Abstract
Background:
Evidence has suggested that cognitive decline may be a risk factor for freezing of gait (FOG) in Parkinson’s disease (PD). Complex and challenging exercises have been suggested as potential rehabilitation strategies to decrease FOG severity and improve cognition; however, it is unknown whether improvement in cognition would explain decreased FOG severity following exercise.
Objective:
In this secondary analysis, we evaluated the effects of the adapted resistance training with instability (ARTI-complex and challenging exercises) compared with traditional motor rehabilitation (TMR-without challenging exercises) on cognitive function in people with FOG of PD. We also verified whether cognitive improvement explains the decrease in FOG previously published.
Methods:
Participants were randomized to either the experimental group (ARTI, n=17) or the active control group (TMR, n=15). Both training groups exercised three times a week for 12 weeks (80-90 min each session). FOG severity (FOG ratio from inertial sensors during a 360-degree turning-in-place task), frontal lobe function (Frontal Assessment Battery-FAB), global cognition (Montreal Cognitive Assessment-MoCA), attention and psychomotor speed (Digit Symbol Substitution Test-DSST) were evaluated before and after interventions.
Results:
Only the ARTI group improved FAB, MoCA, and DSST scores at posttraining. In addition, ARTI was more effective than TMR in improving FAB scores at posttraining. The changes in FAB scores explained the changes in FOG ratio following ARTI (R2=0.43, P<0.01).
Conclusions:
This pilot study suggests that ARTI, a complex and challenging training, improves cognition in people with FOG of PD. Improvements in frontal lobe function with ARTI help explain decreased FOG severity.
Keywords: frontal lobe function, cognitive inhibition, global cognition, attention, freezing of gait, Parkinson’s disease
INTRODUCTION
Cognitive impairment is a common non-motor symptom in Parkinson’s Disease (PD)1 that can occur prior to, at the time, or years after diagnosis of PD.1 Longitudinal studies showed that 47% of 141 people with PD with normal cognition develop cognitive impairment within 1-6 years,2 and 48% of 149 people with PD with normal cognition at the diagnosis fulfilled the criteria for dementia within 15 years3. Importantly, cognitive impairment has been suggested as a risk factor for freezing of gait (FOG) in PD.4 5, 6
FOG is the most disabling symptom of PD7 and a common cause of falls.7–9 Although FOG is a complex phenomenon and its pathophysiology remains unknown,7 a recent meta-analysis of 145 studies showed that people with PD and FOG (PD+FOG) have worse cognition than those without FOG (PD-FOG) across frontal lobe function, executive function, global cognition, attention, language, memory, and visuospatial domains,5 which shows a strong link between FOG and cognition.
The effects of pharmacological treatments for FOG and cognition are still inconclusive.7 Levodopa has only a limited impact on FOG, which is considered a dopamine-resistant phenomenon in PD.7 In fact, FOG severity has been more common in levodopa-treated cohorts compared to levodopa-naïve ones.10 Antiparkinsonian medication has limited and complex effects on cognition, varying depending on the cognitive domain tested and the type of medication (e.g., levodopa vs. dopamine agonists).11, 12 Thus, exercise interventions aimed at decreasing FOG severity and improving cognition are needed.
Cognitive and challenging exercises have been suggested as potential rehabilitation strategies to decrease FOG severity and improve cognition in PD+FOG,13–15 although empirical findings are equivocal. Walton et al.,13 showed that 12 sessions (120 min each) of cognitive training (specific computer-based cognitive tasks) reduced FOG severity (percentage time spent frozen during a gait task) and improved cognitive processing speed (Trial Making Test part [TMT] A) compared to active control (non-specific, computer-based tasks) in PD+FOG assessed ON medication. However, other cognitive domains, such as global cognition, cognitive flexibility, memory, executive function, and inhibitory control, did not show any change after cognitive training.13 King et al.,14 showed that 18 sessions (80 min each) of challenging cognitive-mobility exercises improved perceived FOG severity (New FOG Questionnaire – NFOGQ), global cognition, cognitive flexibility, and cognitive inhibition in PD+FOG assessed OFF medication. Additionally, this study showed that objective FOG values (360-degree turning in place) at baseline were associated with larger improvements with the challenging cognitive-mobility exercises but not with the control group. Silva-Batista et al.,16 showed that 36 sessions (80-90 min each) of complex and challenging adapted resistance training with instability (ARTI) decreased FOG severity (360-degree turning in place) compared to active control (traditional motor rehabilitation -TMR) in PD+FOG assessed ON medication. ARTI improved cognitive inhibition16 and cognitive flexibility15 in PD+FOG. Although these previous studies show the likely potential of challenging, complex, and cognitive exercises in improving cognition and decreasing FOG, no study has explored whether exercise-induced cognitive function improvements may decrease FOG severity. Although previous studies showed improvements in executive control such as inhibition14, 16 and cognitive flexibility14, 16 after challenging exercises, no study has investigated the effect of those exercises on frontal lobe function in PD+FOG, as the dysfunction in frontostriatal pathways has been implicated in FOG pathophysiology.7, 17, 18
The Frontal Assessment Battery (FAB) assesses frontal lobe functions and has previously been validated in PD.19 Frontal lobe function regulates higher-order processes that play crucial roles in behavioral, psychological, and executive processes required to elaborate goal-directed behaviors and adapts the subjects’ responses to new or challenging situations. These roles are mediated by several different frontal regions (e.g., dorsolateral, medial frontal, and prefrontal) with distinct functions.19–24 Evidence that the FAB measures frontal lobe function comes from older people with cognitive impairment20 and those with Alzheimer’s Disease21 who have low FAB scores and show hypoperfusion in frontal regions (left lateral and right medial frontal regions) and frontal gyrus (left middle and right superior), respectively. In addition, FAB is associated with lower gray matter density in the prefrontal areas of people with PD22 and with frontal areas (dorsolateral and medial frontal cortex) of people with frontotemporal dementia.23 PD+FOG have lower FAB scores and reduced functional connectivity between the right middle frontal gyrus and the angular gyrus compared to PD-FOG.24 These results suggest that FAB is modulated by frontal lobe regions, PD+FOG have frontal lobe dysfunction, and interventions are needed to improve FAB scores in PD+FOG.
Recently, we published the results of our Adapted Resistance Training with Instability Trial in FOG (ARTIT-FOG)15, 16 comparing the effects of ARTI, a complex, challenging, and coordinative intervention, and TMR, an active control group without complex and challenging exercises, on the FOG severity and executive function (cognitive inhibition and cognitive flexibility). Although ARTI decreased FOG severity as assessed objectively 16 and improved executive function15, 16, it is unknown whether ARTI improves other cognitive domains related to FOG, such as frontal lobe function, global cognition, attention, and psychomotor speed.5 Also, it is unknown whether improved cognition would partially explain the variance in the decreased FOG severity following ARTI.
ARTI requires challenging motor complexity exercises that simultaneously require high demands on cognition, proprioception, coordination, and motor control.15, 16, 25, 26 A meta-analysis of 80 randomized controlled trials showed greater benefits of coordinative and complex exercises on cognitive function compared with other exercise protocols (e.g., aerobic).27 In addition, previous studies have shown more brain activation in cognitive and motor areas after high, rather than low, motor complexity exercises.16, 28, 29 Thus, we hypothesized that ARTI will be more effective than TMR (active control group) in improving cognition (frontal lobe function, global cognition, and attention and psychomotor speed) of PD+FOG due to challenging and complex/coordination exercises included in the ARTI but not in the TMR program. We also hypothesize that the mitigation of frontal lobe dysfunction after ARTI likely explains the decrease in FOG severity we have previously published16. FOG is strongly linked to dysfunction in frontostriatal pathways.7, 30–32 Increasing the complexity of a task may demand multiple processes in the frontal lobe related to executive function33, as required during ARTI.
