Abstract
A previous study [Mole et al. (1971) Biochem. J. 124, 301-318] showed several differences in sequence between the variable (V) regions of rabbit immunoglobulin Aa1 and Aa3 heavy chains. The inheritance of one such difference has been followed in a family of 38 rabbits by a radioautographic peptide-'map' technique and is shown to segregate in a Mendelian fashion. This clearly demonstrates the presence of a genetic marker in the rabbit heavy-chain V region, although the finding that Aa2 and Aa3 heavy chains have identity of sequence in the region studied obscures the relationship of this genetic marker to the a locus.
Full text
PDF



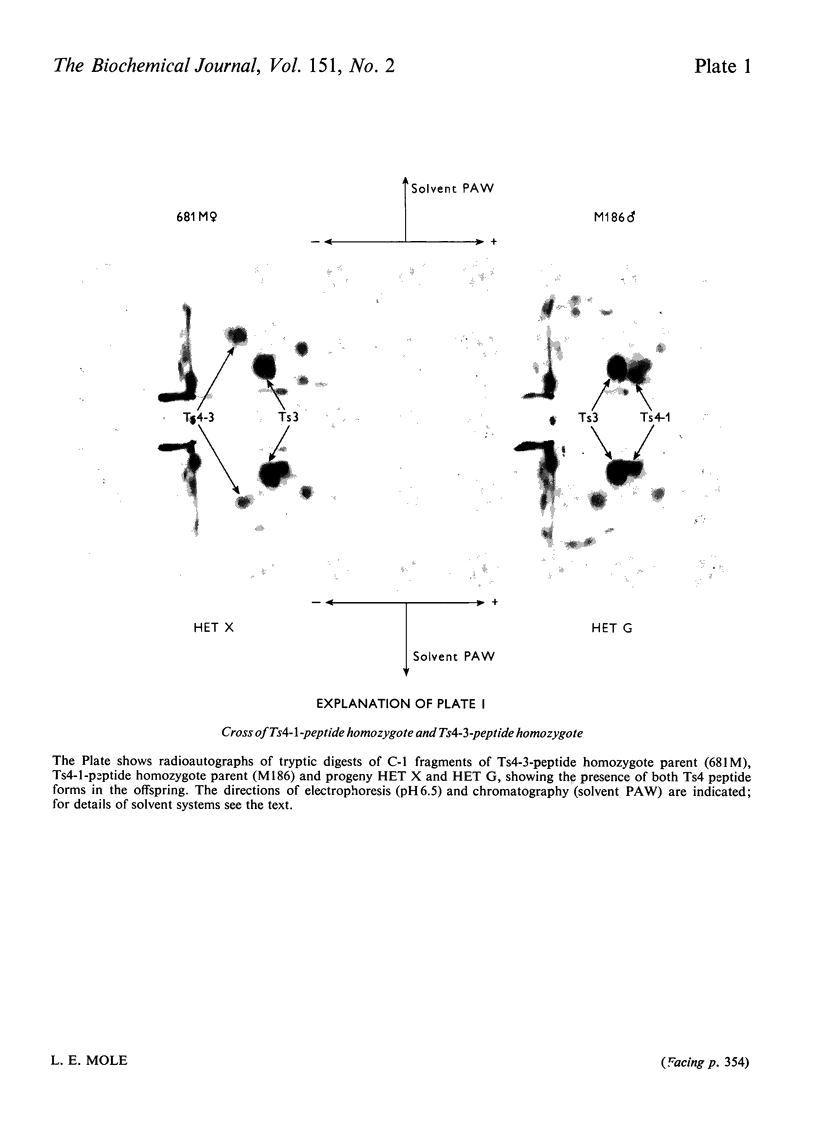
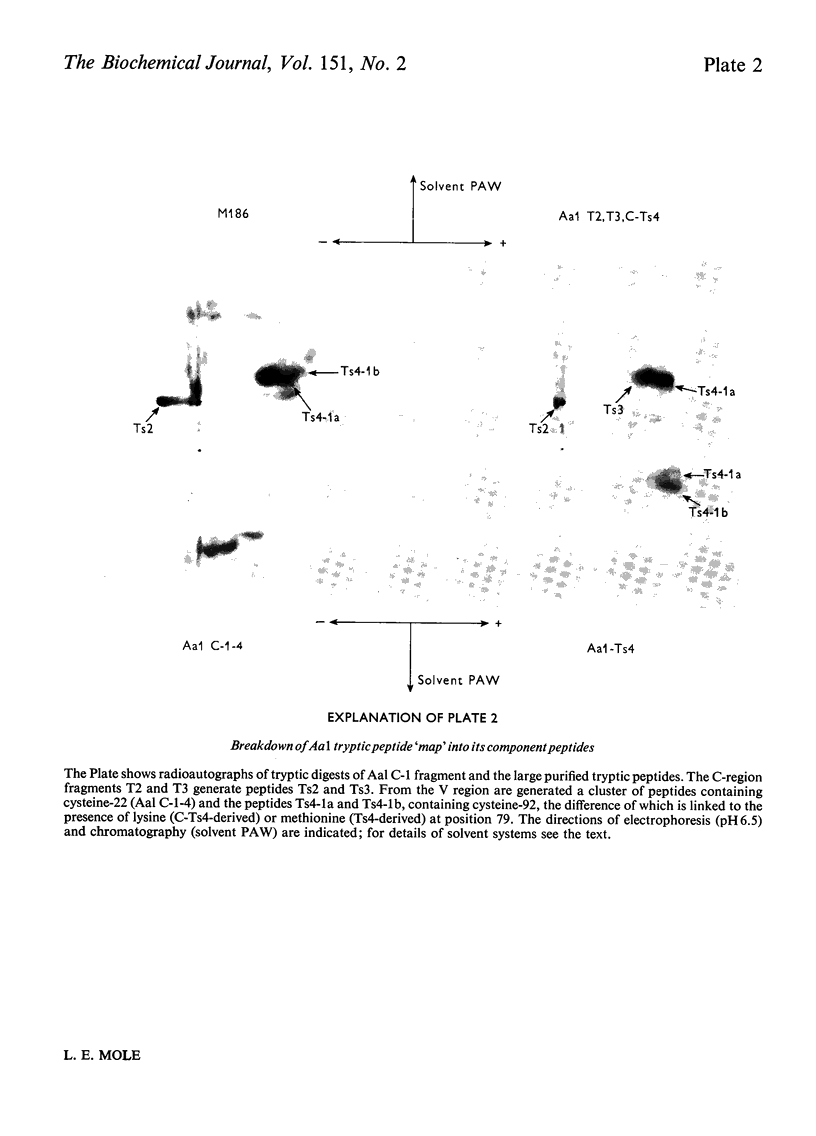
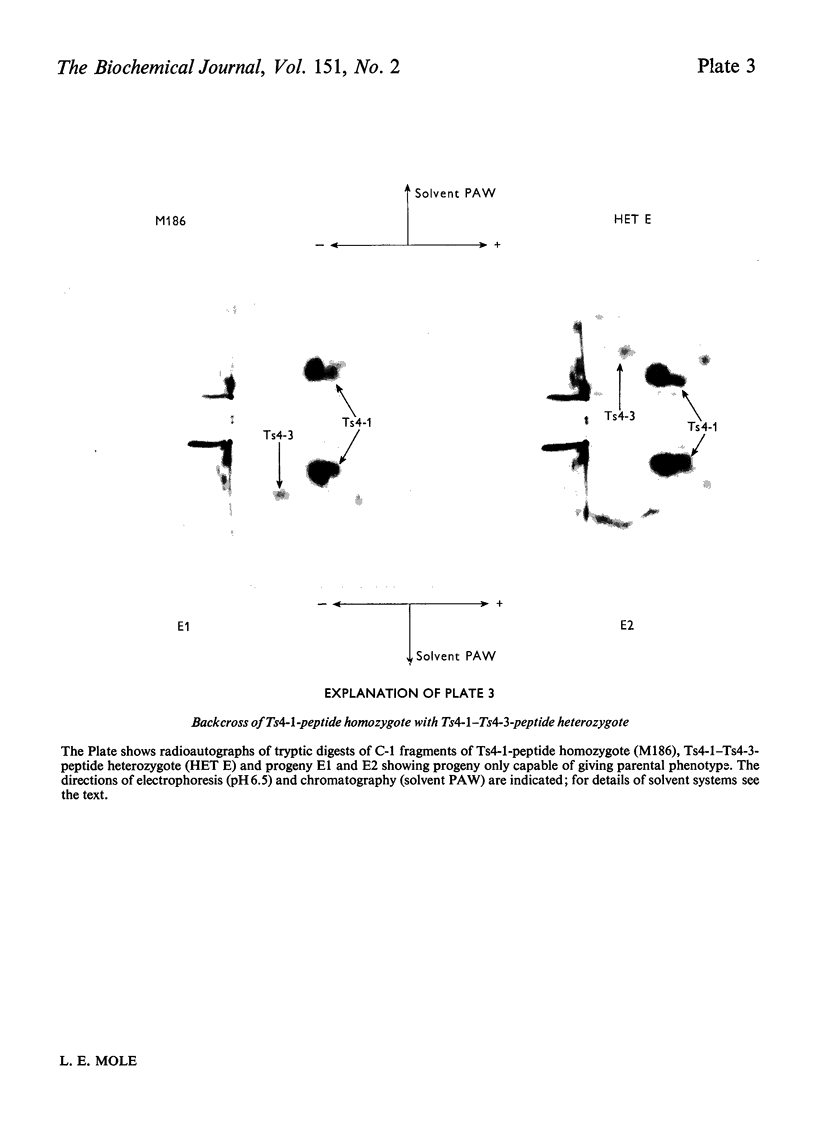
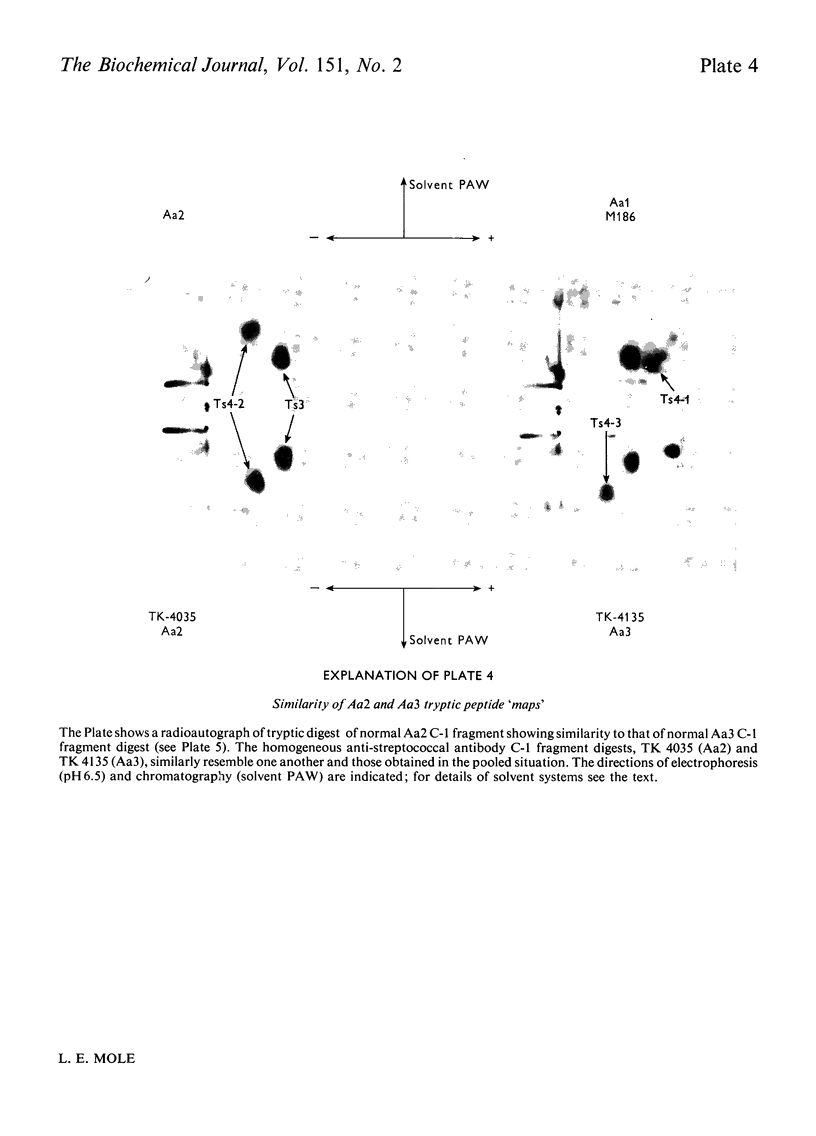
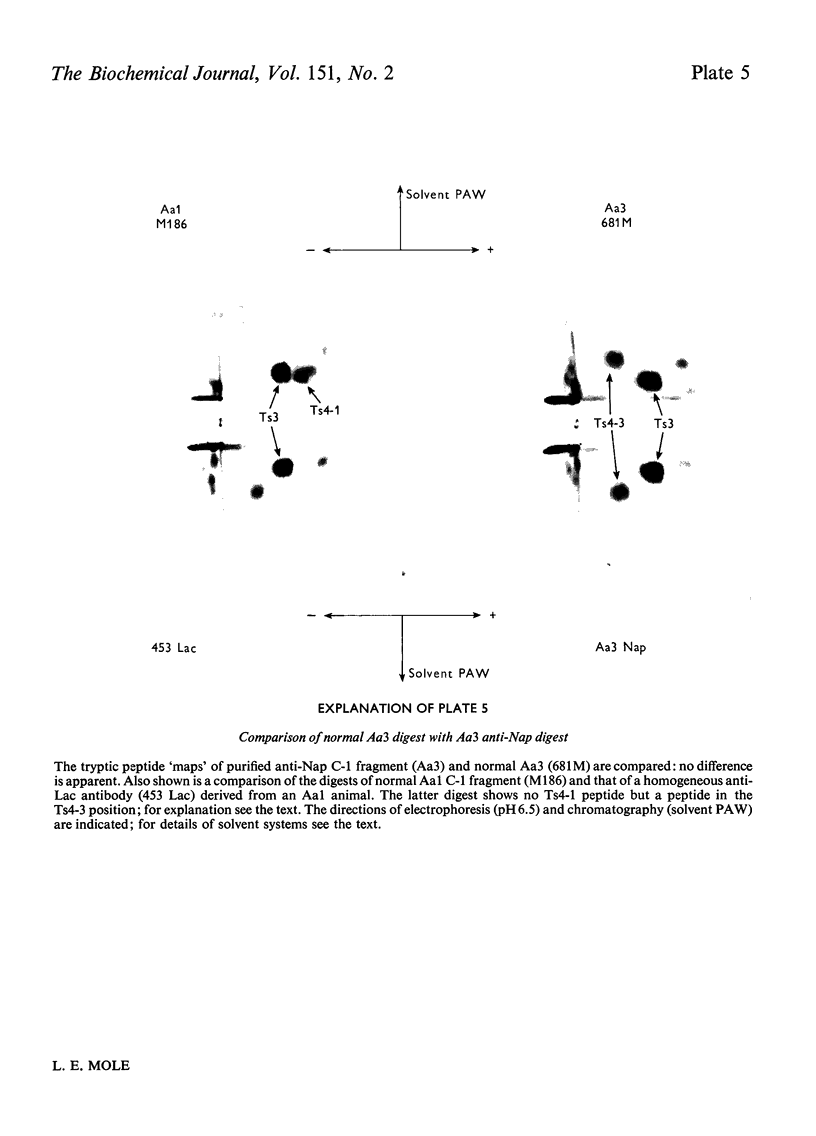

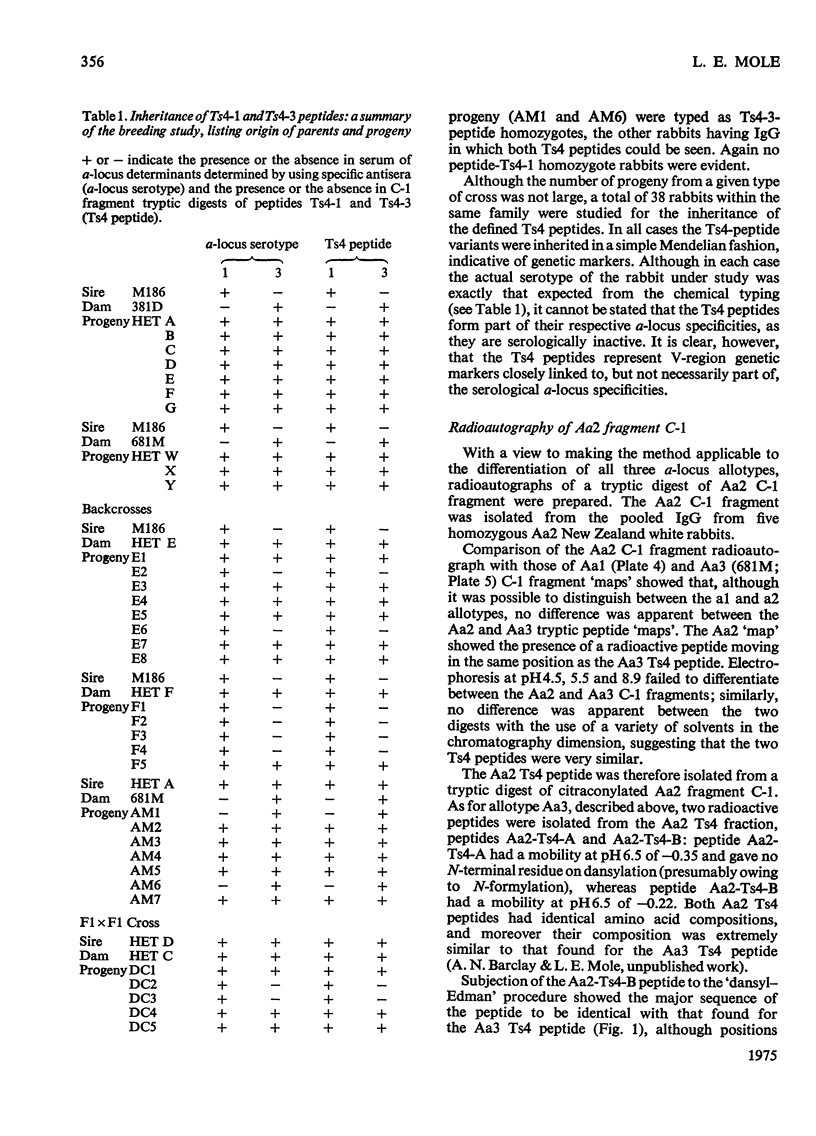



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodmer W. F. New genetic model for allelism at histocompatibility and other complex loci: polymorphism for control of gene expression. Transplant Proc. 1973 Dec;5(4):1471–1475. [PubMed] [Google Scholar]
