Abstract
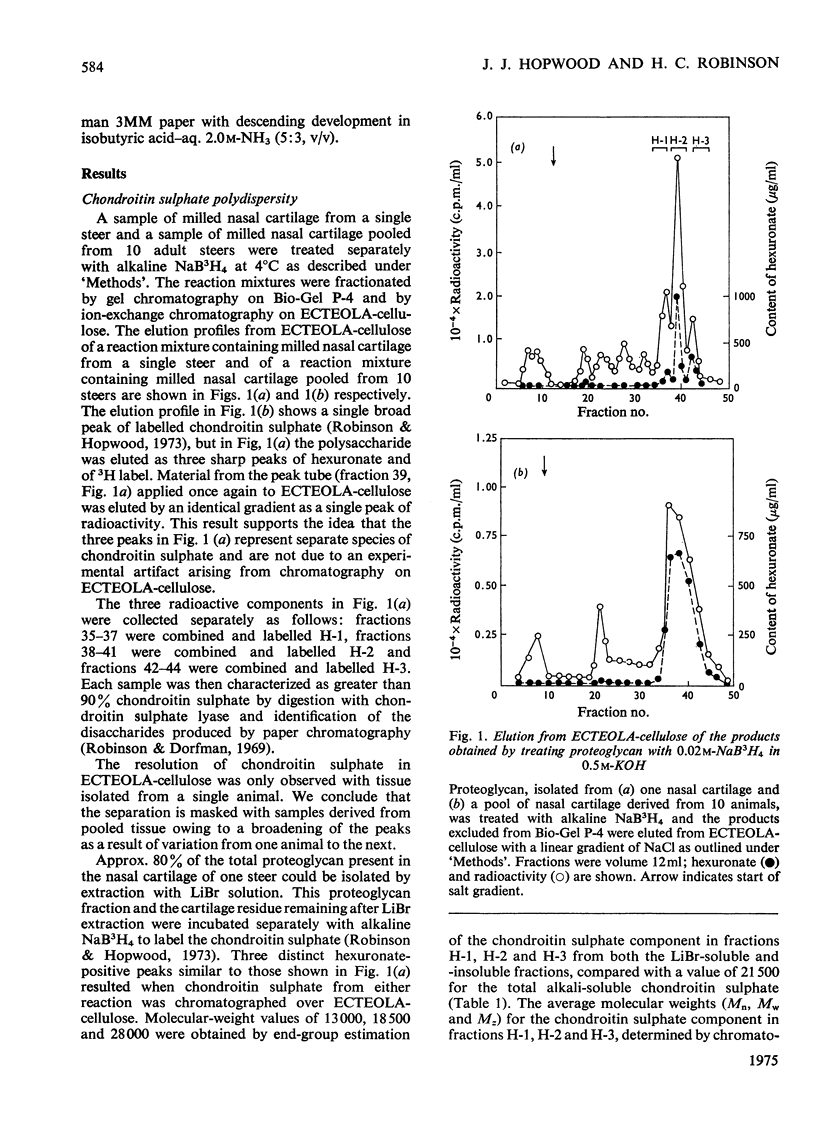
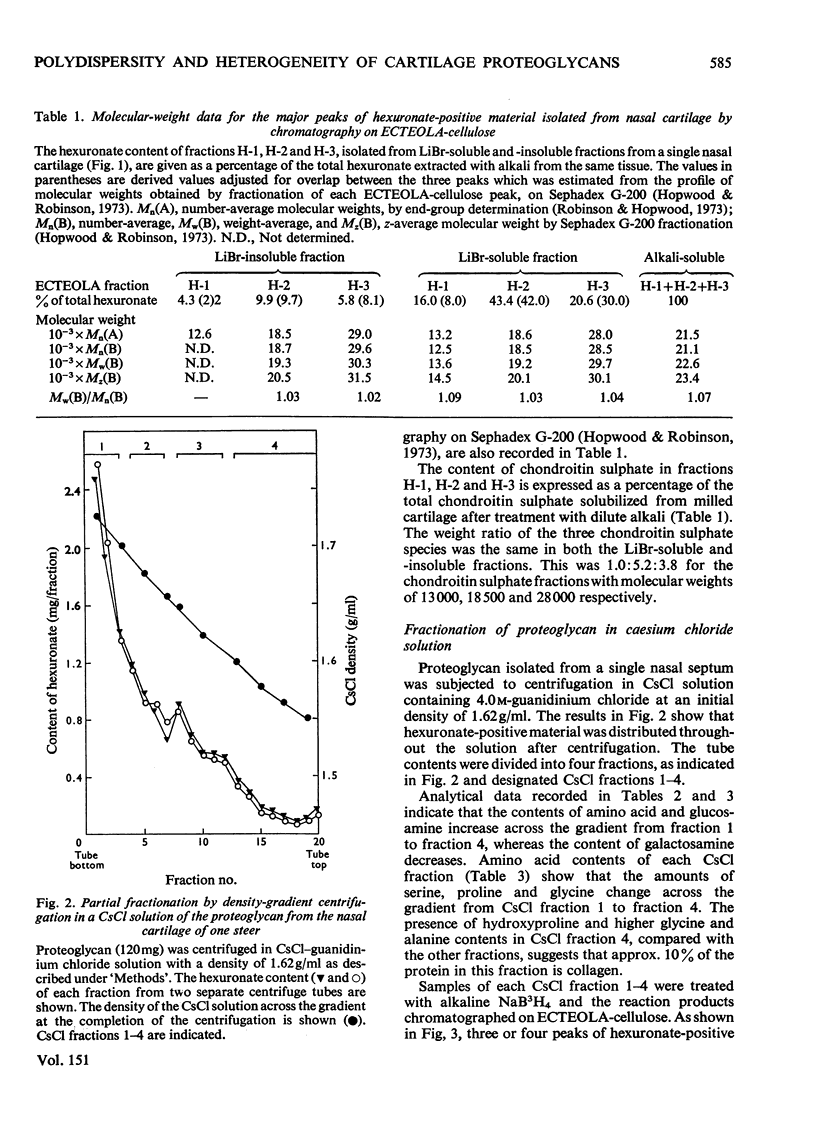
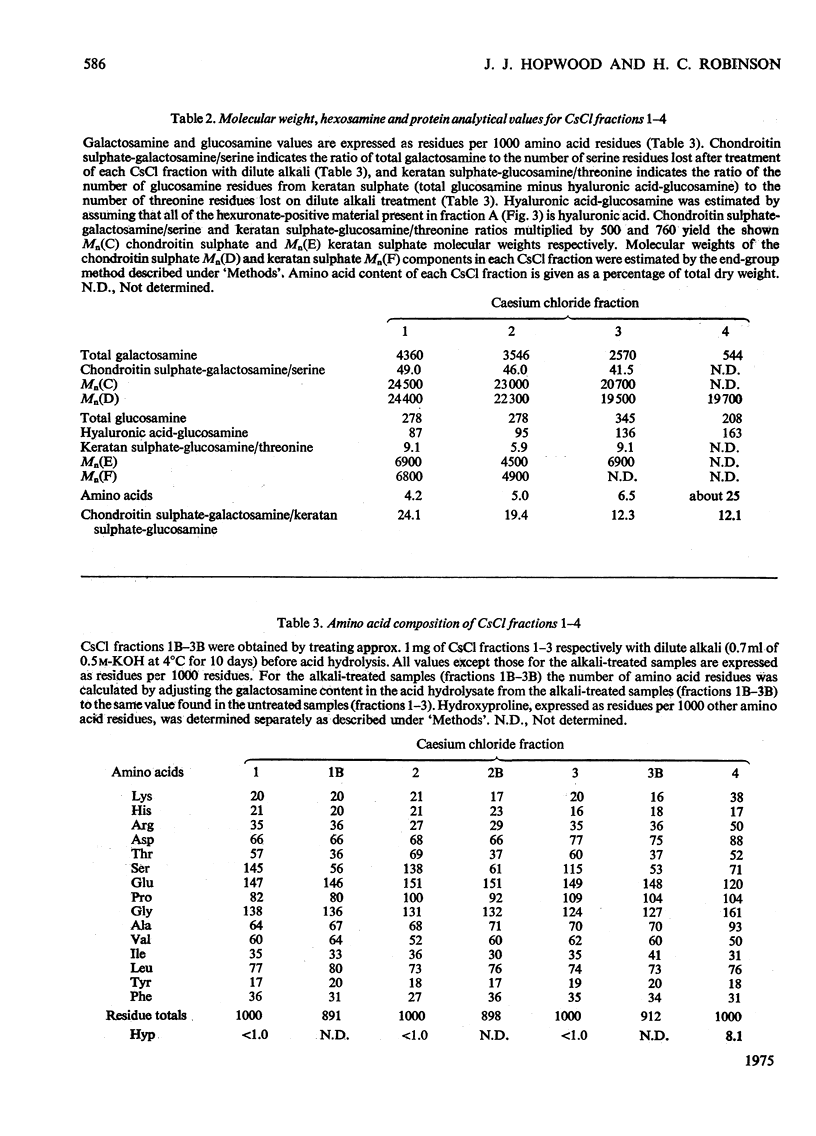
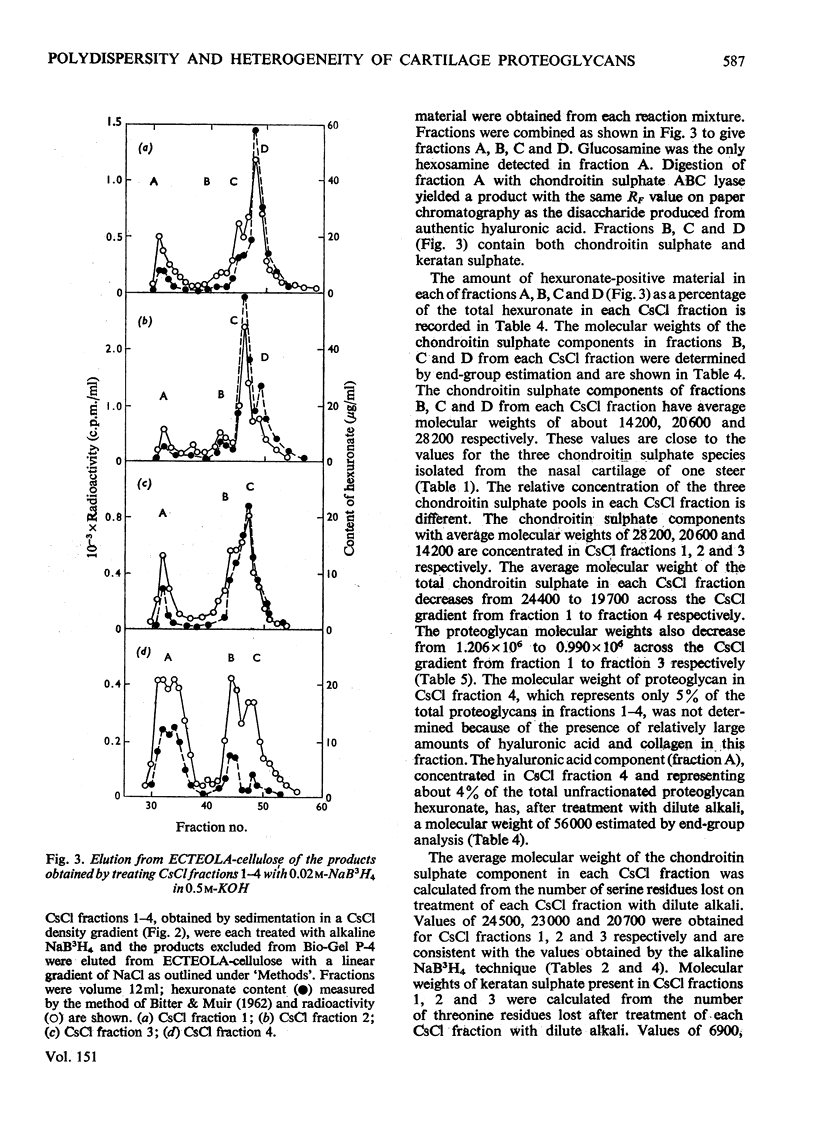
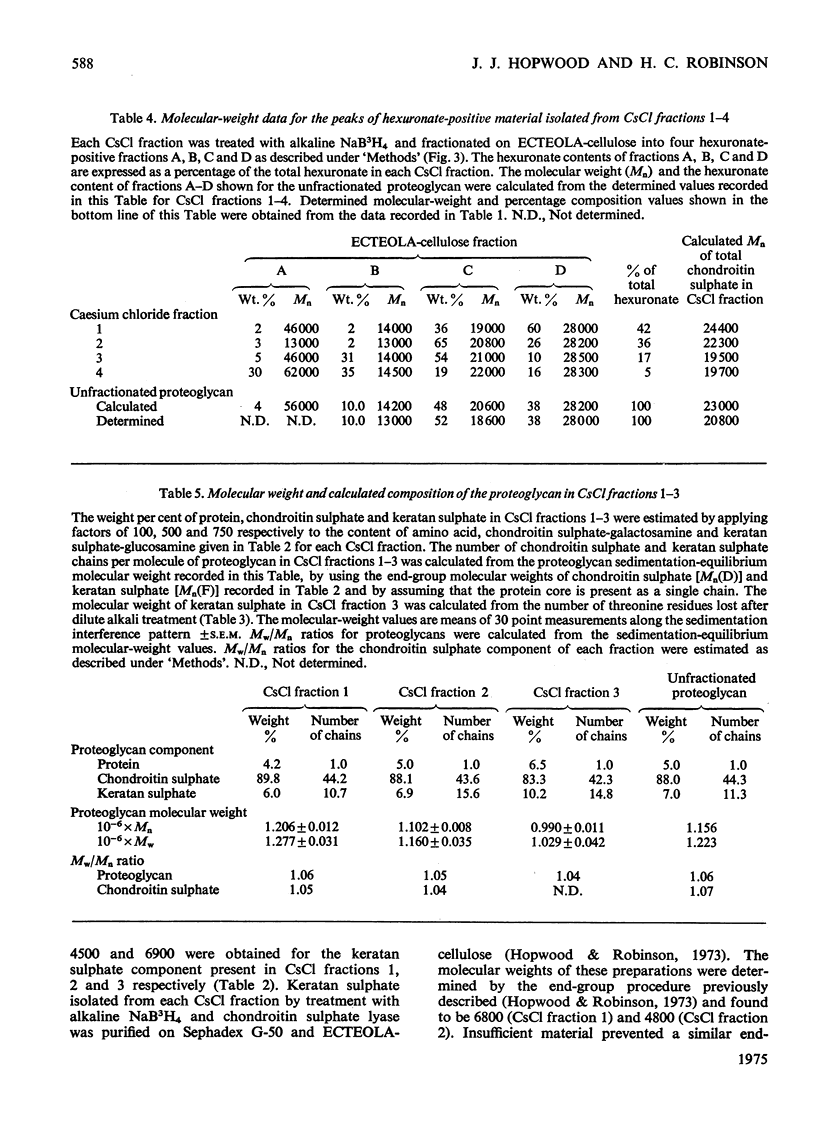
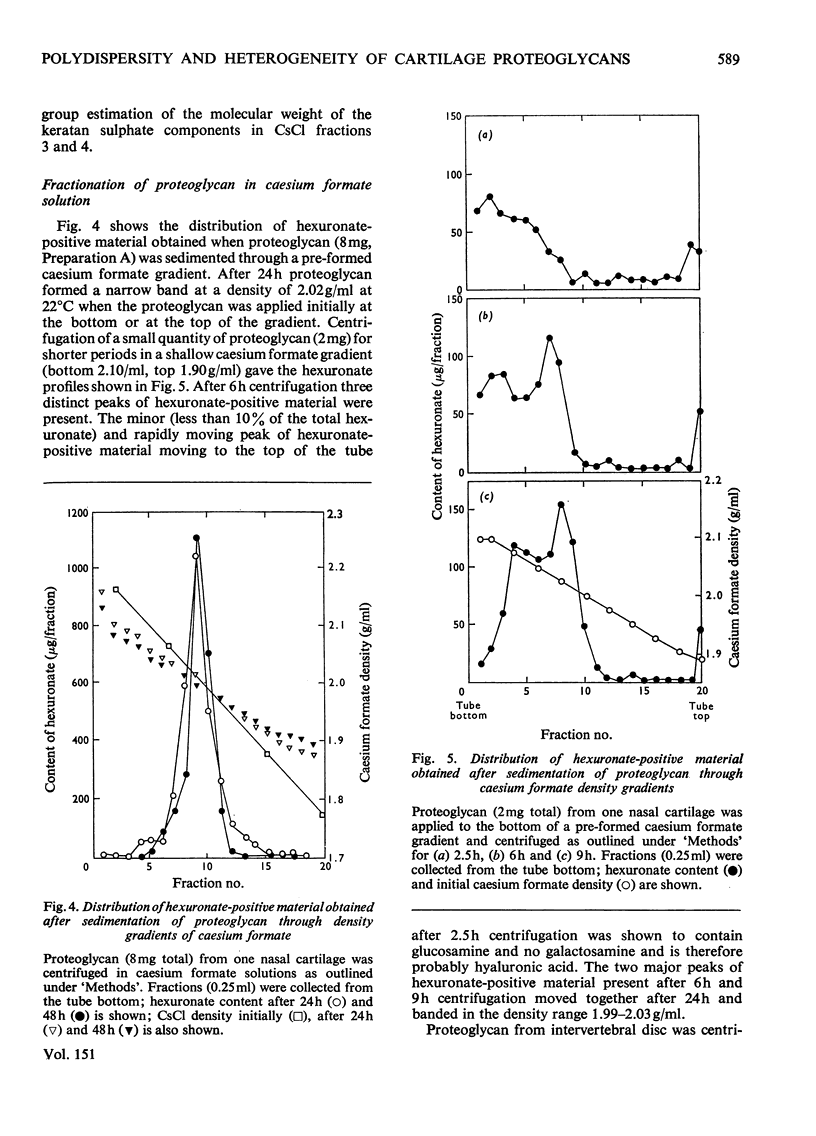
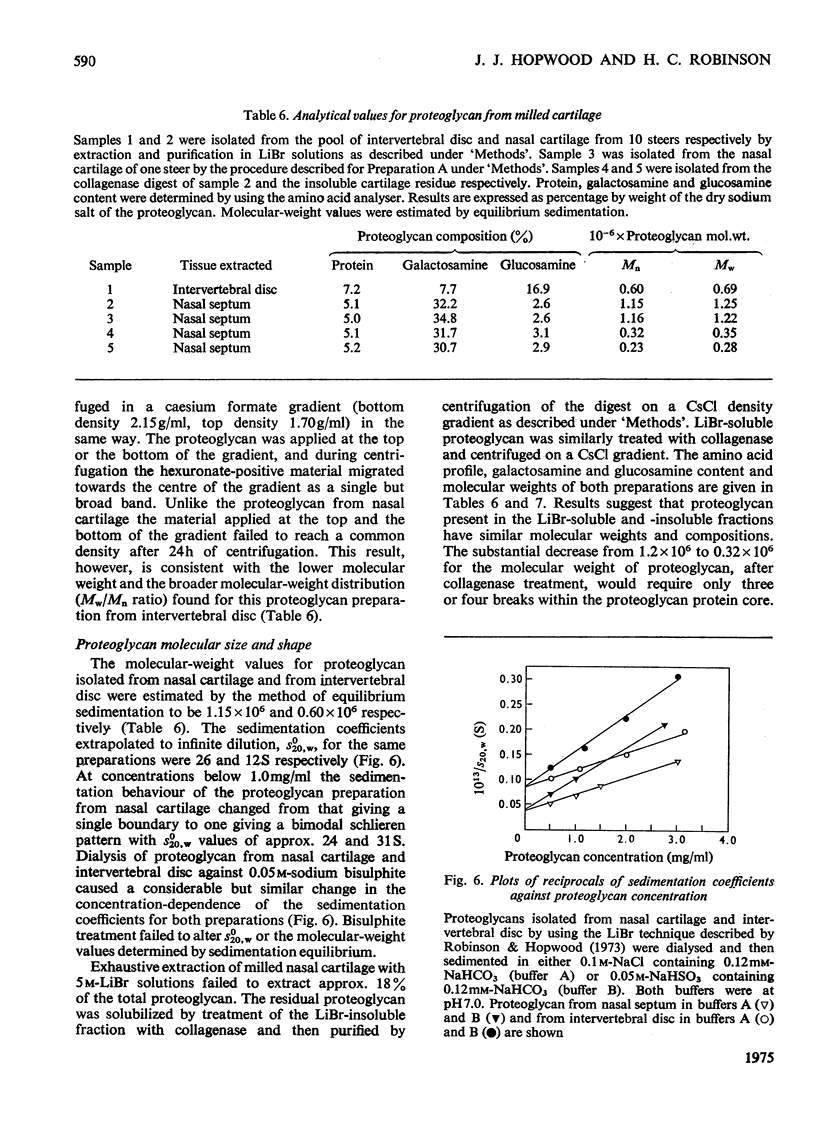
1. Three chondroitin sulphate components were isolated from adult bovine nasal cartilage after treatment with alkaline NaB3H. Average molecular weights of 13000, 18 600 and 28 000 were obtained for chondroitin sulphate species representing 10, 52 and 38% (w/w) of the total chondroitin sulphate respectively. Each chondroitin sulphate pool has a narrow molecular-weight distribution. 2. A proteoglycan subunit preparation, isolated from one nasal cartilage by extraction and density-gradient fractionation in dissociative solvents, partitioned on a CSCl density