Therefore, this study uses secondary analyses of our original trial (RBR-83VB6B) to investigate two aims. First, we compared the effects of ARTI and TMR on secondary cognitive outcomes (frontal lobe function, global cognition, and attention and psychomotor speed). Second, we tested whether improved cognition could explain the decrease in FOG severity (objectively assessed during a 360-degree turning-in-place task) previously reported.16
MATERIALS AND METHODS
Study Design
The ARTIT-FOG was a prospective, single-center, parallel-group, single-blinded randomized controlled trial approved by the university’s ethical committee (School of Physical Education and Sport) and at the Brazilian Clinical Trials Registry (RBR-83VB6B; Universal Trial Number-U1111-1215-9956). The ARTIT-FOG was conducted between June 2018 and April 2019. The primary outcome of the ARTIT-FOG trial was FOG severity.16 In the present study, we analyzed secondary, cognitive outcomes (frontal lobe function, global cognition, and attention and motor speed) of the ARTIT-FOG.16
Participants
All participants were recruited from the Movement Disorders Clinic in the School of Medicine at the University of São Paulo. The diagnosis of idiopathic PD was confirmed by a movement disorders specialist following UK Parkinson’s Disease Society Brain Bank diagnostic criteria.18 All eligibility criteria were measured in the ON medication status: 1) Hoehn and Yahr stage range 3-4; 2) stable dopaminergic therapy 2 months before and during the period of study; 3) 49-85 years of age; 4) able to walk for 20 meters without walking aids; 5) absence of neurological disorders (other than PD); 6) absence of significant arthritis, musculoskeletal or vestibular disorders, severe tremor, claustrophobia, and metal in the body; 7) Mini-Mental State Examination score >23;21 8) no physical exercise practice in the three months preceding study commencement. Individuals gave their written informed consent to participate. All participants were classified as PD+FOG during ON medication status if FOG was observed in the videos (e.g., step-over obstacles, turning clockwise and counterclockwise, and walking through a doorway) and if they answered affirmatively item 1 (Do you experience FOG?) and scored >1 on item 3 (How frequently do you experience freezing episodes during turning?) and 5 (How frequently do you experience episodes of freezing when initiating the first step?) of the NFOGQ.19
Study procedures
PD+FOG were assessed and trained fully medicated (on-state) within 1.5 to 2h of taking their morning dose of dopaminergic medication. Secondary outcome measures of cognition of the ARTIT-FOG trial included frontal lobe function assessed by the Frontal Assessment Battery (FAB)19, global cognition assessed by the Montreal Cognitive Assessment (MoCA)34, and attention and psychomotor speed assessed by the Digit Symbol Substitution Test (DSST).35 A researcher, trained by a neuropsychologist, blind to the experimental design assessed cognition. See Supplementary Material for a detailed description of the assessments.
As well as determining whether ARTIT improves secondary cognitive outcomes (FAB, MoCA, DSST), the second aim of this study is to test if improved cognition following exercise could explain the decrease in FOG severity we have previously published.16 We used the 2-minute turning task (alternating 360-degree turns to the right and the left) as previously published.16 Higher FOG-ratio scores indicate greater FOG severity (see more details in Supplementary Material). Also, for this second aim, we used executive functions of cognitive inhibition (Stroop-III) and cognitive flexibility (TMT part B-A [TMTB-A]) previously published that improved only after ARTI.15, 16
Randomization
After baseline assessments, PD+FOG were classified (a statistician blind to experimental design) into quartiles regarding their NFOGQ scores. PD+FOG from each quartile were randomly assigned to the active control group (TMR) or the experimental group (ARTI). Post-test assessments were performed 24 hours after the end of the training period (12 weeks).
Interventions
See Supplementary Material for details of the ARTI and TMR programs. Briefly, both TMR and ARTI groups trained 3 days per week for 12 weeks (36 training sessions) in different facilities. Each training session lasted between 80-90 minutes. TMR, an active control group, consisted of exercises with a focus on stretching, gait, balance, posture, and lower- and upper-limbs free weight exercises. ARTI is an adaptation of our RTI program for PD (PD-FOG) that has been previously published.26 ARTI consisted of seven lower-limb and upper-limb free weight exercises (half-squat, plantar flexion, chest press, knee-lifting stand, lunge, reverse fly, and dual-task squat) combined with unstable devices (i.e., foam pad, dyna discs, balance discs, BOSU®, and Swiss Ball). Throughout the 12-wk period, there was a progressive increase in motor complexity simultaneously, in terms of attentional, proprioceptive (unstable devices progression), coordinative, dual task, and motor control demands only for the ARTI (see Supplementary Table 1 and Figure 1 for exercise progression and pictures, respectively).
Figure 1.
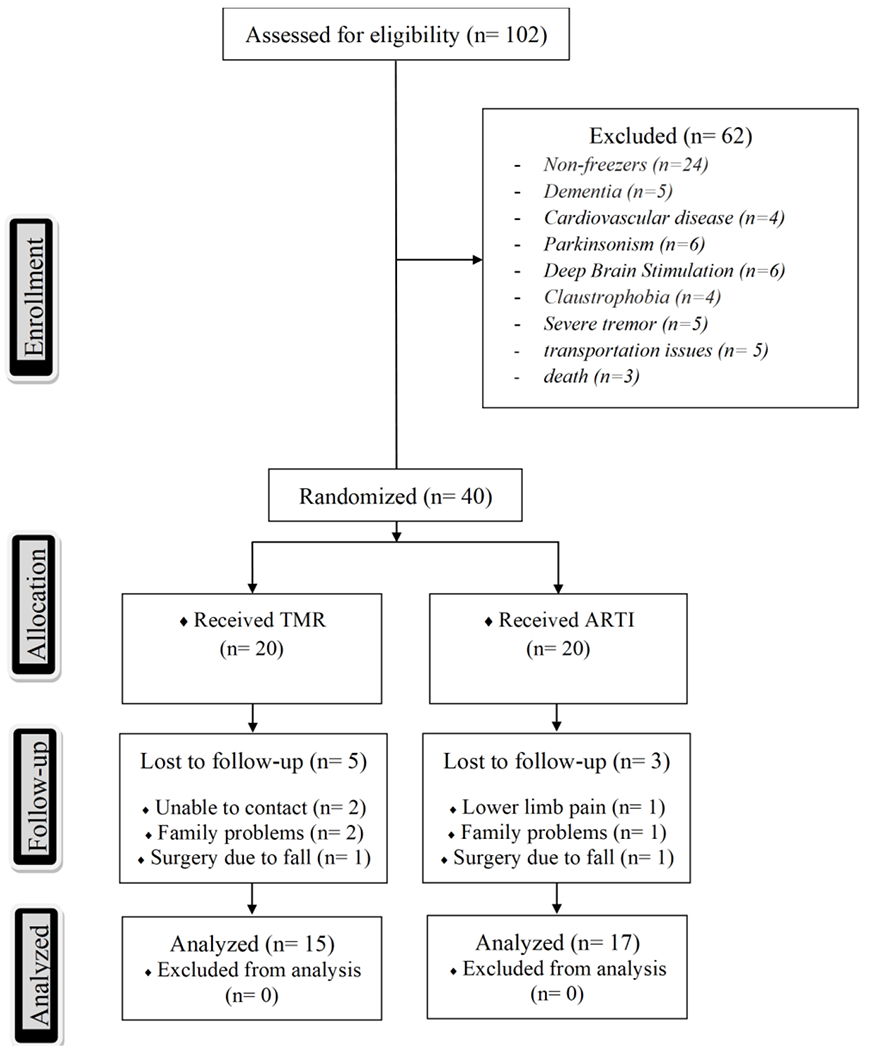
Trial profile with schematic representation of participant recruitment and allocation. TMR, traditional motor rehabilitation; ARTI, adapted resistance training with instability.