- Edelman G. M., Gottlieb P. D. A genetic marker in the variable region of light chains of mouse immunoglobulins. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1192–1199. doi: 10.1073/pnas.67.3.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fleischman J. B. A partial amino acid sequence in the heavy chain of a rabbit antibody to group C streptococcal carbohydrate. Biochemistry. 1971 Jul 6;10(14):2753–2761. doi: 10.1021/bi00790a016. [DOI] [PubMed] [Google Scholar]
- Jaton J. C., Braun D. G., Strosberg A. D., Haber E., Morris J. E. Restricted rabbit antibodies: amino acid sequences of rabbit H chains of allotype a1, a2, and a3 in the region 80 to 94. J Immunol. 1973 Dec;111(6):1838–1843. [PubMed] [Google Scholar]
- Jaton J. C., Haimovich J. Partial amino acid sequence in the N-terminal region of an anti-pneumococcal immunoglobulin heavy chain of allotype alpha2. Biochem J. 1974 Apr;139(1):281–283. doi: 10.1042/bj1390281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B. S., Dray S. Identification and genetic control of allotypic specificities on two variable region subgroups of rabbit immunoglobulin heavy chains. Eur J Immunol. 1972 Dec;2(6):509–514. doi: 10.1002/eji.1830020608. [DOI] [PubMed] [Google Scholar]
- Kindt T. J., Klapper D. G., Waterfield M. D. An idiotypic cross-reaction between allotype a3 and allotype a negative rabbit antibodies to streptococcal carbohydrate. J Exp Med. 1973 Mar 1;137(3):636–648. doi: 10.1084/jem.137.3.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt T. J., Seide R. K., Lackland H., Thunberg A. L. Serologic identity of the b4 allotypic determinants present on homogeneous rabbit light chains with different N-terminal amino acid sequences. J Immunol. 1972 Oct;109(4):735–741. [PubMed] [Google Scholar]
- Kindt T. J., Seide R. K., Tack B. F., Todd C. W. Diverse expression of group a allotype specificities on the heavy chains of homogeneous rabbit antibodies. J Exp Med. 1973 Jul 1;138(1):33–43. doi: 10.1084/jem.138.