Abstract
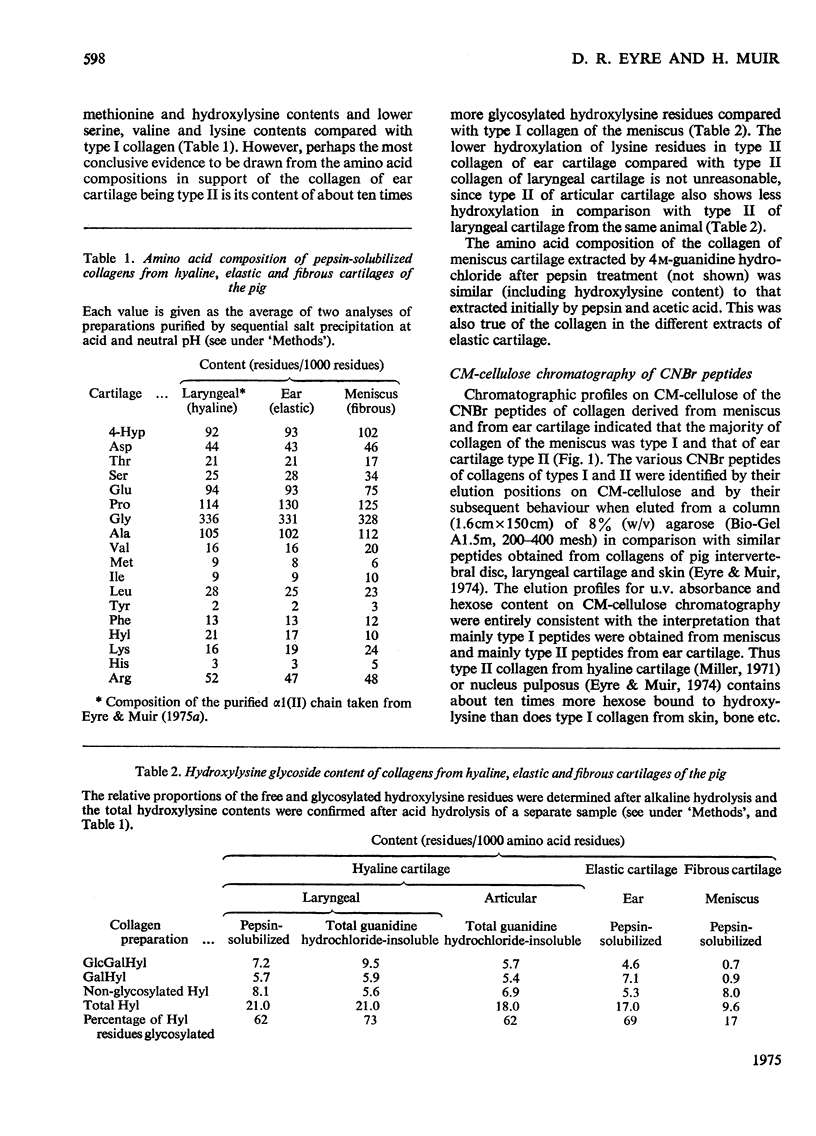
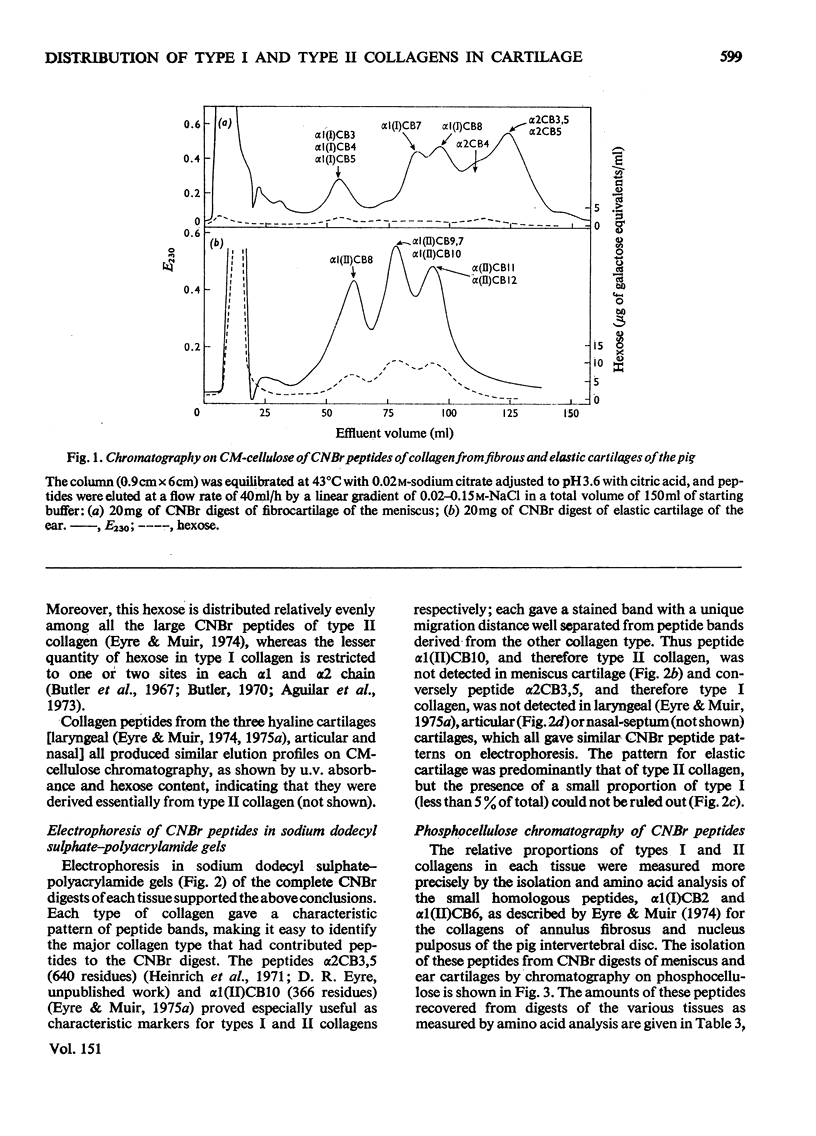
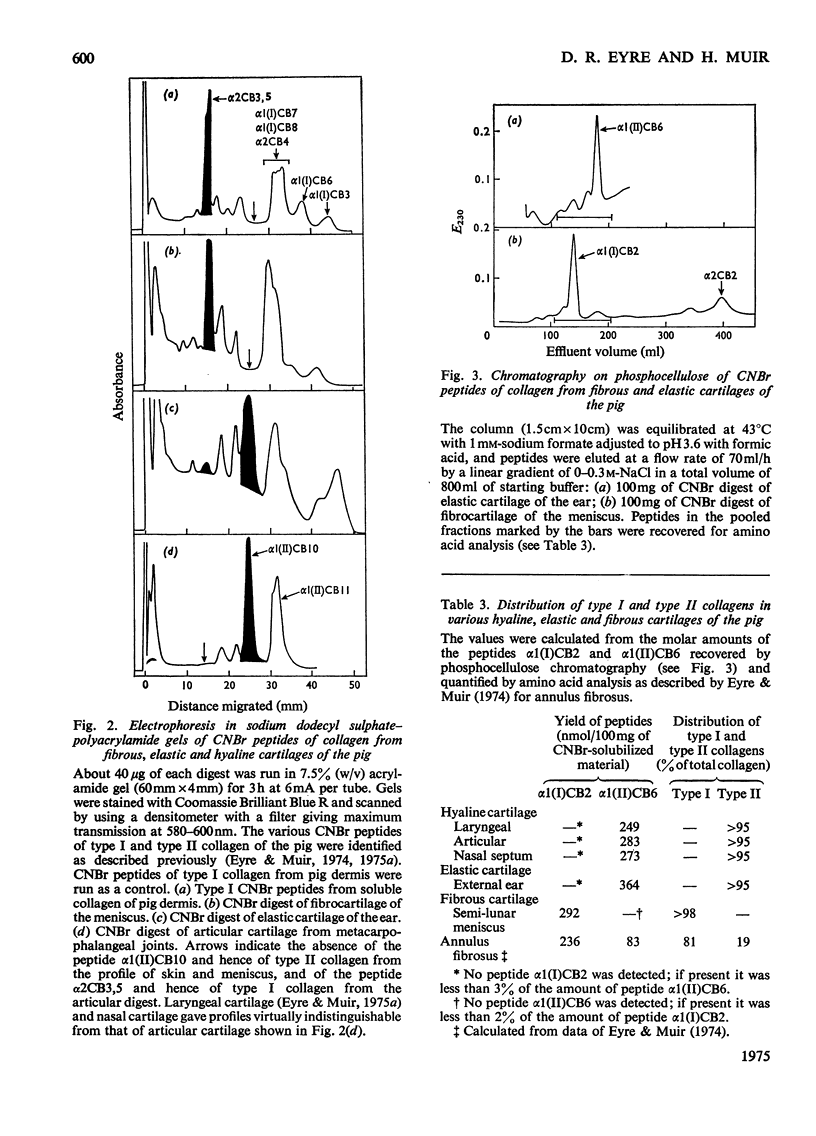
The distribution of type II collagen, considered to be characteristic of cartilaginous tissues, was determined in various specialized cartilages of the mature pig. The tissues examined were: (1) fibrocartilage of the semilunar meniscus of the knee; (2) elastic cartilage of the external ear; (3) hyaline cartilage of (a) the synovial joint (b) the thyroid plate of the larynx, and (c) the nasal septum. The predominant species of collagen in each tissue, whether type I or type II, was appraised semi-quantitatively by analysis of purified collagen solubilized by pepsin and of peptide fragments produced by cyanogen bromide. Cyanogen bromide-derived peptides were characterized by column chromatography on CM-cellulose and by electrophoresis in sodium dodecyl sulphate-polyacrylamide gels. The proportion of each type of collagen was determined precisely by isolating the homologous small peptides alpha1(II)CB6 [nomenclature of Miller (1973) Clin. Orthop. 92, 260-280], by column chromatography on phosphocellulose and determining their relative proportions by amino acid analysis. Thus collagen of the fibrocartilage of the meniscus proved to be all type I; type II was not detected. In contrast, collagen of elastic cartilage of the outer ear, after rigorous exclusion of perichondrium, was type II. Similarly, type II was the only collagen detected in all the mature hyalline cartilages examined.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar J. H., Jacobs H. G., Butler W. T., Cunningham L. W. The distribution of carbohydrate groups in rat skin collagen. J Biol Chem. 1973 Jul 25;248(14):5106–5113. [PubMed] [Google Scholar]
