Abstract
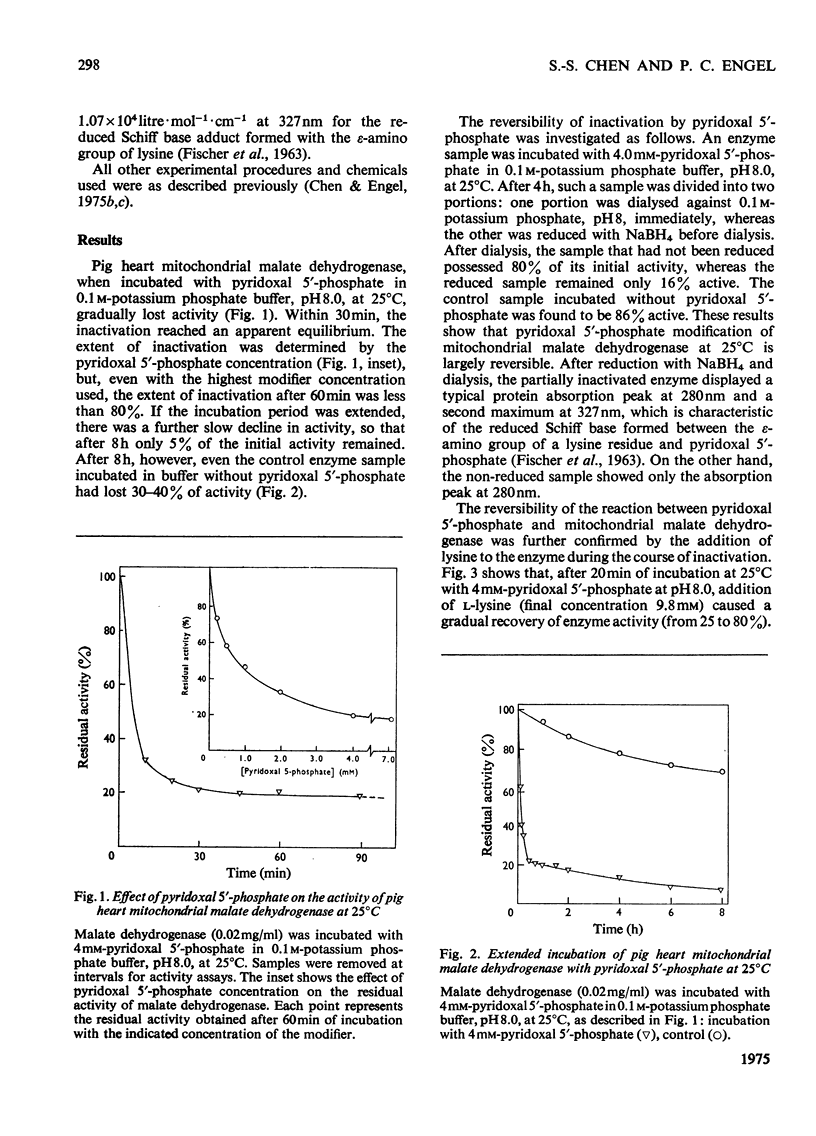
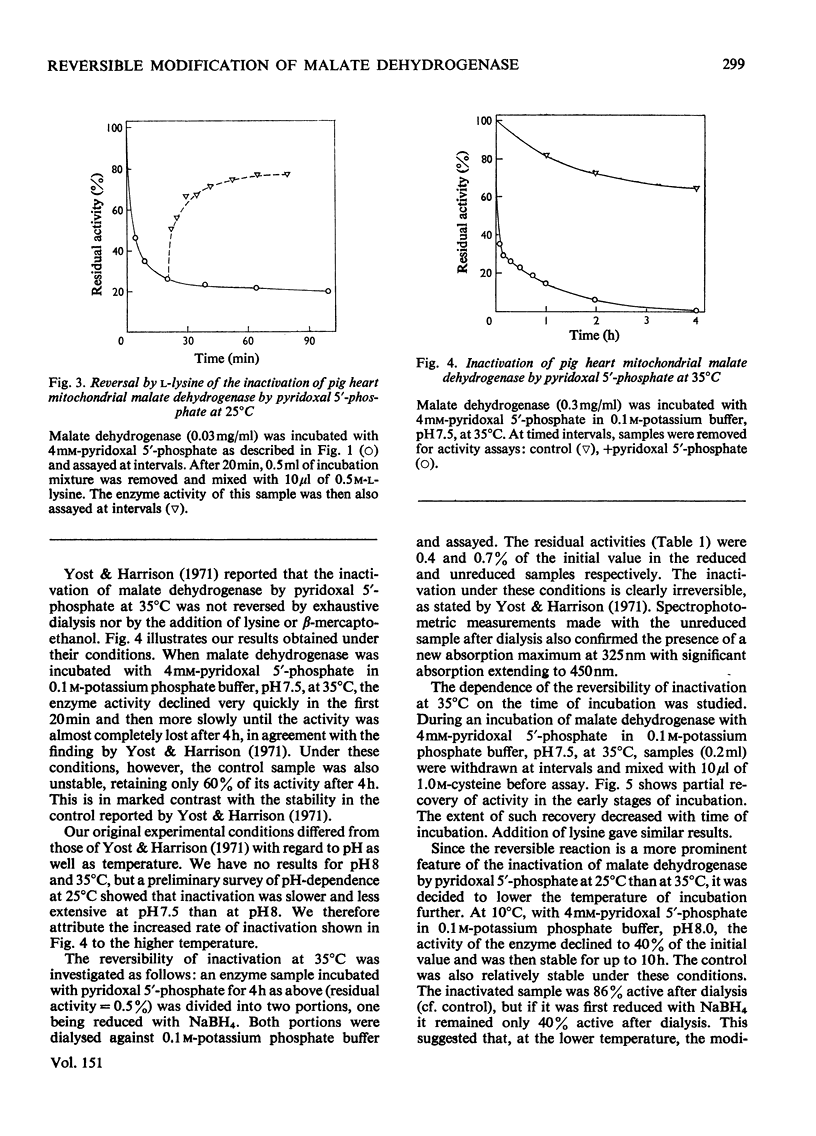
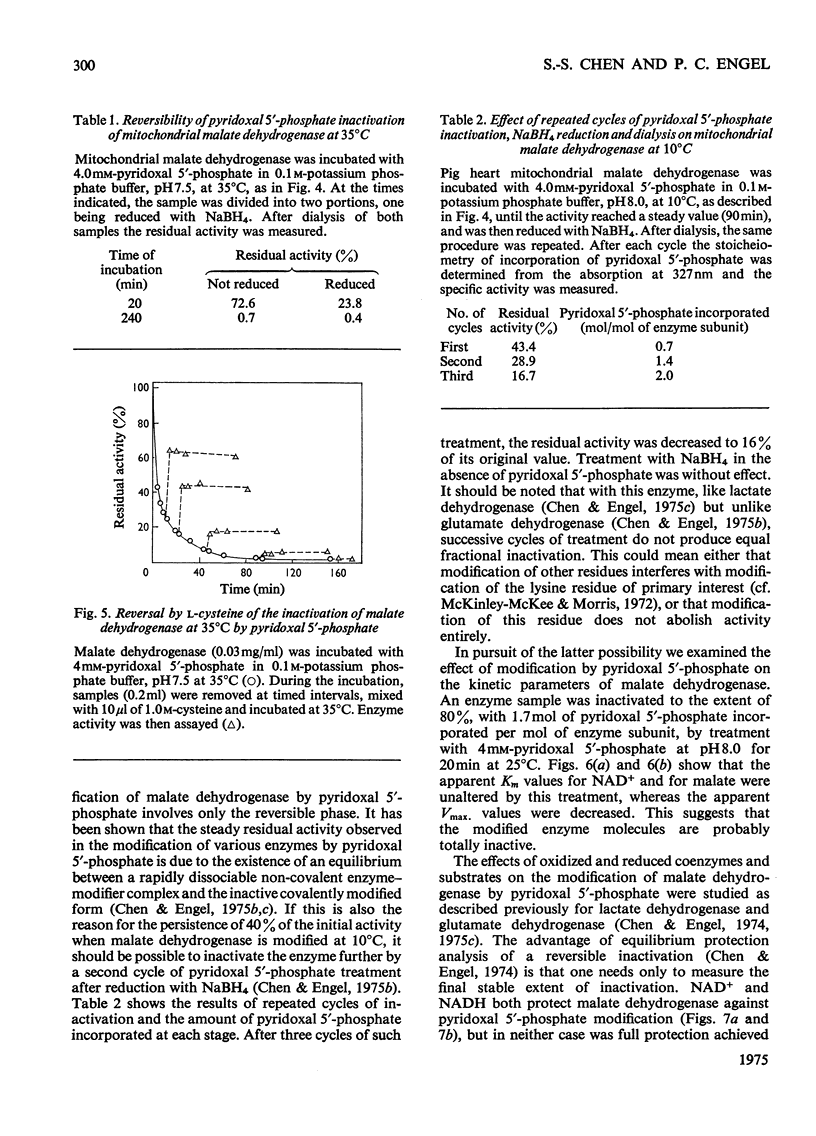
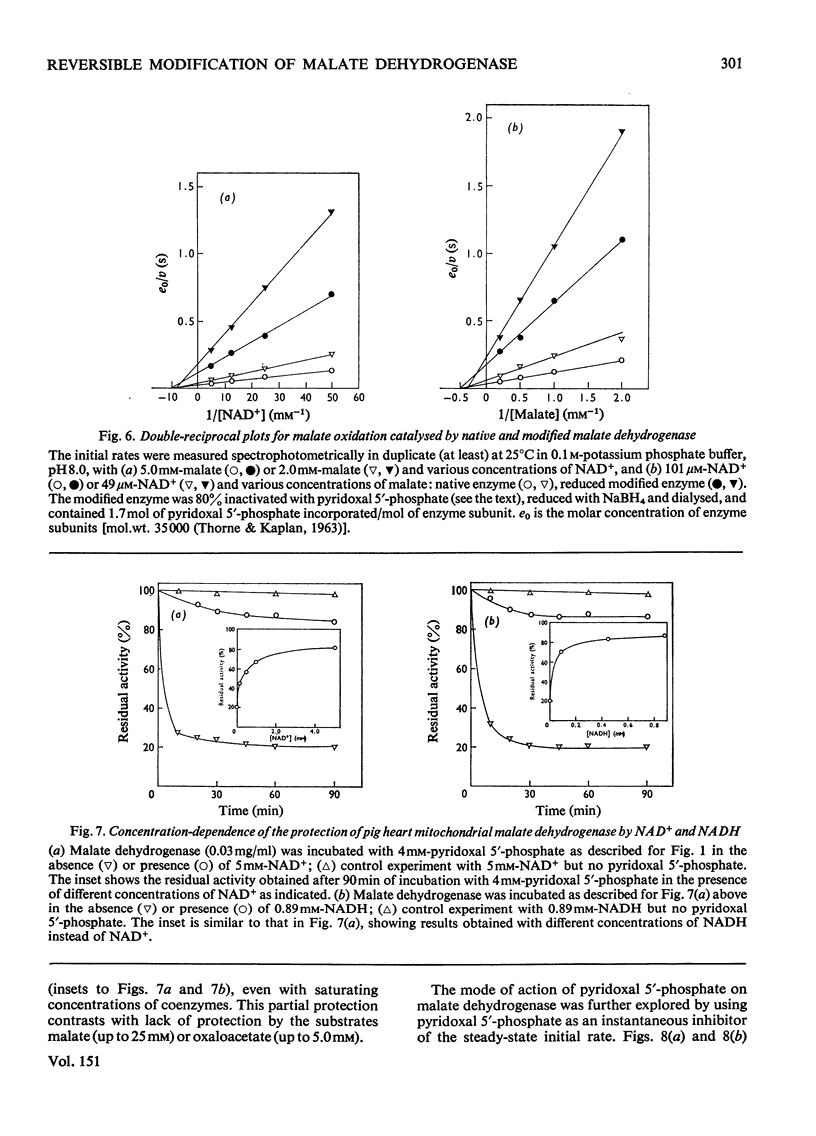
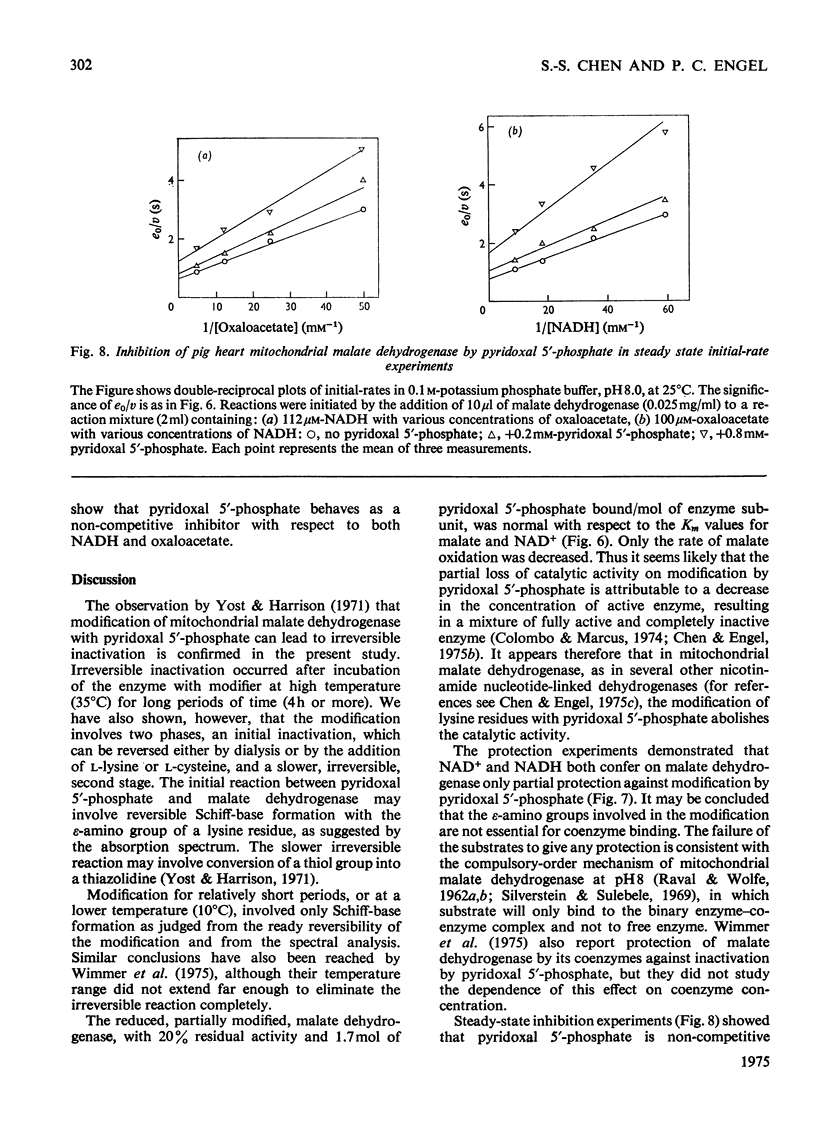
1. Pig heart mitochondrial malate dehydrogenase incubated with pyridoxal 5'-phosphate at pH 8.0 and 25 degrees C gradually loses activity. Such inactivation can be largely reversed by dialysis or by addition of L-lysine or L-cysteine, and can be made permanent by NaBH4 reduction. 2. Modification of malate dehydrogenase with pyridoxal 5'-phosphate at 35 degrees C involves two phases, an initial inactivation which is reversible and a slower irreversible second stage. 3. The initial reaction between pyridoxal 5'-phosphate and malate dehydrogenase appears to involve reversible formation of a Schiff base with the epsilon-amino group of a lysine residue. 4. Inactivation of malate dehydrogenase by pyridoxal 5'-phosphate at 10 degrees C involves only the reversible reaction. 5. At 10 degrees C repeated cycles of treatment with pyridoxal 5'-phosphate and NaBH4 reduction lead to a stepwise decline in residual activity. 6. Apparent Km values for malate and NAD+ are unaltered in the partially inactivated enzyme. 