ABSTRACT
Invasive liver abscess syndrome due to hypervirulent Klebsiella pneumoniae poses significant mortality risk, particularly in immunocompromised patients. Early recognition in non‐endemic regions is crucial for prompt antibiotic therapy and source control, highlighting the need for increased suspicion and aggressive management of this rare disease to improve patient outcomes.
Keywords: Hypervirulent klebsiella pneumoniae , invasive liver abscess syndrome, splenectomy, uncontrolled diabetes mellitus
1. Introduction
Invasive liver abscess syndrome (ILAS) caused by Klebsiella pneumoniae ( K. pneumoniae ) is a rare clinical condition characterized by bacteremia and metastatic infections with disseminated abscesses [1]. Extrahepatic metastatic infections may result in severe complications and represent a poor prognosis if not appropriately treated [2]. The invasive nature of the disease is due to certain subtypes of K. pneumoniae with virulence characteristics such as hypermucoviscosity phenotypes [1].
ILAS is prevalent mostly in Asian and African countries, but only a handful of cases have been reported in Western countries [3]. In immunocompetent individuals, these infections usually respond to an extended course of antibiotics, and mortality rates range from 4% to 11% [2]. However, in the context of immunocompromised individuals, the mortality rates can be significantly higher [4]. Herein, we present a case of ILAS caused likely by the hypervirulent mucus phenotype of K. pneumoniae in the background of multiple risk factors, including a history of splenectomy and newly diagnosed uncontrolled type 2 diabetes mellitus.
2. Case History
A 34‐year‐old male with a history of immigration from the Republic of Congo presented with complaints of abdominal pain and shortness of breath for 2 days. He had a past medical history of significant recurrent alcoholic pancreatitis leading to splenic vein thrombosis and splenectomy. He was noted to be febrile (102.2 F), tachycardic, tachypneic, and hypoxic, requiring 2 L of oxygen supplementation.
3. Methods
His laboratory investigations were notable for high anion gap metabolic acidosis, elevated blood sugars, glycosylated hemoglobin of 19%, and ketonuria with positive beta‐hydroxybutyrate. His initial CT imaging revealed bilateral lower lobe consolidations and also an abscess in the liver (Figures 1 and 2). He was started on an insulin infusion and fluid resuscitated for diabetic ketoacidosis and anti‐bacterial coverage with Piperacillin‐Tazobactam for the management of pneumonia and liver abscess. The patient tested negative for HIV, hepatitis B, and C, TB by quantiferon analysis, Neisseria gonorrhoeae , MRSA, COVID‐19, and EBV.
FIGURE 1.
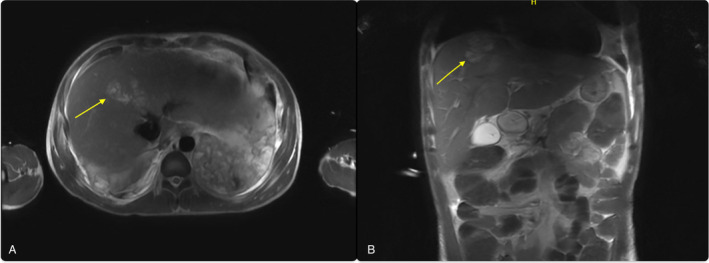
Magnetic resonance imaging (A) Coronal view (B) Sagittal view, showing liver abscess.
FIGURE 2.
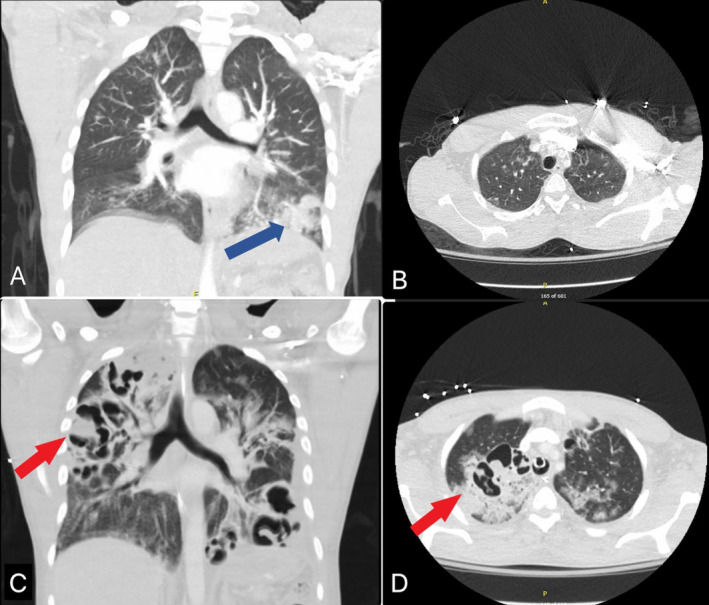
CT imaging of the chest (A) Sagittal view, and (B) Coronal view showing disease process at presentation with large left lower lobe consolidation (Blue arrow); (C) Sagittal view, and (D) Coronal view showing (red arrows) areas of cavitations and multifocal pneumonia.