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland M. E., Englberger F. M., Shapanka R. Location of amino acid differences in the subunits of three rabbit antibodies. Biochemistry. 1966 Feb;5(2):641–651. doi: 10.1021/bi00866a034. [DOI] [PubMed] [Google Scholar]
- Krause R. M. The search for antibodies with molecular uniformity. Adv Immunol. 1970;12:1–56. doi: 10.1016/s0065-2776(08)60167-4. [DOI] [PubMed] [Google Scholar]
- Mole L. E., Jackson S. A., Porter R. R., Wilkinson J. M. Allotypically related sequences in the Fd fragment of rabbit immunoglobulin heavy chains. Biochem J. 1971 Sep;124(2):301–318. doi: 10.1042/bj1240301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell I. J., Frangione B., Porter R. R. The disulphide bonds of the heavy chain of rabbit immunoglobulin G. Biochem J. 1970 Jan;116(2):261–268. doi: 10.1042/bj1160261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OUDIN J. Allotypy of rabbit serum proteins. I. Immuno-chemical analysis leading to the individualization of seven main allotypes. J Exp Med. 1960 Jul 1;112:107–124. doi: 10.1084/jem.112.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Prahl J. W., Porter R. R. Allotype-related sequence variation of the heavy chain of rabbit immunoglobulin G. Biochem J. 1968 May;107(6):753–763. doi: 10.1042/bj1070753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahl J. W., Tack B. F., Todd C. W. Rabbit immunoglobulin lacking group a allotypic specificities. 3. Variable region structure and genetic control. Biochemistry. 1973 Dec 4;12(25):5181–5186. doi: 10.1021/bi00749a026. [DOI] [PubMed] [Google Scholar]
- Pratt D. M., Mole L. E. Sequence studies on the constant region of the Fd sections of rabbit immunoglobulin G of different allotype. Biochem J. 1975 Nov;151(2):337–349. doi: 10.1042/bj1510337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press E. M., Piggot P. J., Porter R. R. The N- and c-terminal amino acid sequences of the heavy chain from a pathological human immunoglobulin IgG. Biochem J. 1966 May;99(2):356–366. doi: 10.1042/bj0990356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivat L., Gilbert D., Ropartz C. Immunoglobulin allotypic specificities in mixed leucocyte cultures. Immunology. 1973 Jun;24(6):1041–1049. [PMC free article] [PubMed] [Google Scholar]
- Sargent J. R., Vadlamudi B. P. Electrophoresis of peptides on thin layers of silica gel. Anal Biochem. 1968 Oct 24;25(1):583–587. doi: 10.1016/0003-2697(68)90137-1. [DOI] [PubMed] [Google Scholar]
- Sie H. G., Hablanian A. Depletion of glycogen synthetase and increase of glucose 6-phosphate dehydrogenase in livers of ethionine-treated mice. Biochem J. 1965 Oct;97(1):32–36. doi: 10.1042/bj0970032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strosberg A. D., Hamers-Casterman C., Van der Loo W., Hamers R. A rabbit with the allotypic phenotype: ala2a3 b4b5b6. J Immunol. 1974 Oct;113(4):1313–1318. [PubMed] [Google Scholar]
- Todd C. W. Genetic control of H chain biosynthesis in the rabbit. Fed Proc. 1972 Jan-Feb;31(1):188–192. [PubMed] [Google Scholar]
- Wilkinson J. M. Variation in the N-terminal sequence of heavy chains of immunoglobulin G from rabbits of different allotype. Biochem J. 1969 Apr;112(2):173–185. doi: 10.1042/bj1120173. [DOI] [PMC free article] [PubMed] [Google Scholar]