- Butler W. T. Chemical studies on the cyanogen bromide peptides of rat skin collagen. The covalent structure of alpha 1-CB5, the major hexose-containing cyanogen bromide peptide of alpha 1. Biochemistry. 1970 Jan 6;9(1):44–50. doi: 10.1021/bi00803a006. [DOI] [PubMed] [Google Scholar]
- Butler W. T., Piez K. A., Bornstein P. Isolation and characterization of the cyanogen bromide peptides from the alpha-1 chain of rat skin collagen. Biochemistry. 1967 Dec;6(12):3771–3780. doi: 10.1021/bi00864a022. [DOI] [PubMed] [Google Scholar]
- Byers P. H., McKenney K. H., Lichtenstein J. R., Martin G. R. Preparation of type III procollagen and collagen from rat skin. Biochemistry. 1974 Dec 3;13(25):5243–5248. doi: 10.1021/bi00722a030. [DOI] [PubMed] [Google Scholar]
- Chung E., Miller E. J. Collagen polymorphism: characterization of molecules with the chain composition (alpha 1 (3)03 in human tissues. Science. 1974 Mar;183(130):1200–1201. doi: 10.1126/science.183.4130.1200. [DOI] [PubMed] [Google Scholar]
- Epstein E. H., Jr (Alpha1(3))3 human skin collagen. Release by pepsin digestion and preponderance in fetal life. J Biol Chem. 1974 May 25;249(10):3225–3231. [PubMed] [Google Scholar]
- Eyre D. R., Glimcher M. J. Collagen cross-linking. Isolation of cross-linked peptides from collagen of chicken bone. Biochem J. 1973 Nov;135(3):393–403. doi: 10.1042/bj1350393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre D. R., Muir H. Characterisation of the major CNBr-Derived peptides of porcine type II collagen. Connect Tissue Res. 1975;3(2):165–170. doi: 10.3109/03008207509152175. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Muir H. Collagen polymorphism: two molecular species in pig intervertebral disc. FEBS Lett. 1974 Jun 1;42(2):192–196. doi: 10.1016/0014-5793(74)80783-0. [DOI] [PubMed] [Google Scholar]