gradient according to size and composition. Variation of proteoglycan molecular weight across the gradient was directly related to the average chondrotin sulphate chain length, which in turn reflected the relative proportion of the three chondroitin sulphate pools in each proteoglycan fraction. Consideration of proteoglycan molecular parameters, compositions and behaviour on sedimentation leads to a proposal that nasal cartilage contains 3 distinct proteoglycan pools, each of which has a constant number of chondroitin sulphate side chains of different average molecular weight. 3. Molecular-weight distribution parameters for these proteoglycan preparations indicate that all serine residues on the protein core capable of initiating chondroitin sulphate biosynthesis are occupied and that proteoglycan polydispersity results directly from the polydispersity of the attached chondroitin sulphate component.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Baker J. R., Rodén L., Stoolmiller A. C. Biosynthesis of chondroitin sulfate proteoglycan. Xylosyl transfer to Smith-degraded cartilage proteoglycan and other exogenous acceptors. J Biol Chem. 1972 Jun 25;247(12):3838–3847. [PubMed] [Google Scholar]
- Dunstone J. R., Franek M. D. Connective tissue proteinpolysaccharides. Physical characterization of proteinpolysaccharide fractions obtained from bovine nasal cartilage by density gradient sedimentation. J Biol Chem. 1969 Jul 10;244(13):3654–3659. [PubMed] [Google Scholar]
- Eyring E. J., Yang J. T. Conformation of protein-polysaccharide complex from bovine nasal septum. J Biol Chem. 1968 Mar 25;243(6):1306–1311. [PubMed] [Google Scholar]
