Abstract
Introduction:
Systemic cystic angiomatosis is an exceedingly rare condition characterized by widespread cystic vascular lesions involving multiple organs. Its clinical presentation can be non-specific, often leading to diagnostic challenges. This report discusses the case of a 72-year-old female with a long-standing history of diabetes mellitus who presented with non-specific symptoms, ultimately diagnosed with systemic cystic angiomatosis after an initial misdiagnosis of vascular neoplasia. The role of advanced imaging techniques and a multidisciplinary, individualized management approach is emphasized.
Case Report:
A 72-year-old female with long-standing diabetes presented with non-specific symptoms, including fatigue, fever, and rashes on the upper limbs. Initial physical examination revealed anemia and severe thrombocytopenia with a leukoerythroblastic blood picture. Despite unremarkable initial imaging studies, a bone marrow biopsy suggested vascular neoplasia. Further evaluation with a positron emission tomography (PET) scan revealed multiple non-avid lytic skeletal areas and cystic liver lesions, leading to a diagnosis of systemic cystic angiomatosis. A conservative management approach with danazol and eltrombopag was adopted. The patient later developed an acute-on-chronic subdural hematoma, a severe complication of the condition.
Conclusion:
This case highlights the diagnostic complexity and the necessity for individualized management strategies in systemic cystic angiomatosis. It underscores the importance of considering rare diagnoses when faced with non-specific symptoms and atypical laboratory findings. Advanced imaging techniques, such as PET scans, and a multidisciplinary approach are crucial for accurate diagnosis and effective management of this rare condition.
Keywords: Systemic cystic angiomatosis, vascular neoplasia, advanced imaging, positron emission tomography scan, multidisciplinary approach, individualized management, rare diagnoses, danazol, eltrombopag, subdural hematoma
Learning Point of the Article:
This case underscores the importance of considering rare diagnoses, employing advanced imaging techniques, and utilizing a multidisciplinary, personalized treatment approach to ensure accurate diagnosis and effective management of systemic cystic angiomatosis.
Introduction
Systemic cystic angiomatosis is an extremely rare condition characterized by the proliferation of cystic blood vessels affecting various organs. Due to its rarity and non-specific presentation, it often poses significant diagnostic challenges. This case report documents a unique presentation of systemic cystic angiomatosis in an elderly female initially misdiagnosed as having a vascular neoplasm, highlighting the importance of comprehensive diagnostic workup and multidisciplinary management.
Case Report
A 72-year-old female with a history of long-standing diabetes presented with fatigue, fever, and rashes on the upper limbs for 1 week. She denied any history of weight loss or loss of appetite. On physical examination, she exhibited no signs of distress, cyanosis, clubbing, raised jugular venous pressure, pedal edema, or lymphadenopathy. Her chest wall and spine were non-tender on palpation, and examinations of the abdominal, cardiovascular, pulmonary, and neurological systems were unremarkable.
Laboratory investigations showed anemia (Hemoglobin: 6.4 g/dL) and severe thrombocytopenia (platelet count: 6000/μL) with a leukoerythroblastic blood picture on peripheral smear. Computed tomography (CT) scans of the abdomen and chest were unrevealing. Bone marrow aspiration and biopsy showed marrow spaces completely replaced by proliferating thin-walled vessels. In certain areas, clusters of plump spindle cells, likely endothelial cells, were observed. Immunohistochemical staining demonstrated that both the vessels and the spindle cells were strongly positive for CD31 and CD34, all suggestive of vascular neoplasia (Fig. 1). However, further positron emission tomography (PET) scan investigation revealed multiple non-avid lytic areas involving almost the entire visualized skeleton, suggestive of skeletal angiomatosis, and non-avid cystic lesions in the liver indicative of visceral angiomatosis, leading to the diagnosis of systemic cystic angiomatosis (Fig. 2-5).
Figure 1.
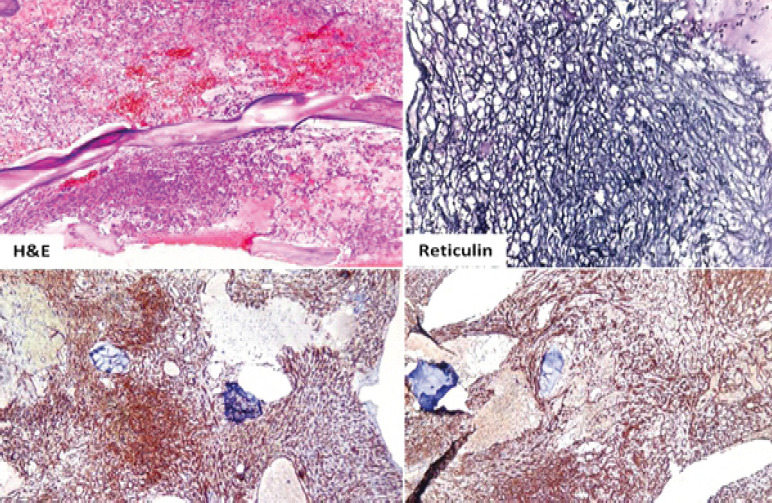
Histopathology slide.