- Furthmayr H., Timpl R. Characterization of collagen peptides by sodium dodecylsulfate-polyacrylamide electrophoresis. Anal Biochem. 1971 Jun;41(2):510–516. doi: 10.1016/0003-2697(71)90173-4. [DOI] [PubMed] [Google Scholar]
- Habuchi H., Yamagata T., Iwata H., Suzuki S. The occurrence of a wide variety of dermatan sulfate-chondroitin sulfate copolymers in fibrous cartilage. J Biol Chem. 1973 Sep 10;248(17):6019–6028. [PubMed] [Google Scholar]
- Heinrich W., Lange P. M., Stirtz T., Iancu C., Heidemann E. Isolation and characterization of the large cyanogen bromide peptides from the alpha1- and alpha2-chains of pig skin collagen. FEBS Lett. 1971 Jul 15;16(1):63–67. doi: 10.1016/0014-5793(71)80687-7. [DOI] [PubMed] [Google Scholar]
- Hoffman P., Linker A., Meyer K. Uronic Acid of Chondroitin Sulfate B. Science. 1956 Dec 21;124(3234):1252–1252. doi: 10.1126/science.124.3234.1252. [DOI] [PubMed] [Google Scholar]
- Hough A. J., Sokoloff L. Tissue sampling as a potential source of error in experimental studies of cartilage. Connect Tissue Res. 1975;3(1):27–31. doi: 10.3109/03008207509152338. [DOI] [PubMed] [Google Scholar]
- Houston L. L. Amino acid analysis of stained bands from polyacrylamide gels. Anal Biochem. 1971 Nov;44(1):81–88. doi: 10.1016/0003-2697(71)90348-4. [DOI] [PubMed] [Google Scholar]
- Hunter J. A., Finlay B. Scanning electron microscopy of connective tissues in health and disease. Int Rev Connect Tissue Res. 1973;6:217–255. doi: 10.1016/b978-0-12-363706-2.50011-8. [DOI] [PubMed] [Google Scholar]
- Kefalides N. A. Structure and biosynthesis of basement membranes. Int Rev Connect Tissue Res. 1973;6:63–104. doi: 10.1016/b978-0-12-363706-2.50008-8. [DOI] [PubMed] [Google Scholar]
- Layman D. L., Sokoloff L., Miller E. J. Collagen synthesis by articular in monolayer culture. Exp Cell Res. 1972 Jul;73(1):107–112. doi: 10.1016/0014-4827(72)90107-3. [DOI] [PubMed] [Google Scholar]