Statistical analyses
This study is a secondary analysis of our previous research,15, 16 then, no power analysis was conducted.
To test our first hypothesis, the effects of training protocols on secondary cognitive outcomes of the present study (FAB, MoCA, and DSST) were verified using a linear mixed model having two fixed factors: training groups (ARTI and TMR) and time (pre and post), and subjects as a random factor. A Tukey’s adjustment was used for multicomparison purposes whether a significant F value was obtained. Kenward and Roger’s method for correcting the degrees of freedom of the fixed effects was applied to cater to the small sample size. Although our previous study did not show significant between-groups differences for FOG ratio at baseline,16 we used ANCOVA analyses to rule out the possibility that FOG ratio values at baseline between the groups could be affecting our results. Then, we controlled (ANCOVA tests) for baseline FOG ratio values. Effect Size (ES) for within-group (pre- to post-changes) and between-group (post-changes) comparisons, using Cohen’s d36, were used for each cognitive outcome. ESs were classified as small (ES ≤0.49), medium (ES, 0.50–0.79), and large (ES ≥0.80).
Chi-square was used to determine whether the proportion of PD+FOG with frontal lobe dysfunction (score ≤12 in the FAB)37 and mild cognitive impairment (score ≤25 in the MoCA)34 decreased after interventions.
Independent t tests were used to compare the changes (Δ) in each secondary cognitive outcome between the groups and compare between-groups differences for demographic characteristics and clinical variables at baseline.
To test our second hypothesis, a linear multiple regression, forward stepwise method was used to verify if changes in cognition could explain changes in FOG severity (FOG ratio – dependent variable) following ARTI. For this analysis, we used FAB, MoCA, and DSST of the present study, and previous executive tests (Stroop-III and [TMTB-A]) and FOG ratio we have previously published that improved only after ARTI.15, 16 Only independent variables (FAB, MoCA, DSST, Stroop-III, TMTB-A) with low collinearity (<0.7)38 with the dependent variable (FOG ratio) entered the regression model. We also included baseline FOG ratio as a continuous predictor in the linear multiple regression.
Normality and the presence of extreme observations were assessed through the Shapiro-Wilk test and box-plots, respectively. Non-normal data (FOG ratio) were log-transformed. Results are presented as mean and standard deviation (SD). The significance level was set at P≤0.05. Statistical procedures were implemented using SAS 9.2®, with exception of linear multiple regression, which was performed with the IBM SPSS Statistics (version 27).
RESULTS
Participants
Forty individuals performed baseline testing and were randomized into each group (TMR or ARTI). Thirty-two participants (TMR n=15; ARTI n=17) composed the final sample as 8 participants dropped out before post-assessment (TMR n=5; ARTI n=3) (Figure 1). Of the TMR group, we were unable to contact two participants; two participants had family problems; and one participant had surgery due to a fall in the house. Of the ARTI group, one participant experienced lower limb pain (no medical intervention required) and dropped his consent to participate; one participant dropped out due to family problems; and one participant had surgery due to a fall getting out of the car.
Baseline values
As demonstrated in Table 1, there were no between-group differences in demographic, anthropometric, clinical characteristics, and outcomes (FAB, MoCA, DSST, Stroop-III, TMTB-A, FOG ratio) at baseline.
Table 1.
Characteristics, clinical, and cognitive variables of people with Parkinson’s disease and freezing of gait at baseline, by group (mean ± SD).
| TMR (n=15) | ARTI (n=17) | P value | |
|---|---|---|---|
| Men/women (number) | 9/6 | 12/5 | |
| Age (years) | 66.8 ± 8.9 | 64.6 ± 10.5 | 0.542 |
| Educational level (years) | 10.5 ± 5.8 | 12.9 ± 5.8 | 0.253 |
| Body mass (kg) | 67.5 ± 9.1 | 73.4 ± 13.5 | 0.162 |
| Height (cm) | 1.6 ± 0.1 | 1.6 ± 0.1 | 0.577 |
| Body mass index (kg/m2) | 25.3 ± 2.7 | 26.8 ± 4.1 | 0.235 |
| Mini-Mental State Examination (score) | 25.6 ± 2.0 | 25.5 ± 1.7 | 0.986 |
| Years since diagnosis (years) | 10.0 ± 5.6 | 7.7 ± 4.0 | 0.204 |
| Hoehn and Yahr staging scale (a.u) | 3.2 ± 0.4 | 3.1 ± 0.3 | 0.296 |
| Hoehn and Yahr stage 3 (number) | 11 | 15 | |
| Hoehn and Yahr stage 4 (number) | 4 | 2 | |
| Symptom-dominant side (R/L/B) | 1/3/11 | 3/2/12 | |
| Postural instability and gait disturbance (score) | 8.9 ± 2.6 | 7.7 ± 1.9 | 0.153 |
| L-Dopa equivalent daily dose (mg/day) | 854.8 ± 251.9 | 772.5 ± 275.8 | 0.342 |
| UPDRS-III (score) | 51.4 ± 10.6 | 46.5 ± 11.4 | 0.212 |
| NFOGQ (score) | 22.3 ± 5.9 | 21.6 ± 5.7 | 0.743 |
| FOG ratio during 360-degrees turning task (a.u.) | 12.8 ± 8.8 | 9.8 ± 8.2 | 0.311 |
| FAB (score) | 12.0 ± 1.5 | 11.8 ± 2.4 | 0.744 |
| MoCA (score) | 21.1 ± 1.9 | 20.4 ± 2.3 | 0.374 |
| DSST (points) | 15.5 ± 7.1 | 12.8 ± 8.2 | 0.347 |
| Stroop-III test (a.u.) | 72.1 ± 44.4 | 79.1 ± 40.3 | 0.712 |
| TMTB-A (seconds) | 72.0 ± 68.2 | 79.3 ± 56.8 | 0.653 |
TMR= Traditional Motor Rehabilitation; ARTI= adapted resistance training with instability; L-Dopa = levodopa; a.u = Arbitrary units; R = right; L = left; B = both; UPDRS-III = Unified Parkinson’s Disease Rating Scale part III motor subscale score; NFOGQ = New Freezing of Gait Questionnaire; FOG ratio = freezing-of-gait ratio; FAB = Frontal Assessment Battery; MoCA = Montreal Cognitive Assessment; DSST = Digit Symbol Substitution Test; TMTB-A= the difference in time to completion between Trail Making Tests B and A.
ARTI improves frontal lobe function
The FAB scores showed a significant group × time interaction (F[1, 30]=4.16, P=0.01). Only the ARTI group increased the FAB scores from pre- to post-training (P<0.01, ES=1.15) presenting higher scores than the TMR group at post-training (P=0.05, ES=1.00). Improvements in FAB scores after ARTI were observed in the inhibitory control subscores (P<0.01, ES=1.20) presenting higher scores than the TMR group (P<0.01, ES=1.36) at post-training, and a trend toward higher subscores in the mental flexibility (P=0.08, ES=0.73) from pre- to post-training. See Tables 2 and 3 for details.
Table 2.