7. NAD+ and NADH give only partial protection against pyridoxal 5'-phosphate inactivation. Substrates give no effect.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderton B. H. Identification of an essential, reactive histidine in pig heart mitochondrial malate dehydrogenase. Eur J Biochem. 1970 Sep;15(3):562–567. doi: 10.1111/j.1432-1033.1970.tb01041.x. [DOI] [PubMed] [Google Scholar]
- Anderton B. H., Rabin B. R. Alkylation studies on a reactive histidine in pig heart malate dehydrogenase. Eur J Biochem. 1970 Sep;15(3):568–573. doi: 10.1111/j.1432-1033.1970.tb01042.x. [DOI] [PubMed] [Google Scholar]
- Cassman M. Beef heart malic dehydrogenases. VI. A pH-dependent transition observed in the presence of reduced diphosphopyridine nucleotide. J Biol Chem. 1967 May 10;242(9):2013–2020. [PubMed] [Google Scholar]
- Chen S. S., Engel P. C. Inactivation of nicotinamide--adenine dinucleotide-linked dehydrogenases by pyridoxal 5'-phosphate. Biochem Soc Trans. 1975;3(1):80–82. doi: 10.1042/bst0030080. [DOI] [PubMed] [Google Scholar]
- Chen S. S., Engel P. C. Modification of pig M4 lactate dehydrogenase by pyridoxal 5'-phosphate. Demonstration of an essential lysine residue. Biochem J. 1975 Jul;149(1):107–113. doi: 