoing benign hysterectomy (N = 117,691) as well as the number of preoperative variables (16) that were able to be analyzed with regard to operative time. Additionally, given the regional variability and number of sites contributing data to the NSQIP database, the results of our analysis can be considered generalizable to the general population. We were able to stratify by route of surgery and use BMI as a continuous variable, which allowed the predictive models to better fit real-life clinical situations, rather than typically used cutoffs for BMI with the WHO classification, that does not account for the increasing number of patients with class III obesity, which has no upper limit (i.e., BMI 40 kg/m2 and beyond). Our study should also be interpreted in the context of its limitations. Our analysis was restricted to the variables within the NSQIP data set, introducing the possibility that there are other attainable nontracked variables contributing significantly to the variability in OT. In addition, the ACS-NSQIP relies on voluntary participation, and may not be representative of all hospital settings (eg, rural or community hospitals). Tertiary care and/or academic medical centers serve as a referral center for patients of increasing medical complexity, such as increasing BMI, which may be contributing to the variability and subsequent inability to capture the additional factors at play.
Previous studies have demonstrated that surgeons can accurately predict their OT within 14 minutes of their historic time.17 While we could not develop a simple model for clinical implementation, knowledge of the variability in OT based on route of surgery may aid the individual surgeon in accounting for changes in OT based on patient’s BMI, among other factors.
CONCLUSION
BMI and operative time for benign hysterectomy are positively correlated across all routes of surgery. Increasing BMI differentially affects the operative time in abdominal hysterectomy, greater than laparoscopic and vaginal hysterectomy. However, operative time for hysterectomy is highly variable, and its estimation based on patient characteristics and other preoperative variables is difficult to reliably predict using this national dataset.
Footnotes
Presented at the 48th Annual Scientific Meeting, Society of Gynecologic Surgeons, March 27–30, 2022, San Antonio, Texas.
Funding sources: none.
Disclosure: none.
Conflict of interests: none.
Contributor Information
A. Caroline Cochrane, Wake Forest University Health Sciences, Department of Obstetrics and Gynecology, Winston-Salem, NC. (Drs. Cochrane and Moulder).
Evan Olson, The Iowa Clinic, Department of Obstetrics and Gynecology, West Des Moines, IA. (Dr. Olson).
Tim Craven, Wake Forest School of Medicine, Department of Biostatistics and Data Science, Winston-Salem, NC. (Dr. Craven).
Erica F. Robinson, University of South Carolina School of Medicine-Greenville, Prisma Health Upstate, Department of Obstetrics and Gynecology, Greenville, SC. (Dr. Robinson).
Janelle K. Moulder, Wake Forest University Health Sciences, Department of Obstetrics and Gynecology, Winston-Salem, NC. (Drs. Cochrane and Moulder).
References:
- 1.Su M-C, Lai S-C, Wang P-C, Hsieh Y-Z, Lin S-C. A SOMO-based approach to the operating room scheduling problem. Expert Syst Appl. 2011;38(12):15447–15454. [Google Scholar]
- 2.Levine WC, Dunn PF. Optimizing operating room scheduling. Anesthesiol Clin. 2015;33(4):697–711. [DOI] [PubMed] [Google Scholar]
- 3.Joustra P, Meester R, van Ophem H. Can statisticians beat surgeons at the planning of operations? Empir Econ. 2013;44(3):1697–1718. [Google Scholar]
- 4.Eijkemans MJ, van Houdenhoven M, Nguyen T, Boersma E, Steyerberg EW, Kazemier G. Predicting the unpredictable: a new prediction model for operating room times using individual characteristics and the surgeon’s estimate. Anesthesiology. 2010;112(1):41–49. [DOI] [PubMed] [Google Scholar]
- 5.Driessen SR, Sandberg EM, la Chapelle CF, Twijnstra AR, Rhemrev JP, Jansen FW. Case-mix variables and predictors for outcomes of laparoscopic hysterectomy: a systematic review. J Minim Invasive Gynecol. 2016;23(3):317–330. [DOI] [PubMed] [Google Scholar]
- 6.Siedhoff MT, Carey ET, Findley AD, Riggins LE, Garrett JM, Steege JF. Effect of extreme obesity on outcomes in laparoscopic hysterectomy. J Minim Invasive Gynecol. 2012;19(6):701–707. [DOI] [PubMed] [Google Scholar]
- 7.Burneikis D, Morris-Stiff G, Chalikonda S. Time attributable to obesity in surgery: a multi-specialty report on day-of-surgery resource utilization from 189,264 cases. World J Surg. 2018;42(10):3125–3133. [DOI] [PubMed] [Google Scholar]
- 8.Deshpande NA, Labora A, Sammel MD, Schreiber CA, Sonalkar S. Relationship between body mass index and operative time in women receiving immediate postpartum tubal ligation. Contraception. 2019;100(2):106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawn MT, Bian J, Leeth RR, et al. Impact of obesity on resource utilization for general surgical procedures. Ann Surg. 2005;241(5):821–826; discussion 826–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Neveu M, AlAshqar A, Kohn J, Tambovtseva A, Wang K, Borahay M. Impact of obesity on clinical and financial outcomes of minimally invasive hysterectomy for benign conditions. J Obstet Gynaecol Can. 2022;44(9):953–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020(360):1–8. [PubMed] [Google Scholar]
- 12.Wang JL, Gadinsky NE, Yeager AM, Lyman SL, Westrich GH. The increased utilization of operating room time in patients with increased BMI during primary total hip arthroplasty. J Arthroplasty. 2013;28(4):680–683. [DOI] [PubMed] [Google Scholar]
- 13.Shah DK, Van Voorhis BJ, Vitonis AF, Missmer SA. Association between body mass index, uterine size, and operative morbidity in women undergoing minimally invasive hysterectomy. J Minim Invasive Gynecol. 2016;23(7):1113–1122. [DOI] [PubMed] [Google Scholar]
- 14.Catanzarite T, Saha S, Pilecki MA, Kim JY, Milad MP. Longer operative time during benign laparoscopic and robotic hysterectomy is associated with increased 30-day perioperative complications. J Minim Invasive Gynecol. 2015;22(6):1049–1058. [DOI] [PubMed] [Google Scholar]
- 15.AlAshqar A, Goktepe ME, Kilic GS, Borahay MA. Predictors of the cost of hysterectomy for benign indications. J Gynecol Obstet Hum Reprod. 2021;50(2):101936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothstein DH, Raval MV. Operating room efficiency. Semin Pediatr Surg. 2018;27(2):79–85. [DOI] [PubMed] [Google Scholar]
- 17.Cassling C, Shay R, Strassle PD, et al. Use of historic surgical times to predict duration of hysterectomy: stratifying by uterine weight. J Minim Invasive Gynecol. 2019;26(7):1327–1333. [DOI] [PubMed] [Google Scholar]