- Miller E. J. A review of biochemical studies on the genetically distinct collagens of the skeletal system. Clin Orthop Relat Res. 1973 May;(92):260–280. doi: 10.1097/00003086-197305000-00024. [DOI] [PubMed] [Google Scholar]
- Miller E. J. Isolation and characterization of a collagen from chick cartilage containing three identical alpha chains. Biochemistry. 1971 Apr 27;10(9):1652–1659. doi: 10.1021/bi00785a024. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Lunde L. G. Isolation and characterization of the cyanogen bromide peptides from the alpha 1(II) chain of bovine and human cartilage collagen. Biochemistry. 1973 Aug 14;12(17):3153–3159. doi: 10.1021/bi00741a003. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Mathews M. B. Characterization of notochord collagen as a cartilage-type collagen. Biochem Biophys Res Commun. 1974 Sep 9;60(1):424–430. doi: 10.1016/0006-291x(74)90221-6. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Matukas V. J. Biosynthesis of collagen. The biochemist's view. Fed Proc. 1974 May;33(5):1197–1204. [PubMed] [Google Scholar]
- Miller E. J. Structural studies on cartilage collagen employing limited cleavage and solubilization with pepsin. Biochemistry. 1972 Dec 19;11(26):4903–4909. doi: 10.1021/bi00776a005. [DOI] [PubMed] [Google Scholar]
- Muir H. Chemistry and metabolism of connective tissue glycosaminoglycans (mucopolysaccharides). Int Rev Connect Tissue Res. 1964;2:101–154. doi: 10.1016/b978-1-4831-6751-0.50009-4. [DOI] [PubMed] [Google Scholar]
- Nimni M., Deshmukh K. Differences in collagen metabolism between normal and osteoarthritic human articular cartilage. Science. 1973 Aug 24;181(4101):751–752. doi: 10.1126/science.181.4101.751. [DOI] [PubMed] [Google Scholar]
- STEGEMANN H. Mikrobestimmung von Hydroxyprolin mit Chloramin-T und p-Dimethylaminobenzaldehyd. Hoppe Seylers Z Physiol Chem. 1958;311(1-3):41–45. [PubMed] [Google Scholar]