Frontal Assessment Battery (FAB), Montreal Cognitive Assessment (MoCA), Digit Symbol Substitution Test (DSST), Stroop-III test, and Trial Making Test part B-A (TMTB-A) at pre- and post-training assessments for each training group.
| Outcomes | TMR | ARTI | Group × Time interaction | Change from pre- to post-training | Difference at post-training ARTI vs TMR | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| F value | P value | TMR P value | ARTI P value | P value | |||
| FAB (scores) | 4.16 | 0.01 | |||||
| Pre | 11.9 ± 1.5 | 11.8 ± 2.5 | |||||
| Post | 12.3 ± 2.1 | 14.6 ± 2.7 | 0.86 | <.01 | 0.05 | ||
| MoCA (scores) | 6.00 | 0.02 | |||||
| Pre | 21.1 ± 1.9 | 20.4 ± 2.2 | |||||
| Post | 22.7 ± 1.3 | 24.1 ± 1.9 | 0.08 | <.01 | 0.12 | ||
| DSST (score) | 9.30 | <.01 | |||||
| Pre | 15.5 ± 7.1 | 13.2 ± 7.7 | |||||
| Post | 15.7 ± 6.9 | 16.3 ± 6.8 | 0.97 | <.01 | 0.91 | ||
| Stroop-III test (a.u.) | 22.3 | <.01 | |||||
| Pre | 72.1 ± 44.4 | 79.1 ± 40.3 | |||||
| Post | 72.5 ± 41.1 | 65.1 ± 31.4 | 0.99 | <.01 | 0.95 | ||
| TMTB-A (seconds) | 14.6 | <.01 | |||||
| Pre | 72.0 ± 68.2 | 79.3 ± 56.8 | |||||
| Post | 86.9 ± 70.5 | 59.8 ± 62.6 | 0.13 | 0.02 | 0.63 | ||
a.u., arbitrary units
Table 3.
Subscores of Frontal Assessment Battery (FAB) at pre- and post-training assessments for each training group.
| Outcomes | TMR | ARTI | Group × Time interaction | Change from pre- to post-training | Difference at post-training ARTI vs TMR | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| F value | P value | TMR P value | ARTI P value | P value | |||
| Conceptualization (subscore) | 0.81 | 0.37 | |||||
| Pre | 1.5 ± 0.6 | 1.8 ± 0.6 | |||||
| Post | 2.3 ± 0.8 | 2.8 ± 0.4 | <.01a | <.01a | 0.14 | ||
| Mental flexibility (subscore) | 7.11 | 0.01 | |||||
| Pre | 2.4 ± 0.6 | 2.1 ± 0.5 | |||||
| Post | 2.2 ± 0.7 | 2.5 ± 0.7 | 0.55 | 0.08 | 0.62 | ||
| Programming (subscore) | 3.22 | 0.08 | |||||
| Pre | 2.6 ± 0.6 | 2.4 ± 0.7 | |||||
| Post | 2.3 ± 0.8 | 2.5 ± 0.7 | 0.28 | 0.87 | 0.73 | ||
| Sensitivity to interference (subscore) | 1.62 | 0.21 | |||||
| Pre | 1.2 ± 0.4 | 1.4 ± 0.7 | |||||
| Post | 1.5 ± 0.6 | 2.1 ± 0.9 | <.01a | <.01a | 0.06 | ||
| Inhibitory control (subscore) | 8.09 | <.01 | |||||
| Pre | 1.2 ± 0.5 | 1.3 ± 0.7 | |||||
| Post | 1.2 ± 0.7 | 2.1 ± 0.9 | 0.99 | <.01 | <.01 | ||
| Environmental autonomy (subscore) | 1.67 | 0.20 | |||||
| Pre | 2.9 ± 0.3 | 2.8 ± 0.4 | |||||
| Post | 2.9 ± 0.3 | 2.6 ± 0.7 | 1.00 | 0.25 | 0.17 | ||
Main time effect
ARTI improves global cognition
The MoCA scores showed a significant group × time interaction (F[1, 30]=6.00, P=0.02). Only the ARTI group increased the MoCA scores (P<0.01, ES=1.68), although the TMR group presented a trend toward higher scores from pre- to post-training (P=0.08, ES=0.86). Improvements in MoCA scores after ARTI were observed in the visuo-executive subscores (P<0.01, ES=1.19), presenting a stronger trend toward higher scores than the TMR group (P=0.06, ES=1.19) at post-training. See Table 2 and 4 for details.
Table 4.
Subscores of Montreal Cognitive Assessment (MoCA) at pre- and post-training assessments for each training group.
| Outcomes | TMR | ARTI | Group × Time interaction | Change from pre- to post-training | Difference at post-training ARTI vs TMR | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| F value | P value | TMR P value | ARTI P value | P value | |||
| Visuo-executive (subscore) | 7.15 | 0.01 | |||||
| Pre | 2.3 ± 0.8 | 2.0 ± 0.9 | |||||
| Post | 2.3 ± 0.6 | 3.1 ± 0.9 | 1.0 | <.01 | 0.06 | ||
| Naming (subscore) | 0.58 | 0.45 | |||||
| Pre | 2.7 ± 0.6 | 2.4 ± 1.0 | |||||
| Post | 2.9 ± 0.3 | 2.8 ± 0.5 | 0.75 | 0.15 | 0.96 | ||
| Attention (subscore) | 1.70 | 0.20 | |||||
| Pre | 3.5 ± 1.2 | 3.1 ± 2.0 | |||||
| Post | 4.4 ± 0.5 | 4.8 ± 0.9 | <.01a | <.01a | 0.86 | ||
| Language (subscore) | 0.12 | 0.73 | |||||
| Pre | 2.9 ± 0.3 | 2.9 ± 0.3 | |||||
| Post | 2.9 ± 0.3 | 2.8 ± 0.4 | 1.00 | 0.95 | 0.98 | ||
| Abstraction (subscore) | 01.3 | 0.71 | |||||
| Pre | 1.9 ± 0.4 | 1.7 ± 0.4 | |||||
| Post | 1.9 ± 0.3 | 1.8 ± 0.7 | 0.91 | 0.61 | 0.99 | ||
| Delayed recall (subscore) | 1.39 | 0.25 | |||||
| Pre | 1.7 ± 0.8 | 2.2 ± 0.9 | |||||
| Post | 1.9 ± 0.7 | 2.7 ± 0.7 | <.01a | <.01a | 0.06 | ||
| Orientation (subscore) | 2.45 | 0.12 | |||||
| Pre | 5.4 ± 0.7 | 5.0 ± 1.1 | |||||
| Post | 5.7 ± 0.6 | 5.1 ± 1.1 | 0.16 | 1.00 | 0.29 | ||
Main time effect
ARTI improves attention and psychomotor speed
The DSST scores showed a significant group × time interaction (F[1, 30]=9.30, P<0.01). Only the ARTI group increased the DSST scores from pre- to post-training (P<0.01, ES=0.36). See Table 2 for details.
ARTI improves executive function
As previously published15, 16, the Stroop-III (F[1, 30]=22.28, P<0.01) and TMTB-A (F[1, 30]=14.59, P<0.01) showed significant group × time interactions. Only the ARTI group decreased the Stroop-III test (P<0.01, ES=−0.35) and the TMTB-A values (P=0.01, ES=−0.34). from pre- to post-training. See Table 2 for details.
ARTI maintains effects on secondary cognitive outcomes even after controlling for baseline FOG ratio
FOG-ratio at baseline was used as a covariate and it did not present any significant influence on the secondary cognitive outcomes (P>0.05). See details in the Supplementary ANCOVA results.
ARTI decreases the proportion of people with cognitive impairments
Our Chi-squared analysis showed that the proportion of PD+FOG who scored ≤ 12 on the FAB significantly decreased from 76.4% (13 PD+FOG) to 23.5% (4 PD+FOG) for the ARTI group from pre- to post-training (CI=−0.81 to −0.24) but not for the TMR group (CI=0.07 to −0.60) where the FAB decreased from 73.3% (11 PD+FOG) to 46.6% (7 PD+FOG). The proportion of PD+FOG who scored ≤ 25 on the MoCA decreased from 100% (17 PD+FOG) to 70.5% (12 PD+FOG) for the ARTI group from pre- to post-training (CI= −0.51 to −0.07) but not for the TMR group, where the proportion remained the same (100%) pre- to post-training.
ARTI shows large improvements in secondary cognitive outcomes and FOG ratio
The ARTI group presented larger improvements in FAB (P=0.01), MoCA (P=0.01), DSST (P<0.01), Stroop-III (P<0.01), TMTB-A (P<0.01), and FOG ratio (P<0.01) compared with the TMR group. See details in Supplementary Figure 2.