Due to worsening hypoxia and increased work of breathing, the patient was intubated 2 days into his admission. Bronchoscopy with serial washings was done, and bronchoalveolar cultures were positive for K. pneumoniae growth. These were also negative for acid‐fast bacilli and fungi. Ultrasound‐guided drainage of the liver abscess was done, and the drain was left in situ. The liver‐drained abscess culture was also positive for K. pneumoniae . The patient continued to be febrile with worsening of leukocytosis with a predominant left shift (18,000/mm3; normal: 4500‐11,000/mm3). Due to his worsening clinical condition, antibiotics were adjusted to meropenem and linezolid with antifungal coverage with micafungin. Repeat CT imaging revealed multiple lung cavitations and abscesses (Figure 2). The patient's oxygen requirements continued to increase on mechanical ventilation. Due to the multiplicity and extensiveness of the lung abscesses, surgical options were very limited and deferred. Due to increasing suspicion of hypervirulent K. pneumoniae with high mucus phenotype, antibiotics were further escalated to include tobramycin and rifampin.
Despite multiple bronchial washings, drainage of liver abscess, and 27 days of antibiotic therapy, the patient's condition continued to decline. Eventually, he was noted to be in distributive shock secondary to severe sepsis, requiring three pressor support with phenylephrine, norepinephrine, and vasopressin. His course was further complicated by renal failure, and continuous renal replacement therapy was attempted. The patient demised in spite of maximum support and medical therapy.
4. Conclusion
This case highlights the extremely high mortality in ILAS with multiple risk factors. Despite aggressive attempts at source control and antibiotic regimens, due to the significantly immunocompromised state and extensiveness of the disease, our patient deteriorated rapidly, leading to mortality. Timely diagnosis and a low threshold of suspicion for these rare subtypes/phenotypes of K. pneumoniae , especially in non‐endemic regions like the United States, is crucial to initiate prompt treatment and source control.
5. Discussion
This case details ILAS in a 34‐year‐old man with substantial medical comorbidities, including newly diagnosed uncontrolled diabetes (hemoglobin A1c > 19%) and a remote history of splenectomy. Due to the extensive damage to the liver by the invasive bacterial species, he also developed hypoproteinemia and hypoalbuminemia during the hospital course, worsening his immunological status. These put him in an immunocompromised state, worsening the trajectory of his illness, and leading to mortality despite early identification of the offending organism and aggressive management with multiple antibiotics regimens. Furthermore, this case presents a unique challenge regarding source control due to rapidly progressing disseminated abscesses involving the liver and multiple segments of both lungs within a very short period. The lesions' severity and anatomical distribution made abscess drainage a challenging and non‐applicable option.
Most community‐acquired K. pneumoniae infections cause pneumonia and urinary tract infections; however, a rare and more distinct invasive syndrome has been associated with this infection in the last two decades [5, 6]. ILAS, defined by the development of one or more abscesses inside the liver, was found to be caused by a hypervirulent strain of K. pneumonia that had serious and often life‐threatening complications. These infections have more commonly been reported in the Southeast Asian subcontinent and their incidence over the last two decades has only been increasing. In a retrospective study conducted on patients in Taipei with liver abscesses, 78% of the abscesses were caused by K. pneumoniae [6].