- Seyer J. M., Brickley D. M., Glimcher M. J. The identification of two types of collagen in the articular cartilage of postnatal chickens. Calcif Tissue Res. 1974;17(1):43–55. doi: 10.1007/BF02547213. [DOI] [PubMed] [Google Scholar]
- Seyer J. M., Brickley D. M., Glimcher M. J. The isolation of two types of collagen from embryonic bovine epiphyseal cartilage. Calcif Tissue Res. 1974;17(1):25–41. doi: 10.1007/BF02547212. [DOI] [PubMed] [Google Scholar]
- Strawich E., Nimni M. E. Properties of a collagen molecule containing three identical components extracted from bovine articular cartilage. Biochemistry. 1971 Oct 12;10(21):3905–3911. doi: 10.1021/bi00797a017. [DOI] [PubMed] [Google Scholar]
- Swann D. A., Constable I. J., Harper E. Vitreous structure. 3. Composition of bovine vitreous collagen. Invest Ophthalmol. 1972 Sep;11(9):735–738. [PubMed] [Google Scholar]
- Thompson M. F., Bachelard H. S. Cerebral-cortex hexokinase. Comparison of properties of solubilized mitochondrial and cytoplasmic activities. Biochem J. 1970 Jun;118(1):25–34. doi: 10.1042/bj1180025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelstad R. L. Human aorta collagens: evidence for three distinct species. Biochem Biophys Res Commun. 1974 Apr 8;57(3):717–725. doi: 10.1016/0006-291x(74)90605-6. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L., Kang A. H., Igarashi S., Gross J. Isolation of two distinct collagens from chick cartilage. Biochemistry. 1970 Dec 8;9(25):4993–4998. doi: 10.1021/bi00827a025. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L., Kang A. H., Toole B. P., Gross J. Collagen heterogeneity. High resolution separation of native ( 1(I) 2 2 and ( 1(II) 3 and their component chains. J Biol Chem. 1972 Oct 25;247(20):6469–6473. [PubMed] [Google Scholar]


