Abstract
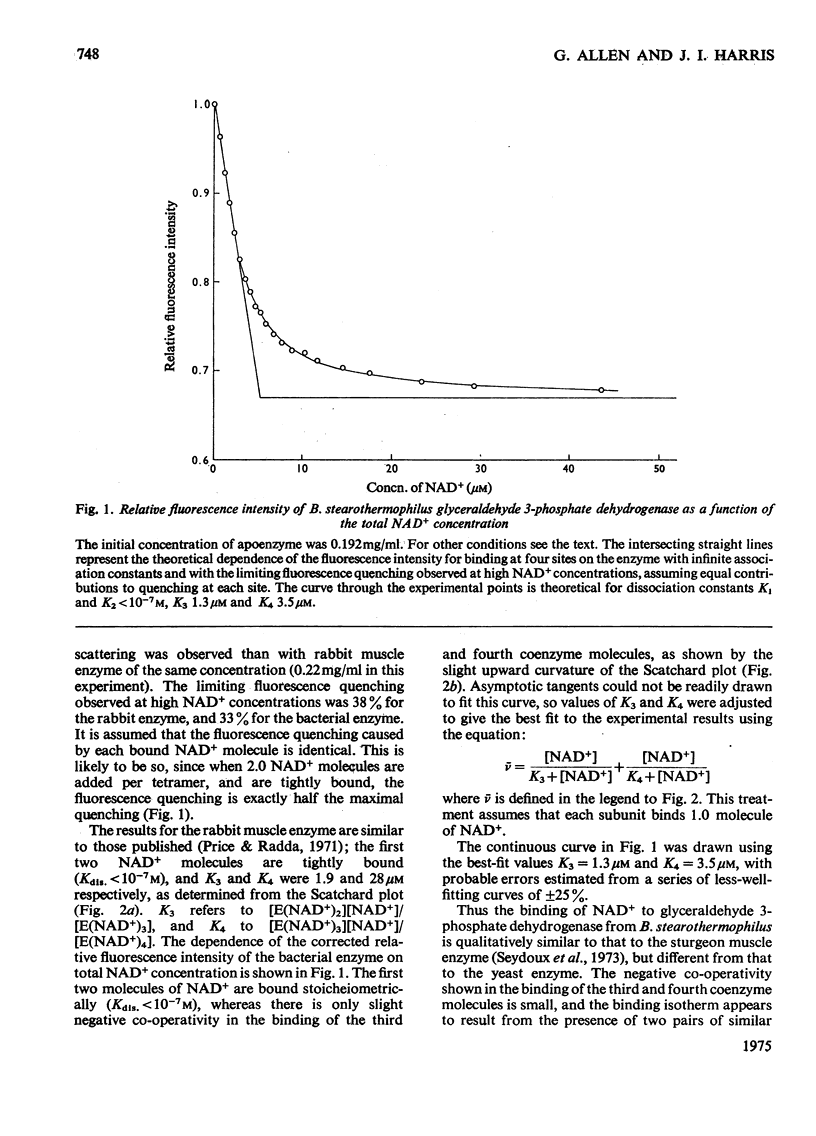
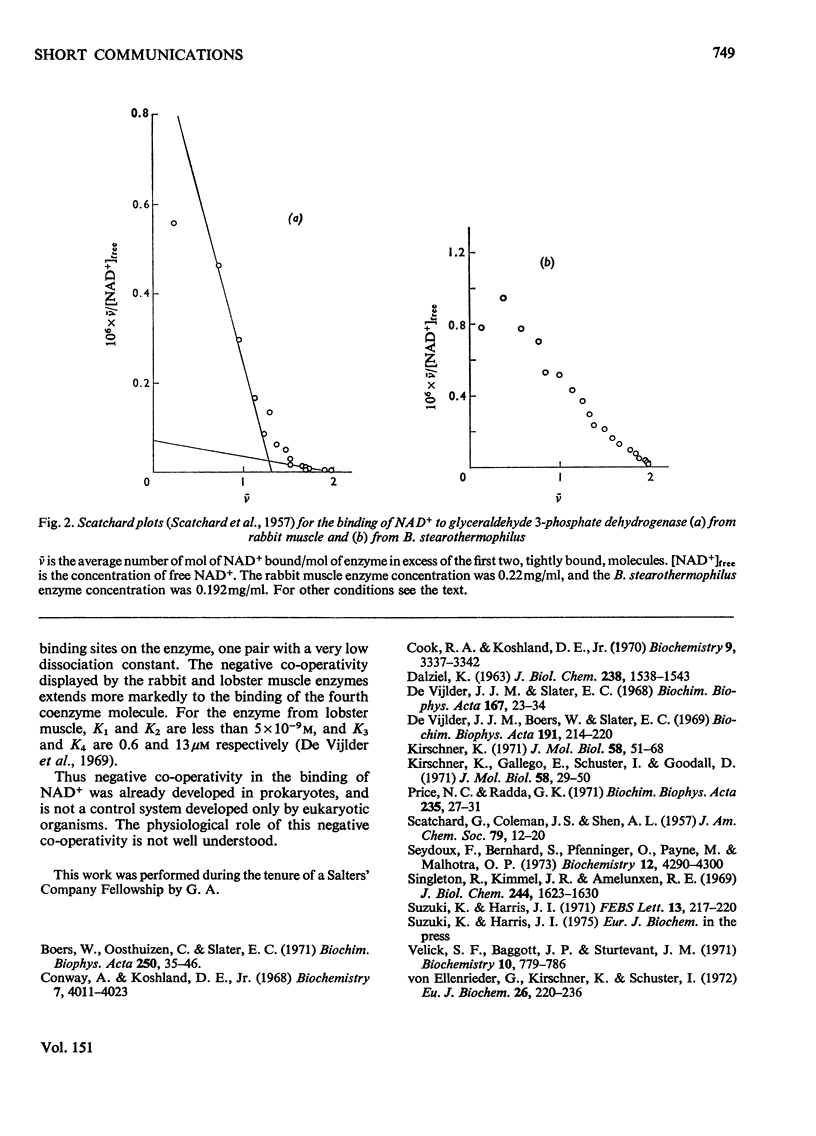
The binding of NAD+ to glyceraldehyde 3-phosphate dehydrogenase (EC 1.2.1.12) from Bacillus stearothermophilus has been studied by measurement of protein fluorescence quenching. Slight negative co-operativity was observed in the binding of the third and fourth coenzyme molecules to the tetrameric enzyme. The first two coenzyme molecules were tightly bound. In this respect the enzyme resembles that from sturgeon muscle rather than that from yeast.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boers W., Oosthuizen C., Slater E. C. Binding of NAD + and NADH to rabbit-muscle glyceraldehydephosphate dehydrogenase. Biochim Biophys Acta. 1971 Oct;250(1):35–46. doi: 10.1016/0005-2744(71)90117-3. [DOI] [PubMed] [Google Scholar]
- Conway A., Koshland D. E., Jr Negative cooperativity in enzyme action. The binding of diphosphopyridine nucleotide to glyceraldehyde 3-phosphate dehydrogenase. Biochemistry. 1968 Nov;7(11):4011–4023. doi: 10.1021/bi00851a031. [DOI] [PubMed] [Google Scholar]
- Cook R. A., Koshland D. E., Jr Positive and negative cooperativity in yeast glyceraldehyde 3-phosphate dehydrogenase. Biochemistry. 1970 Aug 18;9(17):3337–3342. doi: 10.1021/bi00819a007. [DOI] [PubMed] [Google Scholar]
- DALZIEL K. The purification of nicotinamide adenine dinucleotide and kinetic effects of nucleotide impurities. J Biol Chem. 1963 Apr;238:1538–1543. [PubMed] [Google Scholar]
- Kirschner K. Co-operative binding of nicotinamide-adenine dinucleotide to yeast glyceraldehyde-3-phosphate dehydrogenase. II. Stopped-flow studies at pH 8-5 and 40 degrees C. J Mol Biol. 1971 May 28;58(1):51–68. doi: 10.1016/0022-2836(71)90231-2. [DOI] [PubMed] [Google Scholar]
- Kirschner K., Gallego E., Schuster I., Goodall D. Co-operative binding of nicotinamide-adenine dinucleotide to yeast glyceraldehyde-3-phosphate dehydrogenase. I. Equilibrium and temperature-jump studies at pH 8-5 and 40 degrees C. J Mol Biol. 1971 May 28;58(1):29–50. doi: 10.1016/0022-2836(71)90230-0. [DOI] [PubMed] [Google Scholar]