Hypervirulent strains often result in more extensive infections, resulting in pyogenic liver abscesses, necrotizing fasciitis, meningitis, myositis, and endophthalmitis [7]. Serotypes K1 and K2 are most often associated with hypervirulent strain. Unlike classical K. pneumoniae , which are nosocomial in nature, hypervirulent strains are most commonly community‐acquired [8]. These are usually more invasive and have a tendency to become disseminated. The bacterium's capacity to proliferate and elude the host immune system is greatly enhanced by the acquisition of large plasmids that enhance capsule formation and siderophore encoding, which are genetic elements that are partially responsible for this hypervirulence [7].
Notably, compared to those with liver abscesses caused by other organisms, individuals with liver abscesses from K. pneumoniae had a higher prevalence of diabetes mellitus (66% vs. 19%) [6]. Regardless of the microbiological source, diabetic patients, such as in the present case, had longer fever durations and required prolonged hospital stays [6]. This is likely because hyperglycemia can increase the risk of infection in patients with diabetes through several different processes, including hyperglycemia‐induced delayed chemotactic response, phagocytosis, and neutrophil activity [9].
Due to the infections' rapid progression and significant resistance profile, the diagnostic strategy for these infections is still complicated and requires timely microbial culture, sensitivity testing, and molecular approaches to detect particular resistance genes and virulence components [10]. Patients often endorse nonspecific symptoms of fever, chills, and abdominal pain [11]. Other potential findings include elevated transaminases and CRP of greater than 20 [2]. Patients with diabetes mellitus who are noted to have K. pneumoniae bacteremia, meningitis, or endophthalmitis should be evaluated for a potential liver abscess [1]. Abdominal CT is the preferred diagnostic study compared to ultrasound [12]. Abscesses secondary to K. pneumoniae aggregate in a single lobe and are solid in characteristic and multilocular [12].
Antimicrobial treatment is usually tailored according to the sensitivity pattern of the bacteria. A third‐generation cephalosporin is often used; however, ampicillin‐sulbactam, aztreonam, or quinolone can be considered. Carbapenems can be used with strains that produce extended‐spectrum beta‐lactamases (ESBL) [1]. Rifampin has also been shown to have anti‐mucoviscous activity against hvKp, and some cases have documented clinical improvement in ILAS with a combination of meropenem and rifampin [13]. Some case reports have used either a combination or sequential use of the above antibiotics with varying success rates [13, 14]. For diabetic patients, strict glycemic control is also an important part of management. Percutaneous drainage can be done in the setting of limited abscesses to improve clinical response [15]. The K. pneumoniae isolated in this patient was resistant only to ampicillin; thus, multiple antibiotic regimens were attempted, including Piperacillin‐Tazobactam, meropenem, rifampin, and tobramycin.
It is important to recognize the pattern of dealing with liver abscess and pneumonia at time of diagnosis. Promoting the physician to suspect highly resistant strains that likely require an early and aggressive antibiotic regimen and possibly surgical drainage of the abscess to achieve better source control as this might change the outcome.
Author Contributions
Amey Joshi: conceptualization, data curation, formal analysis, investigation, methodology, supervision, visualization, writing – original draft, writing – review and editing. Eki Wari: conceptualization, investigation, project administration, validation, visualization, writing – original draft, writing – review and editing. Harith Ghnaima: investigation, project administration, supervision, visualization, writing – original draft, writing – review and editing. Georgette Nader: investigation, methodology, project administration, supervision, validation, writing – original draft, writing – review and editing. Surya Chennupati: conceptualization, data curation, supervision, visualization, writing – original draft, writing – review and editing. Nagham Jafar: conceptualization, formal analysis, investigation, methodology, project administration, resources, software, supervision, writing – review and editing.
Consent
Written informed consent was obtained from the patient's family to publish this report as the patient was critically ill and unfortunately demised. This case report has been completely anonymized.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
This project was funded by the Michigan State University Foundation.
Funding: This work was supported by Michigan State University Research Foundation.
Data Availability Statement
The data supporting the findings of the present study are available from corresponding author upon request.