10.1042/bj1490107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. S., Engel P. C. The equilibrium position of the reaction of bovine liver glutamate dehydrogenase with pyridoxal5'-phosphate. A demonstration that covalent modification with this reagent completely abolishes catalytic activity. Biochem J. 1975 May;147(2):351–358. doi: 10.1042/bj1470351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Engel P. C. Protection of glutamate dehydrogenase by nicotinamide-adenine dinucleotide against reversible inactivation by pyridoxal 5'-phosphate as a sensitive indicator of conformational change induced by substrates and substrate analogues. Biochem J. 1974 Dec;143(3):569–574. doi: 10.1042/bj1430569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G., Marcus F. Modification of fructose-1,6-diphosphatase with pyridoxal 5'-phosphate. Evidence for the participation of lysyl residues at the active site. Biochemistry. 1974 Jul 16;13(15):3085–3091. doi: 10.1021/bi00712a014. [DOI] [PubMed] [Google Scholar]
- DALZIEL K. Kinetic studies of liver alcohol dehydrogenase. Biochem J. 1962 Aug;84:244–254. doi: 10.1042/bj0840244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook J. J., Lodola A., Illsley N. P. Histidine residues and the enzyme activity of pig heart supernatant malate dehydrogenase. Biochem J. 1974 Jun;139(3):797–800. doi: 10.1042/bj1390797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley-McKee J. S., Morris D. L. The lysines in liver alcohol dehydrogenase. Chemical modification with pyridoxal 5'-phosphate and methyl picolinimidate. Eur J Biochem. 1972 Jun 23;28(1):1–11. doi: 10.1111/j.1432-1033.1972.tb01877.x. [DOI] [PubMed] [Google Scholar]
- Munkres K. D. Allosteric and multifunctional properties of Neurospora mitochondrial malate dehydrogenase. Biochim Biophys Acta. 1970 Nov 11;220(2):149–160. doi: 10.1016/0005-2744(70)90002-1. [DOI] [PubMed] [Google Scholar]
- RAVAL D. N., WOLFE R. G. Malic dehydrogenase. II. Kinetic studies of the reaction mechanism. Biochemistry. 1962 Mar;1:263–269. doi: 10.1021/bi00908a012. [DOI] [PubMed] [Google Scholar]
- RAVAL D. N., WOLFE R. G. Malic dehydrogenase. IV. pH dependence of the kinetic parametrs. Biochemistry. 1962 Nov;1:1118–1123. doi: 10.1021/bi00912a024. [DOI] [PubMed] [Google Scholar]
- Silverstein E., Sulebele G. Catalytic mechanism of pig heart mitochondrial malate dehydrogenase studied by kinetics at equilibrium. Biochemistry. 1969 Jun;8(6):2543–2550. doi: 10.1021/bi00834a042. [DOI] [PubMed] [Google Scholar]