Figure 2.
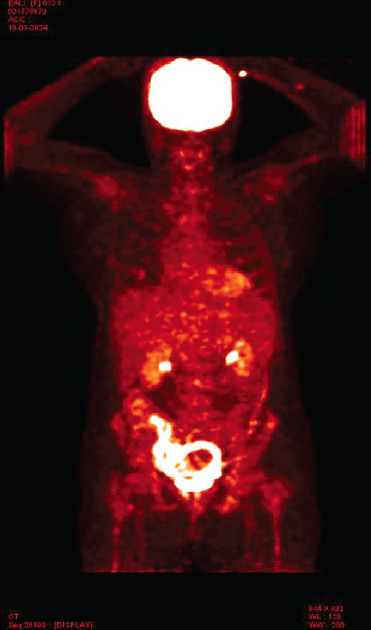
Positron emission tomography scan image-coronal.
Figure 5.
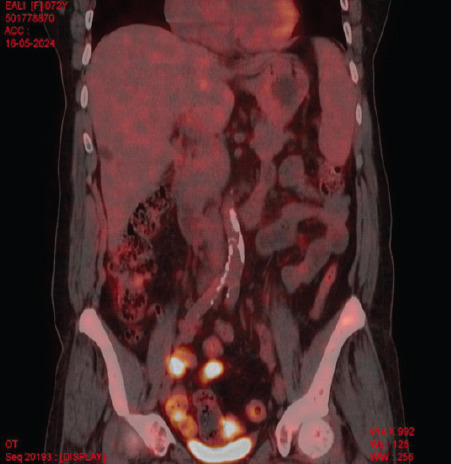
Positron emission tomography scan image-coronal.
Figure 3.
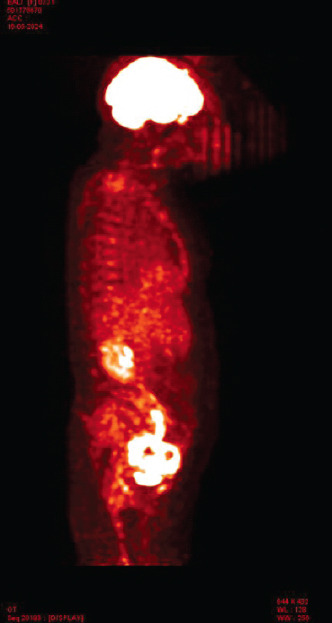
Positron emission tomography scan image-sagittal.
Figure 4.
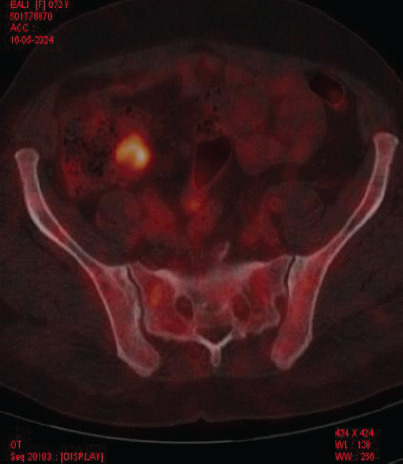
Positron emission tomography scan image-axial.
A multidisciplinary team, including a general internist, orthopedician, and critical care specialist, formulated a conservative treatment plan. After thorough discussions with the patient and her family, she was initiated on danazol and eltrombopag, with careful monitoring for bleeding risks. One month post-discharge, the patient presented with slurred speech and altered sensorium. CT brain imaging revealed an acute-on-chronic subdural hematoma, a rare but severe complication of disseminated cystic angiomatosis. Following the improvement of her sensorium, she was discharged with recommendations for close outpatient follow-up.
Methods
Diagnostic techniques involved in this case included peripheral smear analysis, bone marrow biopsy, and advanced imaging modalities such as PET scan. Histopathological examination of biopsy samples and detailed imaging studies were crucial in differentiating systemic cystic angiomatosis from other vascular neoplasms.
Results
The diagnostic findings that led to the differentiation of systemic cystic angiomatosis from vascular neoplasia included multiple non-avid lytic skeletal areas and non-avid cystic liver lesions on PET scan. The treatment with danazol and eltrombopag, although unconventional, was chosen based on the patient’s preference for conservative management.