References
- 1. Siu L. K., Yeh K. M., Lin J. C., Fung C. P., and Chang F. Y., “ Klebsiella pneumoniae Liver Abscess: A New Invasive Syndrome,” Lancet Infectious Diseases 12 (2012): 881–887. [DOI] [PubMed] [Google Scholar]
- 2. Lee S. S., Chen Y. S., Tsai H. C., et al., “Predictors of Septic Metastatic Infection and Mortality Among Patients With Klebsiella pneumoniae Liver Abscess,” Clinical Infectious Diseases 47 (2008): 642–650. [DOI] [PubMed] [Google Scholar]
- 3. Byarugaba D. K., Erima B., Wokorach G., et al., “Genome Analysis of Klebsiella pneumoniae Reveals International High‐Risk Pandemic MDR Clones Emerging in Tertiary Healthcare Settings in Uganda,” Pathogens 12, no. 11 (2023): 1334, 10.3390/pathogens12111334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ramos‐Castañeda J. A., Ruano‐Ravina A., Barbosa‐Lorenzo R., et al., “Mortality due to KPC Carbapenemase‐Producing Klebsiella pneumoniae Infections: Systematic Review and Meta‐Analysis: Mortality due to KPC Klebsiella pneumoniae Infections,” Journal of Infection 76, no. 5 (2018): 438–448, 10.1016/j.jinf.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 5. Chang F. Y., Chou M. Y., Fan R. L., and Shaio M. F., “A Clinical Study of Klebsiella Liver Abscess,” Taiwan Yi Xue Hui Za Zhi 87 (1988): 282–287. [PubMed] [Google Scholar]
- 6. Chang F. Y. and Chou M. Y., “Comparison of Pyogenic Liver Abscesses Caused by Klebsiella pneumoniae and Non‐ K. pneumoniae Pathogens,” Journal of the Formosan Medical Association 94, no. 5 (1995): 232–237. [PubMed] [Google Scholar]
- 7. Russo T. A. and Marr C. M., “Hypervirulent Klebsiella pneumoniae ,” Clinical Microbiology Reviews 32, no. 3 (2019): e00001‐19, 10.1128/CMR.00001-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jun J. B., “ Klebsiella pneumoniae Liver Abscess,” Infect Chemother 50, no. 3 (2018): 210–218, 10.3947/ic.2018.50.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee C. H., Chen I. L., Chuah S. K., et al., “Impact of Glycemic Control on Capsular Polysaccharide Biosynthesis and Opsonophagocytosis of Klebsiella pneumoniae : Implications for Invasive Syndrome in Patients With Diabetes Mellitus,” Virulence 7, no. 7 (2016): 770–778, 10.1080/21505594.2016.1186315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paczosa M. K. and Mecsas J., “ Klebsiella pneumoniae : Going on the Offense With a Strong Defense,” Microbiology and Molecular Biology Reviews 80, no. 3 (2016): 629–661, 10.1128/MMBR.00078-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang C. C., Yen C. H., Ho M. W., and Wang J. H., “Comparison of Pyogenic Liver Abscess Caused by Non‐Klebsiella pneumoniae and Klebsiella pneumoniae ,” Journal of Microbiology, Immunology, and Infection 37 (2004): 176–184. [PubMed] [Google Scholar]
- 12. Lin A. C., Yeh D. Y., Hsu Y. H., et al., “Diagnosis of Pyogenic Liver Abscess by Abdominal Ultrasonography in the Emergency Department,” Emergency Medicine Journal 26 (2009): 273–275. [DOI] [PubMed] [Google Scholar]
- 13. Alsaif H. S., Venkatesh S. K., Chan D. S., and Archuleta S., “CT Appearance of Pyogenic Liver Abscesses Caused by Klebsiella pneumoniae ,” Radiology 260 (2011): 129–138. [DOI] [PubMed] [Google Scholar]
- 14. Tohda M., Oinuma K. I., Sakiyama A., et al., “Rifampicin Exerts Anti‐Mucoviscous Activity Against Hypervirulent Klebsiella pneumoniae via Binding to the RNA Polymerase β Subunit,” Journal of Global Antimicrobial Resistance 32 (2023): 21–28, 10.1016/j.jgar.2022.11.018. [DOI] [PubMed] [Google Scholar]
- 15. Seo R., Kudo D., Gu Y., et al., “Invasive Liver Abscess Syndrome Caused by Klebsiella pneumoniae With Definite K2 Serotyping in Japan: A Case Report,” Surgical Case Reports 2, no. 1 (2016): 72, 10.1186/s40792-016-0201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of the present study are available from corresponding author upon request.