Improvements in FAB scores explain changes in FOG ratio
This analysis was performed only for the ARTI group due to the significant results for cognition in the present study (FAB, MoCA, and DSST) and our previously published results of other executive tests (Stroop-III and TMTB-A)15, 16 and FOG ratio16 that improved only after ARTI. The linear multiple regression, using baseline FOG ratio as a covariate, showed that changes in FAB score explained 43% (P<0.01) of the changes in FOG ratio following ARTI (Figure 2). See details in Supplementary Table 2.
Figure 2.
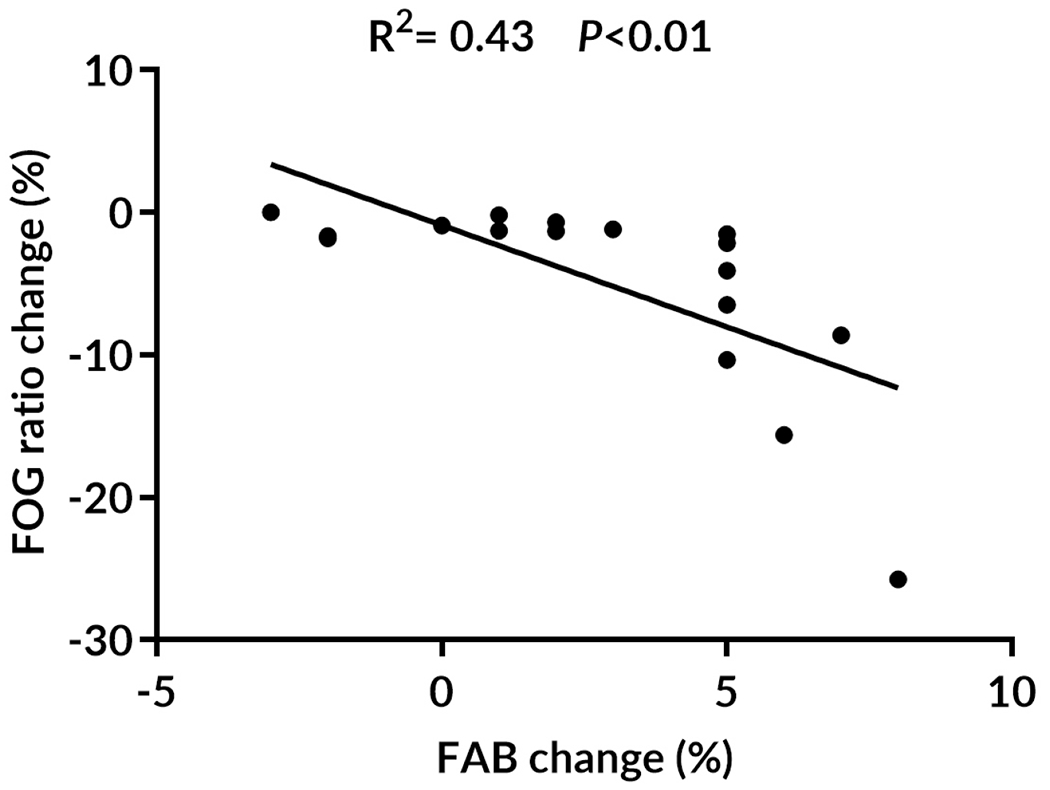
Correlation of changes in freezing of gait (FOG) ratio with changes in Frontal Assessment Battery (FAB) following ARTI, adapted resistance training with instability. R2 and P-values of the linear multiple regression approach are shown.
Adherence and Adverse events
The TMR group performed 35.4±1.3 sessions (98%) and the ARTI group performed 36.0±0.0 sessions (100%) showing high adherence to the training protocols. Only one adverse effect was reported during ARTI sessions (lower limb pain while performing lunge exercise). Finally, no adverse effect was reported during the TMR classes.
DISCUSSION
Our study is the first to show that: a) challenging exercises with high motor complexity (ARTI) are more effective than those without motor complexity in improving frontal lobe function in PD+FOG, and b) improvement in frontal lobe function is correlated to decreased, objectively assessed FOG following ARTI. These findings show the link between frontal lobe function, FOG, and exercise.
ARTI improves frontal lobe function
Our results show that the ARTI program, but not the TMR program significantly improved frontal lobe function as assessed through FAB (Table 2). In addition, ARTI was more effective than TMR in improving FAB scores. Specifically, the ARTI group showed a 68.2% (n=9) reduction in the proportion of PD+FOG with frontal dysfunction (score ≤ 12 in the FAB). A recent meta-analysis, based on data from 69 studies, showed that PD+FOG have lower FAB scores (worse frontal function) than PD-FOG, regardless of disease severity.5 Our study is the first to show exercise-induced improvements in FAB and its association with decreasing PD+FOG severity. Improvements in FAB scores were observed in the inhibitory control (go-no-go task) subscores and a trend for the mental flexibility (lexical fluency) (Table 3). These findings are vital for PD+FOG because frontostriatal dysfunction is one of the hypotheses for FOG pathogenesis.7, 17, 30–32
The literature on the effects of exercise interventions on the frontal lobe function of PD+FOG is unknown. Our16 and previous studies14 have demonstrated that challenging exercises can cause significant improvements in executive function such as cognitive inhibition measured with the Stroop task in PD+FOG. A systematic review of 34 neuroimaging studies showed that conflict-related activity (Stroop task) is related to activity in the anterior cingulate cortex, bilaterally in the lateral prefrontal cortex, the anterior insula, and the parietal lobe.39 In PD+FOG, but not in healthy control subjects or PD-FOG, pedunculopontine nucleus tract laterality is strongly associated with performance on response inhibition tasks (Stroop and Flankers).40 Our previous study showed that 36 sessions of complex and challenging exercises improved cognitive inhibition and mesencephalic locomotor region activity in PD+FOG.16 In the current study, we did an exploratory analysis. We observed a significant association of increased mesencephalic locomotor region activity with improvements in the Stroop-III test (r=0.51, P=0.04) but not with FAB improvements (r=−0.41, P=0.11) following complex and challenging exercises in PD+FOG. These results suggest that complex and challenging exercises cause positive effects on midbrain areas, which seem to modulate only cognitive inhibition.
Our15 and previous studies13, 14 also showed that exercise improves cognitive flexibility measured with the TMT in PD+FOG. A review showed that the rostral anterior cingulate, dorsomedial prefrontal, insular, temporal, and parietal cortex when lesioned, are associated with impaired TMT performance.41 In older adults, significant relationships were found between slower TMT-B completion times and thinner cortex in the frontal, temporal, and inferior parietal regions as well as the Sylvian fissure/insula.42 In PD+FOG, worse set-shifting ability (TMT-B) was associated with decoupling between cognitive and motor networks, with increased coupling between the ventral striatum and the cortical limbic network and between the cerebellum and caudate nucleus.43 Taken together, these findings suggest that complex and challenging exercises may have positive effects on brain areas beyond the frontal lobe that also modulate executive functions. However, the current study focused specifically on the improvement of the FAB scores.
One study showed that 10 sessions of telerehabilitation did not affect FAB scores in PD-FOG.44 This is the first study to investigate the effects of exercises specifically on frontal lobe function in PD+FOG. It has been suggested that FOG episodes may occur due to abnormal patterns of functional connectivity within and between cortico-striatal networks43 due to frontostriatal dysfunction implicated in FOG pathophysiology.7, 17, 18 FOG severity has been associated with greater coupling within the cognitive control network.43 This suggests that although PD+FOG have frontal lobe dysfunction, they still can compensate for frontal circuits. Although we do not know the effects of exercise on brain compensation in PD+FOG, our results support our hypothesis that challenging and complex exercise interventions attenuate frontal lobe dysfunction in PD+FOG.