Discussion
Systemic cystic angiomatosis (SCA) is a rare disorder with multisystemic vascular involvement of the skeletal system and other organ systems, such as the spleen, liver, and lungs. Classically, in the bones, SCA represents a maldeveloped vascular and/or lymphatic system, characterized by multifocal bony cysts with a honeycombed or “bubble” appearance, without aggressive osteolysis with neither peripheral soft-tissue involvement nor a periosteal reaction [1]. Najm et al. conducted a comprehensive review of the existing literature, identifying a total of 48 patients across 36 case reports, which includes their own 4 cases [2]. The mean age at presentation was 22 years; however, there appears to be a bimodal distribution due to cases presenting later in life [2-5]. It is noted that males are more frequently affected than females [6]. The etiology of solitary congenital absence (SCA) remains unclear, but it is believed to result from abnormalities in vascular and/or lymphatic development [7]. The clinical presentation of SCA varies significantly. Most cases involve bony cysts, predominantly affecting the femur and pelvic bones [8, 9]. Spinal involvement is observed in up to 50% of patients [10], while less commonly, it can affect the small bones of the hands [11]. Visceral involvement occurs in approximately 35% of reported cases, with the spleen being the most commonly affected organ, noted in a quarter of cases [2]. Other affected sites include the skin, soft tissues, thymus, liver, kidneys, mediastinum, and mesentery. The lung and pleural involvement are rare [11]. Complications associated with SCA include bony pain, pathological fractures, deformities, splenic rupture, chylothorax, and spinal cord compression [2, 6, 12]. Alkaline phosphatase levels have been reported as elevated in some cases due to extensive lytic osseous lesions, although in many reports, they were within normal limits [13]. Currently, there is no confirmed treatment for SCA. However, spontaneous regression leading to cure has been reported in some cases [14]. Treatment options discussed in the literature include bisphosphonates, interferon, and radiotherapy [15, 16]. Bisphosphonates effectively reduce osteolysis and promote mineralization by inhibiting osteoclastic activity, particularly in states of high bone turnover [17]. They also exert an antiangiogenic effect by inhibiting the proliferation and inducing apoptosis of endothelial cells. Specifically, zoledronic acid controls the expression of angiogenic cytokines such as vascular endothelial growth factor, basic fibroblast growth factor, and platelet-derived growth factor, thereby modulating the migration and adhesion of endothelial cells. This stabilization of bony lesions may result in fewer skeletal-related events [18]. The efficacy of radiation therapy is difficult to assess due to the typically static nature of the disease course [3, 19]. The case presented here underscores the importance of a comprehensive diagnostic approach, which includes advanced imaging techniques such as PET scans. The successful administration of danazol and eltrombopag in this instance, despite its unconventional nature, highlights the need for personalized treatment strategies tailored to patient preferences and the potential for complications. Further research and additional case reports are crucial to deepen our understanding and refine the management of this rare condition.
Conclusion
This case of systemic cystic angiomatosis in a diabetic elderly female highlights the diagnostic complexity and the importance of personalized management strategies. Given the rarity of the condition, further research and additional case reports are necessary to improve our understanding and management of systemic cystic angiomatosis.
Clinical Message.
This case of systemic cystic angiomatosis in an elderly female emphasizes the importance of considering rare diagnoses when faced with non-specific symptoms and atypical laboratory findings. Despite initial misdiagnosis as vascular neoplasm, advanced imaging techniques such as PET scan played a crucial role in identifying systemic cystic angiomatosis. The multidisciplinary approach and personalized management strategy, including the use of danazol and eltrombopag, highlight the necessity for tailored treatment plans that consider patient preferences and potential complications. Clinicians should remain vigilant for rare conditions and utilize comprehensive diagnostic tools to ensure accurate diagnosis and effective management.