- GLAZER A. N., SMITH E. L. Estimation of cystine plus cysteine in proteins by the disulfide interchange reaction. J Biol Chem. 1961 Feb;236:416–421. [PubMed] [Google Scholar]
- GLAZER A. N., SMITH E. L. THE SULFUR DISTRIBUTION OF PAPAIN. J Biol Chem. 1965 Jan;240:201–208. [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Physical properties and polydispersity of proteoglycan from bovine nasal cartilage. J Biol Chem. 1970 Oct 10;245(19):4920–4930. [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Hopwood J. J., Robinson H. C. The molecular-weight distribution of glycosaminoglycans. Biochem J. 1973 Dec;135(4):631–637. doi: 10.1042/bj1350631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscombe M., Phelps C. F. Action of degradative enzymes on the light fraction of bovine septa protein polysaccharide. Biochem J. 1967 Apr;103(1):103–109. doi: 10.1042/bj1030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscombe M., Phelps C. F. The composition and physicochemical properties of bovine nasal-septa protein-polysaccharide complex. Biochem J. 1967 Jan;102(1):110–119. doi: 10.1042/bj1020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALAWISTA I., SCHUBERT M. Chondromucoprotein: new extraction method and alkaline degradation. J Biol Chem. 1958 Jan;230(1):535–544. [PubMed] [Google Scholar]
- MATHEWS M. B., LOZAITYTE I. Sodium chondroitin sulfate-protein complexes of cartilage. I. Molecular weight and shape. Arch Biochem Biophys. 1958 Mar;74(1):158–174. doi: 10.1016/0003-9861(58)90210-8. [DOI] [PubMed] [Google Scholar]
- Mashburn T. A., Jr, Hoffman P. Comparative fractionation studies of cartilage proteinpolysaccharides. J Biol Chem. 1971 Nov;246(21):6497–6506. [PubMed] [Google Scholar]