Our linear multiple regression showed that improvement in frontal lobe function (FAB scores) explained decreased FOG severity following ARTI (Figure 2). Since FOG is strongly linked to frontal lobe dysfunction,7, 32, 45, 46 our results support our hypothesis that increasing the complexity of a task, as when performing ARTI exercises, may demand multiple processes in the frontal lobe related to executive function,33 which are trigger for FOG. For example, performing lunge exercises, forward and backward, on unstable devices (dyna disc and BOSU) while wearing a load and alternating arm movements require: a) coupling of posture with gait (e.g., anticipatory postural adjustments) that involves frontal lobe motor planning and initiation of movements; b) executive control to perform stepping while alternating arms simultaneously (e.g., inhibition of inappropriate actions and set-shifting while performing dual-task exercise); and c) high attentional demand while maintaining balance on unstable surfaces while wearing a load. Thus, our results suggest that ARTI may improve FOG through the high complexity required in the frontal lobe while performing the exercises. Future studies should consider other challenging and complex sensorimotor exercises for improving frontal lobe function in PD+FOG.
ARTI improves global cognition
Our results show that ARTI significantly improved global cognition (MoCA scores) (Table 2). There was a 29.4% (n=5) reduction in the proportion of PD+FOG with mild cognitive impairment (MoCA ≤ 25 scores) for the ARTI group from pre- to post-training. Although the TMR group presented a strong trend toward higher MoCA values from pre- to post-training, the proportion of PD+FOG with mild cognitve impairment remained the same (100%). These results suggest that challenging and complex exercises are important to improve global cognition in PD+FOG. A recent meta-analysis (68 studies) showed worse global cognition (MoCA scores) in PD+FOG than in non-PD+FOG and that disease severity influenced the global cognitive differences between them.5
A recent meta-analysis, including 11 studies, investigated the effects of different exercises programs (e.g., combined exercises, aerobic training, and resistance training) on MoCA scores in PD-FOG.47 In this meta-analysis, interventions combining different types of exercises (e.g. balance with coordination, dance, meditation-based complex exercise programs) but not exercise alone (e.g., aerobic or resistance training) significantly improved global cognition.47 These results reinforce the use of complex and coordinated exercises for improving cognition in PD. A few studies have investigated the effects of exercise on global cognition of PD+FOG.13, 14 Walton et al.,13 did not find any effect of 12 sessions (120 min each) of cognitive training (specific computer-based cognitive tasks) on the MoCA scores of PD+FOG. King et al.,14 found a significant improvement in global cognition (Scales for Outcomes in Parkinson’s disease-COGnition) of PD+FOG after 18 sessions (80 min each) of challenging cognitive-mobility exercises. These previous findings suggest that complex and challenges in mobility and dual-task exercises14, 16 rather than computerized cognitive training tasks without any mobility exercise13 seem to improve global cognitive function in PD+FOG. Our study and the study of King et al.,14 used progression based on the difficulty of challenging exercises. For example, King et al., 14 used motor-cognitive dual-task exercises where the participants were able to progress in the difficulty of their cognitive challenges during the agility/mobility training. Our study used motor-motor dual-task exercises where the participants were able to progress in the difficulty of their coordinative tasks with the arms simultaneously while performing half-squat exercises on the unstable device and wearing a load, which required more complexity of the exercises. Taken together, motor-cognitive dual-task exercises and motor-motor dual-task exercises with complexity are needed to improve global cognition in PD+FOG.
Our previous study in PD-FOG with mild-to-moderate PD showed an improvement of 6 points on the MoCA scores following challenging and complex training.26 Here, we observed an improvement of 3.7 points on MoCA score in PD+FOG with moderate-to-severe PD after challenging and complex training, which suggests that PD+FOG with severe PD may be more resistant in improving global cognition than PD-FOG with less PD severity. A recent study showed that cortical and subcortical atrophy is accelerated early after the onset of PD and becomes prominent in moderate-to-severe PD.48 PD+FOG with severe PD have more and diffuse cortical and subcortical atrophy (e.g., frontal, parietal, occipital and basal ganglia) than PD-FOG.32, 45, 46 A recent study demonstrated that FOG progression was significantly associated with visuo-executive subscores of MoCA.4 Interesting, improvements in MoCA score following ARTI were observed in the visuo-executive subscores (Table 4). Our previous study showed that frontoparietal and visual cortical areas are predictors of responsiveness to challenging and complex exercises in PD+FOG unmatched and matched for motor severity.49 Thus, ARTI may cause neural stimuli to different brain areas in PD+FOG, which could support our hypothesis that challenging and complex exercise interventions are effective in improving global cognition assessed by MoCA in PD+FOG.
ARTI improves attention and psychomotor speed
Our results show that only ARTI improves attention and psychomotor speed assessed with DSST from pre- to post-training (Table 2), although its improvements were not associated with the reduction of FOG. Attentional deficits and affected psychomotor speed are well-known cognitive impairments in individuals with PD.50 Studies have shown that psychomotor slowing in healthy older adults is associated with an increased risk of developing PD.50 For PD+FOG, attentional control plays a major role in gait initiation postural adjustments, as attentional deficits and cognitive overload of attentional brain networks contribute to the gait initiation failure observed in FOG.51, 52 In addition, PD+FOG present worse attentional control to perform dual tasks during postural sway, postural transitions, and walking than PD-FOG.53 This reinforces the importance of ARTI due to dual-task, challenging, and motor complexity exercises which are important for maintaining and enhancing attentional control and psychomotor speed of PD+FOG.
A few studies have investigated the effects of exercise on DSST scores in PD+FOG Carapellotti et al.,54 showed that 20 sessions of the Dance for PD® program did not improve DSST scores in PD+FOG or PD-FOG. Walton et al.,13 showed that 12 sessions (120 min each) of cognitive training (specific computer-based cognitive tasks) but not the active control (non-specific computer-based tasks) improved processing speed assessed by the Trial Making Test part A in PD+FOG. These results suggest that cognitive training13 and ARTI may promote more efficient integration of cognitive and motor brain networks, improving attentional control of movement and psychomotor speed; however, this hypothesis needs to be tested.
Limitations
This study has some limitations. First, only neuropsychological scales were used to evaluate cognition, thus, assumptions on brain cognitive networks involved in the ARTI program are lacking. Second, although some cognitive domains (e.g., visuospatial function) may be underrepresented due to the lack of specific scales, they are included in the MoCA subscores. Third, all participants have mild cognitive impairment by MoCA but not dementia by Mini-Mental State Examination, which was used as screening. Fourth, we did not assess participants after a 12-week wash-out period to determine whether these improvements in cognition from the ARTI program were retained. Fifth, even though our study showed robust changes in secondary cognitive outcomes after ARTI, but not after TMR, the total sample size of the present trial was small. Thus, a larger RCT trial is needed to validate the reported benefits of ARTI on cognition.
CONCLUSIONS
ARTI, a challenging and high motor complexity exercise intervention, improves different cognitive domains in PD+FOG such as frontal lobe function, global cognition, and attention and psychomotor speed. Improvement in frontal lobe function was correlated with decreased FOG ratio objectively assessed following ARTI. These findings show the link between frontal lobe function, FOG, and exercise. Thus, ARTI is an innovative intervention resulting in cognitive improvement in PD+FOG.
Supplementary Material
Acknowledgment
We would like to thank participants from the Movement Disorders Clinic from School of Medicine of the University of São Paulo for their commitment to study, FAPESP, CNPq, and CAPES, PCO Pilot Grant Award, and OHSU-OFDIR.
Funding
This work has been supported by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo under award numbers 2016/13115-9 and 2018/16909-1 for CSB, the Conselho Nacional de Desenvolvimento Científico e Tecnológico under award numbers 303047/2022-4 for CU, National Institutes of Health under award number R01AG006457 for FBH, and Department of Veterans Affairs Merit Award number 5I01RX001075 for FBH, a PCO Pilot Grant Award (CSB), and an OHSU-OFDIR Fellowship (CSB).