- Price N. C., Radda G. K. The binding of NAD+ to rabbit muscle glyceraldehyde-3-phosphate dehydrogenase studied by protein fluorescence quenching. Biochim Biophys Acta. 1971 Apr 14;235(1):27–31. doi: 10.1016/0005-2744(71)90029-5. [DOI] [PubMed] [Google Scholar]
- Seydoux F., Bernhard S., Pfenninger O., Payne M., Malhotra O. P. Preparation and active-site specific properties of sturgeon muscle glyceraldehyde-3-phoshate dehydrogenase. Biochemistry. 1973 Oct 9;12(21):4290–4300. doi: 10.1021/bi00745a038. [DOI] [PubMed] [Google Scholar]
- Singleton R., Jr, Kimmel J. R., Amelunxen R. E. The amino acid composition and other properties of thermostable glyceraldehyde 3-phosphate dehydrogenase from Bacillus stearothermophilus. J Biol Chem. 1969 Mar 25;244(6):1623–1630. [PubMed] [Google Scholar]
- Suzuki Koichi, Ieuan Harris J. Glyceraldehyde-3-phosphate dehydrogenase from Bacillus stearothermophilus. FEBS Lett. 1971 Mar 16;13(4):217–220. doi: 10.1016/0014-5793(71)80539-2. [DOI] [PubMed] [Google Scholar]
- Velick S. F., Baggott J. P., Sturtevant J. M. Thermodynamics of nicotinamide-adenine dinucleotide addition to the glyceraldehyde 3-phosphate dehydrogenases of yeast and of rabbit skeletal muscle. An equilibrium and calorimetric analysis over a range of temperatures. Biochemistry. 1971 Mar 2;10(5):779–786. doi: 10.1021/bi00781a009. [DOI] [PubMed] [Google Scholar]
- de Vijlder J. J., Boers W., Slater E. C. Binding and properties of NAD+ in glyceraldehydephosphate dehydrogenase from lobster-tail muscle. Biochim Biophys Acta. 1969 Nov 4;191(2):214–220. doi: 10.1016/0005-2744(69)90240-x. [DOI] [PubMed] [Google Scholar]
- de Vijlder J. J., Slater E. C. The reaction between NAD+ and rabbit-muscle glyceraldehydephosphate dehydrogenase. Biochim Biophys Acta. 1968 Aug 27;167(1):23–34. doi: 10.1016/0005-2744(68)90274-x. [DOI] [PubMed] [Google Scholar]
- von Ellenrieder G., Kirschner K., Schuster I. The binding of oxidized and reduced nicotinamide adenine-dinucleotide to yeast glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1972 Mar 27;26(2):220–236. doi: 10.1111/j.1432-1033.1972.tb01760.x. [DOI] [PubMed] [Google Scholar]