- THORNE C. J., KAPLAN N. O. Physicochemical properties of pig and horse heart mitochondrial malate dehydrogenase. J Biol Chem. 1963 May;238:1861–1868. [PubMed] [Google Scholar]
- THORNE C. J. Properties of mitochondrial malate dehydrogenases. Biochim Biophys Acta. 1962 Jun 4;59:624–633. doi: 10.1016/0006-3002(62)90642-x. [DOI] [PubMed] [Google Scholar]
- WINER A. D., SCHWERT G. W. Lactic dehydrogenase. IV. The influence of pH on the kinetics of the reaction. J Biol Chem. 1958 Apr;231(2):1065–1083. [PubMed] [Google Scholar]
- WINER A. D., SCHWERT G. W. Lactic dehydrogenase. VII. Fluorescence spectra of ternary complexes of lactic dehydrogenase, reduced diphosphopyridine nucleotide, and carboxylic acids. J Biol Chem. 1959 May;234(5):1155–1161. [PubMed] [Google Scholar]
- Wimmer M. J., Mo T., Sawyers D. L., Harrison J. H. Biphasic inactivation of procine heart mitochondrial malate dehydrogenase by pyridoxal 5'-phosphate. J Biol Chem. 1975 Jan 25;250(2):710–715. [PubMed] [Google Scholar]
- Yost F. J., Jr, Harrison J. H. Interaction of pyridoxal 5' phosphate and malate dehydrogenase. Biochem Biophys Res Commun. 1971 Feb 5;42(3):516–522. doi: 10.1016/0006-291x(71)90401-3. [DOI] [PubMed] [Google Scholar]