- Muir H., Jacobs S. Protein-polysaccharides of pig laryngeal cartilage. Biochem J. 1967 May;103(2):367–374. doi: 10.1042/bj1030367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S., Doganges P. T., Schubert M. The separation of new forms of the proteinpolysaccharides of bovine nasal cartilage. J Biol Chem. 1966 Sep 25;241(18):4261–4266. [PubMed] [Google Scholar]
- Robinson H. C., Dorfman A. The sulfation of chondroitin sulfate in embryonic chick cartilage epiphyses. J Biol Chem. 1969 Jan 25;244(2):348–352. [PubMed] [Google Scholar]
- Robinson H. C., Hopwood J. J. The alkaline cleavage and borohydride reduction of cartilage proteoglycan. Biochem J. 1973 Jul;133(3):457–470. doi: 10.1042/bj1330457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L., Pal S., Beale R., Schubert M. A comparison of proteinpolysaccharides of bovine nasal cartilage isolated and fractionated by different methods. J Biol Chem. 1970 Aug 25;245(16):4112–4122. [PubMed] [Google Scholar]
- Sajdera S. W., Hascall V. C. Proteinpolysaccharide complex from bovine nasal cartilage. A comparison of low and high shear extraction procedures. J Biol Chem. 1969 Jan 10;244(1):77–87. [PubMed] [Google Scholar]
- Stegemann H., Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967 Nov;18(2):267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- Tsiganos C. P., Hardingham T. E., Muir H. Proteoglycans of cartilage: an assessment of their structure. Biochim Biophys Acta. 1971 Feb 16;229(2):529–534. doi: 10.1016/0005-2795(71)90216-9. [DOI] [PubMed] [Google Scholar]
- Woodward C. B., Hranisavljevic J., Davidson E. A. Physical properties of cartilage proteoglycans. Biochemistry. 1972 Mar 28;11(7):1168–1176. doi: 10.1021/bi00757a009. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]