Biography






Footnotes
Conflict of Interest: Nil
Source of Support: Nil
Consent: The authors confirm that informed consent was obtained from the patient for publication of this case report
References
- 1.Levey DS, MacCormack LM, Sartoris DJ, Haghighi P, Resnick D, Thorne R. Cystic angiomatosis:Case report and review of the literature. Skeletal Radiol. 1996;25:287–93. doi: 10.1007/s002560050082. [DOI] [PubMed] [Google Scholar]
- 2.Najm A, Soltner-Neel E, Le Goff B, Guillot P, Maugars Y, Berthelot JM. Cystic angiomatosis, a heterogeneous condition four new cases and a literature review. Medicine (Baltimore) 2016;95:e5213. doi: 10.1097/MD.0000000000005213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clayer M. Skeletal angiomatosis in association with gastro-intestinal angiodysplasia and paraproteinemia:A case report. J Orthop Surg (Hong Kong) 2002;10:85–8. doi: 10.1177/230949900201000115. [DOI] [PubMed] [Google Scholar]
- 4.Devaney K, Vinh TN, Sweet DE. Skeletal-extraskeletal angiomatosis. A clinicopathological study of fourteen patients and nosologic considerations. J Bone Joint Surg. 1994;76:878–91. doi: 10.2106/00004623-199406000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Wallis LA, Asch T, Maisel BW. Diffuse skeletal hemangiomatosis. Report of two cases and review of literature. Am J Med. 1964;37:545–63. doi: 10.1016/0002-9343(64)90068-3. [DOI] [PubMed] [Google Scholar]
- 6.Cohen J, Craig JM. Multiple lymphangiectases of bone. J Bone Joint Surg. 1955;37:585–96. [PubMed] [Google Scholar]
- 7.Dellinger MT, Garg N, Olsen BR. Viewpoints on vessels and vanishing bones in Gorham-Stout disease. Bone. 2014;63:47–52. doi: 10.1016/j.bone.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Boyle WJ. Cystic angiomatosis of bone. A report of three cases and review of the literature. J Bone Joint Surg. 1972;54:626–36. [PubMed] [Google Scholar]
- 9.Jacobs JE, Kimmelstiel P. Cystic angiomatosis of the skeletal system. J Bone Joint Surg. 1953;35:409–64. [PubMed] [Google Scholar]
- 10.Toxey J, Achong DM. Skeletal angiomatosis limited to the hand:Radiographic and scintigraphic correlation. J Nucl Med. 1991;32:1912–14. [PubMed] [Google Scholar]
- 11.Deveci M, Inan N, Çorapçioğlu F, Ekingen G. Gorham-Stout syndrome with chylothorax in a six-year-old boy. Indian J Pediatr. 2011;78:737–9. doi: 10.1007/s12098-010-0328-2. [DOI] [PubMed] [Google Scholar]
- 12.Brower AC, Culver JE, Keats TE, Keats TE. Diffuse cystic angiomatosis of bone. Report of two cases. Am J Roentgenol. 1973;118:456–63. doi: 10.2214/ajr.118.2.456. [DOI] [PubMed] [Google Scholar]
- 13.Ishida T, Dorfman HD, Steiner GC, Norman A. Cystic angiomatosis of bone with sclerotic changes mimicking osteoblastic metastases. Skeletal Radiol. 1994;23:247–52. doi: 10.1007/BF02412356. [DOI] [PubMed] [Google Scholar]
- 14.Schajowicz F, Aiello CL, Francone MV, Giannini RE. Cystic angiomatosis (hamartous haemolymphagiomatosis) of bone. A clinicopathological study of three cases. J Bone Joint Surg Br. 1978;60:100–6. doi: 10.1302/0301-620X.60B1.627569. [DOI] [PubMed] [Google Scholar]
- 15.Iranpour P, Namdari N, Alavi M, Geramizadeh B. Systemic cystic angiomatosis mimicking metastatic cancer. Curr Probl Cancer. 2022;46:100763. doi: 10.1016/j.currproblcancer.2021.100763. [DOI] [PubMed] [Google Scholar]
- 16.Dos Anjos CS, Borges RM, Chaves AC, Fuzita WH, Silva CA, De Oliveira UG. Cystic angiomatosis, pleural effusion and multiple bone lesions mimicking a metastatic malignant neoplasia:A case report. J Med Case Rep. 2019;13:265. doi: 10.1186/s13256-019-2196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moschetta M, Di Pietro G, Ria R, Gnoni A, Mangialardi G, Guarini A, et al. Bortezomib and zoledronic acid on angiogenic and vasculogenic activities of bone marrow macrophages in patients with multiple myeloma. Eur J Cancer. 2010;46:420–9. doi: 10.1016/j.ejca.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 18.Marcucci G, Masi L, Carossino AM, Franchi A, Capanna R, Sinigaglia L, et al. Cystic bone angiomatosis:A case report treated with aminobisphosphonates and review of the literature. Calcif Tissue Int. 2013;93:462–471. doi: 10.1007/s00223-013-9761-3. [DOI] [PubMed] [Google Scholar]
- 19.Aumann WK, Maxfield CM, Heath JL. Asymptomatic skeletal cystic angiomatosis may be managed conservatively with close observation. J Pediatr Hematol Oncol. 2021;43:9. doi: 10.1097/MPH.0000000000001750. [DOI] [PubMed] [Google Scholar]