Footnotes
Declaration of Conflicting Interests
CSB, FA, AB, ERB, and CU declare that they have no conflicts of interest relevant to the content of this review. FBH has a significant financial interest in APDM, a company that may have a commercial interest in the results of this research and technology. FBH also consultants with Biogen, Neuropore, Sanofi, Adamus, Abbott, and Takeda. This potential individual conflict has been reviewed and managed by Oregon Health & Science University.
REFERENCES
- 1.Fengler S, Liepelt-Scarfone I, Brockmann K, Schaffer E, Berg D, Kalbe E. Cognitive changes in prodromal Parkinson’s disease: A review. Movement disorders : official journal of the Movement Disorder Society. Dec 2017;32(12):1655–1666. doi: 10.1002/mds.27135 [DOI] [PubMed] [Google Scholar]
- 2.Pigott K, Rick J, Xie SX, et al. Longitudinal study of normal cognition in Parkinson disease. Neurology. Oct 13 2015;85(15):1276–82. doi: 10.1212/WNL.0000000000002001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hely MA, Morris JG, Reid WG, Trafficante R. Sydney Multicenter Study of Parkinson’s disease: non-L-dopa-responsive problems dominate at 15 years. Movement disorders : official journal of the Movement Disorder Society. Feb 2005;20(2):190–9. doi: 10.1002/mds.20324 [DOI] [PubMed] [Google Scholar]
- 4.Qu Y, Li J, Chen Y, et al. Freezing of gait is a risk factor for cognitive decline in Parkinson’s disease. Journal of neurology. Jan 2023;270(1):466–476. doi: 10.1007/s00415-022-11371-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monaghan AS, Gordon E, Graham L, Hughes E, Peterson DS, Morris R. Cognition and freezing of gait in Parkinson’s disease: A systematic review and meta-analysis. Neurosci Biobehav Rev. Apr 2023;147:105068. doi: 10.1016/j.neubiorev.2023.105068 [DOI] [PubMed] [Google Scholar]
- 6.Peterson DS, King LA, Cohen RG, Horak FB. Cognitive Contributions to Freezing of Gait in Parkinson Disease: Implications for Physical Rehabilitation. Physical therapy. May 2016;96(5):659–70. doi: 10.2522/ptj.20140603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. The Lancet Neurology. Aug 2011;10(8):734–44. doi: 10.1016/S1474-4422(11)70143-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerr GK, Worringham CJ, Cole MH, Lacherez PF, Wood JM, Silburn PA. Predictors of future falls in Parkinson disease. Neurology. Jul 13 2010;75(2):116–24. doi: 10.1212/WNL.0b013e3181e7b688 [DOI] [PubMed] [Google Scholar]
- 9.Allen NE, Schwarzel AK, Canning CG. Recurrent falls in Parkinson’s disease: a systematic review. Parkinson’s disease. 2013;2013:906274. doi: 10.1155/2013/906274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jansen JAF, Capato TTC, Darweesh SKL, et al. Exploring the levodopa-paradox of freezing of gait in dopaminergic medication-naive Parkinson’s disease populations. NPJ Parkinson’s disease. Sep 9 2023;9(1):130. doi: 10.1038/s41531-023-00575-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gul A, Yousaf J. L-Dopa response to Cortical Dysfunctions, health related quality of life and Fatigue Severity in Idiopathic Parkinson’s disease. Pak J Med Sci. Jul-Aug 2018;34(4):1014–1018. doi: 10.12669/pjms.344.14753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moustafa AA, Herzallah MM, Gluck MA. Dissociating the cognitive effects of levodopa versus dopamine agonists in a neurocomputational model of learning in Parkinson’s disease. Neurodegener Dis. 2013;11(2):102–11. doi: 10.1159/000341999 [DOI] [PubMed] [Google Scholar]
- 13.Walton CC, Mowszowski L, Gilat M, et al. Cognitive training for freezing of gait in Parkinson’s disease: a randomized controlled trial. NPJ Parkinson’s disease. 2018;4:15. doi: 10.1038/s41531-018-0052-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King LA, Mancini M, Smulders K, et al. Cognitively Challenging Agility Boot Camp Program for Freezing of Gait in Parkinson Disease. Neurorehabilitation and neural repair. May 2020;34(5):417–427. doi: 10.1177/1545968320909331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vieira-Yano B, Martini DN, Horak FB, et al. The Adapted Resistance Training with Instability Randomized Controlled Trial for Gait Automaticity. Movement disorders : official journal of the Movement Disorder Society. Jan 2021;36(1):152–163. doi: 10.1002/mds.28298 [DOI] [PubMed] [Google Scholar]
- 16.Silva-Batista C, de Lima-Pardini AC, Nucci MP, et al. A Randomized, Controlled Trial of Exercise for Parkinsonian Individuals With Freezing of Gait. Movement disorders : official journal of the Movement Disorder Society. Jun 18 2020;35(9):1607–1617. doi: 10.1002/mds.28128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis SJ, Barker RA. A pathophysiological model of freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord. Jun 2009;15(5):333–8. doi: 10.1016/j.parkreldis.2008.08.006 [DOI] [PubMed] [Google Scholar]
- 18.Shine JM, Moustafa AA, Matar E, Frank MJ, Lewis SJ. The role of frontostriatal impairment in freezing of gait in Parkinson’s disease. Front Syst Neurosci. 2013;7:61. doi: 10.3389/fnsys.2013.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. Dec 12 2000;55(11):1621–6. doi: 10.1212/wnl.55.11.1621 [DOI] [PubMed] [Google Scholar]
- 20.Kume K, Hanyu H, Murakami M, et al. Frontal Assessment Battery and brain perfusion images in amnestic mild cognitive impairment. Geriatr Gerontol Int. Jan 2011;11(1):77–82. doi: 10.1111/j.1447-0594.2010.00645.x [DOI] [PubMed] [Google Scholar]
- 21.Oshima E, Terada S, Sato S, et al. Frontal assessment battery and brain perfusion imaging in Alzheimer’s disease. Int Psychogeriatr. Jun 2012;24(6):994–1001. doi: 10.1017/S1041610211002481 [DOI] [PubMed] [Google Scholar]
- 22.Bezdicek O, Ruzicka F, Fendrych Mazancova A, et al. Frontal Assessment Battery in Parkinson’s Disease: Validity and Morphological Correlates. J Int Neuropsychol Soc. Sep 2017;23(8):675–684. doi: 10.1017/S1355617717000522 [DOI] [PubMed] [Google Scholar]
- 23.Guedj E, Allali G, Goetz C, et al. Frontal Assessment Battery is a marker of dorsolateral and medial frontal functions: A SPECT study in frontotemporal dementia. J Neurol Sci. Oct 15 2008;273(1-2):84–7. doi: 10.1016/j.jns.2008.06.035 [DOI] [PubMed] [Google Scholar]
- 24.Tessitore A, Amboni M, Esposito F, et al. Resting-state brain connectivity in patients with Parkinson’s disease and freezing of gait. Parkinsonism Relat Disord. Jul 2012;18(6):781–7. doi: 10.1016/j.parkreldis.2012.03.018 [DOI] [PubMed] [Google Scholar]
- 25.Silva-Batista C, Corcos DM, Kanegusuku H, et al. Balance and fear of falling in subjects with Parkinson’s disease is improved after exercises with motor complexity. Gait & posture. Mar 2018;61:90–97. doi: 10.1016/j.gaitpost.2017.12.027 [DOI] [PubMed] [Google Scholar]
- 26.Silva-Batista C, Corcos DM, Roschel H, et al. Resistance Training with Instability for Patients with Parkinson’s Disease. Med Sci Sports Exerc. Sep 2016;48(9):1678–87. doi: 10.1249/MSS.0000000000000945 [DOI] [PubMed] [Google Scholar]
- 27.Ludyga S, Gerber M, Puhse U, Looser VN, Kamijo K. Systematic review and meta-analysis investigating moderators of long-term effects of exercise on cognition in healthy individuals. Nat Hum Behav. Jun 2020;4(6):603–612. doi: 10.1038/s41562-020-0851-8 [DOI] [PubMed] [Google Scholar]
- 28.Carey JR, Bhatt E, Nagpal A. Neuroplasticity promoted by task complexity. Exerc Sport Sci Rev. Jan 2005;33(1):24–31. [PubMed] [Google Scholar]
- 29.Muir AL, Jones LM, Signal NEJ. Is neuroplasticity promoted by task complexity? New Zealand Journal of Physiotherapy. Dec 2009;37(3)doi: 10.2522/ptj.20060310.ic [DOI] [Google Scholar]
- 30.Brugger F, Abela E, Hagele-Link S, Bohlhalter S, Galovic M, Kagi G. Do executive dysfunction and freezing of gait in Parkinson’s disease share the same neuroanatomical correlates? J Neurol Sci. Sep 15 2015;356(1-2):184–7. doi: 10.1016/j.jns.2015.06.046 [DOI] [PubMed] [Google Scholar]
- 31.Amboni M, Cozzolino A, Longo K, Picillo M, Barone P. Freezing of gait and executive functions in patients with Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. Feb 15 2008;23(3):395–400. doi: 10.1002/mds.21850 [DOI] [PubMed] [Google Scholar]
- 32.Kostic VS, Agosta F, Pievani M, et al. Pattern of brain tissue loss associated with freezing of gait in Parkinson disease. Neurology. Feb 7 2012;78(6):409–16. doi: 10.1212/WNL.0b013e318245d23c [DOI] [PubMed] [Google Scholar]
- 33.Stuss DT, Alexander MP. Executive functions and the frontal lobes: a conceptual view. Psychol Res. 2000;63(3-4):289–98. doi: 10.1007/s004269900007 [DOI] [PubMed] [Google Scholar]
- 34.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. Apr 2005;53(4):695–9. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 35.Kufman AS. Test Review: Wechsler D. Manual for the Wechsler Adult Intelligence Scale, Revised. New York: Psychological Corporation. Journal of Psychoeducational Assessment. Jun 1983;1(3):309–310. doi: 10.1177/073428298300100310 [DOI] [Google Scholar]
- 36.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale (NJ): L. Erlbaum Associates. 1988:29–35. [Google Scholar]
- 37.Hurtado-Pomares M, Carmen Terol-Cantero M, Sanchez-Perez A, Peral-Gomez P, Valera-Gran D, Navarrete-Munoz EM. The frontal assessment battery in clinical practice: a systematic review. Int J Geriatr Psychiatry. Feb 2018;33(2):237–251. doi: 10.1002/gps.4751 [DOI] [PubMed] [Google Scholar]
- 38.Dormann CF, Elith J, Bacher S, et al. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography. 2012;36:027–046. doi: 10.1111/j.1600-0587.2012.07348.x [DOI] [Google Scholar]
- 39.Roberts KL, Hall DA. Examining a supramodal network for conflict processing: a systematic review and novel functional magnetic resonance imaging data for related visual and auditory stroop tasks. J Cogn Neurosci. Jun 2008;20(6):1063–78. doi: 10.1162/jocn.2008.20074 [DOI] [PubMed] [Google Scholar]
- 40.Fling BW, Cohen RG, Mancini M, Nutt JG, Fair DA, Horak FB. Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain. Aug 2013;136(Pt 8):2405–18. doi: 10.1093/brain/awt172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varjacic A, Mantini D, Demeyere N, Gillebert CR. Neural signatures of Trail Making Test performance: Evidence from lesion-mapping and neuroimaging studies. Neuropsychologia. Jul 1 2018;115:78–87. doi: 10.1016/j.neuropsychologia.2018.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacPherson SE, Cox SR, Dickie DA, et al. Processing speed and the relationship between Trail Making Test-B performance, cortical thinning and white matter microstructure in older adults. Cortex. Oct 2017;95:92–103. doi: 10.1016/j.cortex.2017.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ehgoetz Martens KA, Hall JM, Georgiades MJ, et al. The functional network signature of heterogeneity in freezing of gait. Brain. Apr 1 2018;141(4):1145–1160. doi: 10.1093/brain/awy019 [DOI] [PubMed] [Google Scholar]
- 44.Bianchini E, Onelli C, Morabito C, et al. Feasibility, Safety, and Effectiveness of Telerehabilitation in Mild-to-Moderate Parkinson’s Disease. Frontiers in neurology. 2022;13:909197. doi: 10.3389/fneur.2022.909197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Canu E, Agosta F, Sarasso E, et al. Brain structural and functional connectivity in Parkinson’s disease with freezing of gait. Human brain mapping. Dec 2015;36(12):5064–78. doi: 10.1002/hbm.22994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jha M, Jhunjhunwala K, Sankara BB, et al. Neuropsychological and imaging profile of patients with Parkinson’s disease and freezing of gait. Parkinsonism Relat Disord. Oct 2015;21(10):1184–90. doi: 10.1016/j.parkreldis.2015.08.009 [DOI] [PubMed] [Google Scholar]
- 47.Kim R, Lee TL, Lee H, et al. Effects of physical exercise interventions on cognitive function in Parkinson’s disease: An updated systematic review and meta-analysis of randomized controlled trials. Parkinsonism Relat Disord. Dec 2023;117:105908. doi: 10.1016/j.parkreldis.2023.105908 [DOI] [PubMed] [Google Scholar]
- 48.Filippi M, Sarasso E, Piramide N, et al. Progressive brain atrophy and clinical evolution in Parkinson’s disease. Neuroimage Clin. 2020;28:102374. doi: 10.1016/j.nicl.2020.102374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silva-Batista C, Ragothaman A, Mancini M, et al. Cortical thickness as predictor of response to exercise in people with Parkinson’s disease. Hum Brain Mapp. Jan 2021;42(1):139–153. doi: 10.1002/hbm.25211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amieva H, Meillon C, Proust-Lima C, Dartigues JF. Is Low Psychomotor Speed a Marker of Brain Vulnerability in Late Life? Digit Symbol Substitution Test in the Prediction of Alzheimer, Parkinson, Stroke, Disability, and Depression. Dement Geriatr Cogn Disord. 2019;47(4-6):297–305. doi: 10.1159/000500597 [DOI] [PubMed] [Google Scholar]
- 51.Mandal M, Khan A. Attention switching deficit in patients of Parkinson’s disease who experience freezing of gait. Appl Neuropsychol Adult. Jul-Aug 2023;30(4):389–400. doi: 10.1080/23279095.2021.1951268 [DOI] [PubMed] [Google Scholar]
- 52.Tard C, Dujardin K, Bourriez JL, et al. Attention modulates step initiation postural adjustments in Parkinson freezers. Parkinsonism Relat Disord. Mar 2014;20(3):284–9. doi: 10.1016/j.parkreldis.2013.11.016 [DOI] [PubMed] [Google Scholar]
- 53.de Souza Fortaleza AC, Mancini M, Carlson-Kuhta P, et al. Dual task interference on postural sway, postural transitions and gait in people with Parkinson’s disease and freezing of gait. Gait & posture. Jul 2017;56:76–81. doi: 10.1016/j.gaitpost.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carapellotti AM, Rodger M, Doumas M. Evaluating the effects of dance on motor outcomes, non-motor outcomes, and quality of life in people living with Parkinson’s: a feasibility study. Pilot Feasibility Stud. Feb 9 2022;8(1):36. doi: 10.1186/s40814-022-00982-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


