Abstract
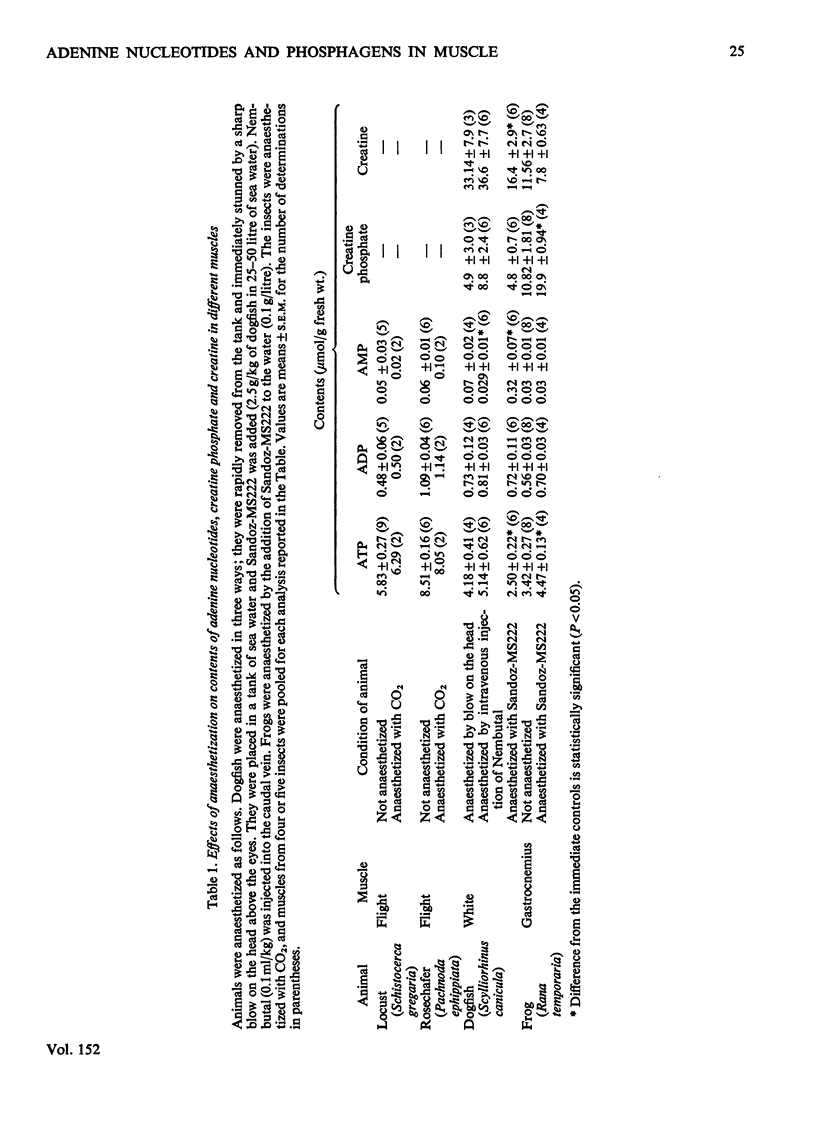
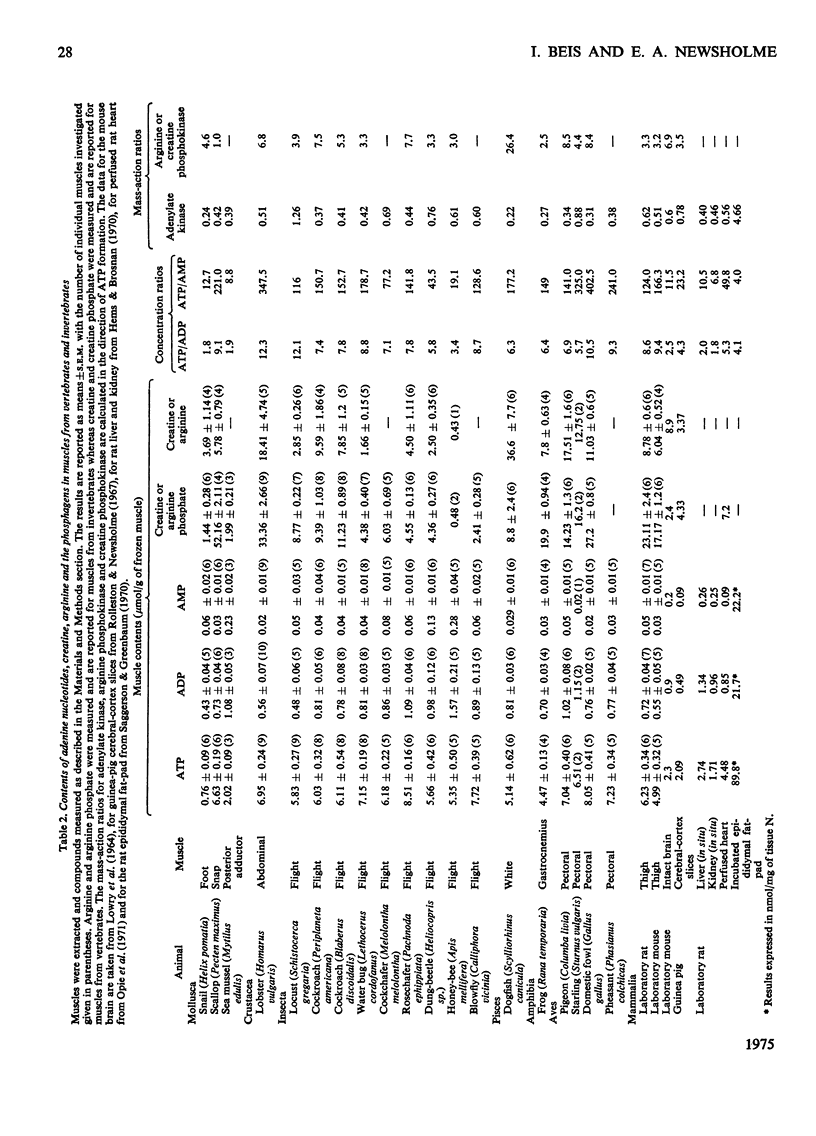
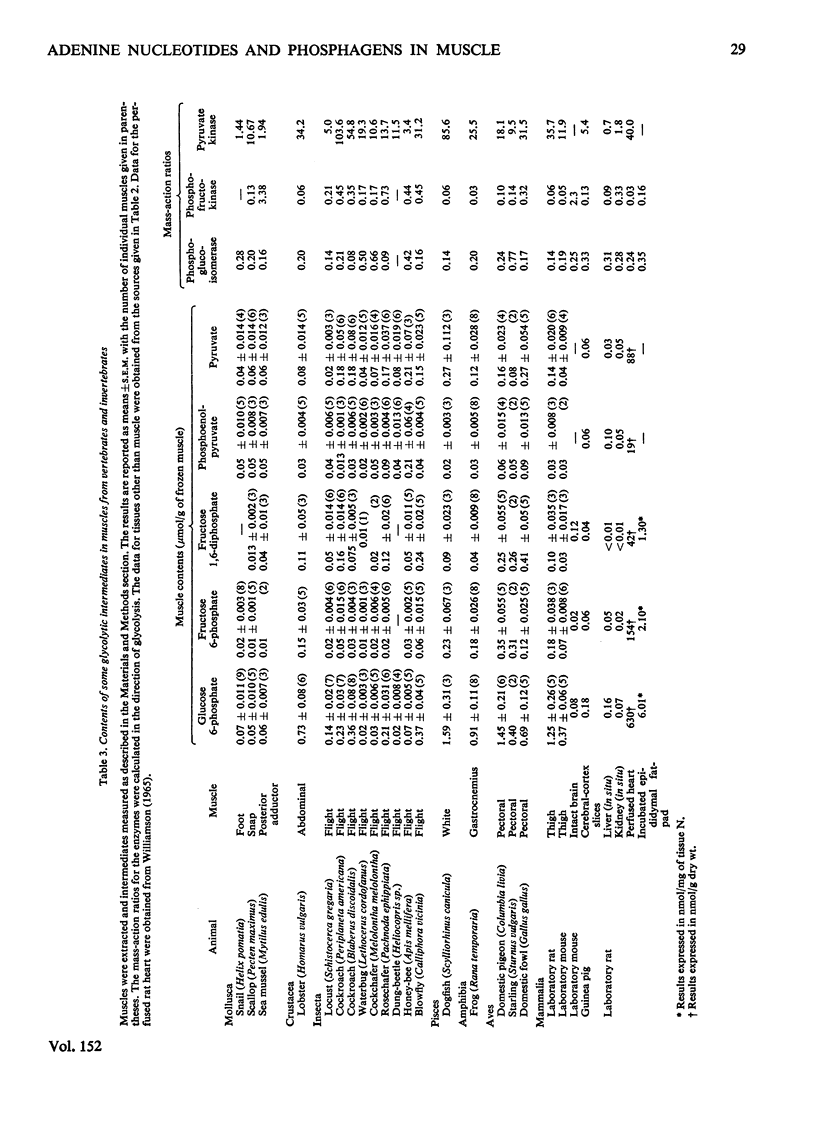
The lowest contents of ATP and the lowest ATP/AMP concentration ratios are observed in the molluscan muscles that have very low rates of energy expenditure during contraction. The highest contents of ATP are observed in the extremely aerobic insect flight muscle and the extremely anaerobic pectoral muscle of the pheasant and domestic fowl. In general, the lowest ATP/AMP concentration ratios are observed for muscle in which the variation in the rate of energy utilization is small (e.g. some molluscan muscles, heart muscle); the highest ratios are observed in muscles in which this variation is large (lobster abdominal muscle, pheasant pectoral muscle, some insect flight muscles). This finding is consistent with the proposed role of AMP and the adenylate kinase reaction in the regulation of glycolysis. However, in the flight muscle of the honey-bee the ATP/AMP ratio is very low, so that glycolysis may be regulated by factors other than the variation in AMP concentration. The variation in the contents of arginine phosphate in muscle from the invertebrates is much larger than the variation in creatine phosphate in muscle from the vertebrates. The contents of hexose monophosphates and pyruvate are, in general, higher in the muscles of vertebrates than in those of the invertebrates. The contents of phosphoenolpyruvate are similar in all the muscles investigated, except for the honey-bee in which it is about 4-10-fold higher. The mass-action ratios for the reactions catalysed by phosphoglucoisomerase and adenylate kinase are very similar to the equilibrium constants for these reactions. Further, the variation in the mass-action ratios between muscles is small. It is concluded that these enzymes catalyse reactions close to equilibrium. However, the mass-action ratios for the reactions catalysed by phosphofructokinase and pyruvate kinase are much smaller than the equilibrium constants. The variation in the ratios between different muscles is large. It is concluded that these enzymes catalyse nonequilibrium reactions. Since the variation in the mass-action ratios for the reactions catalysed by the phosphagen kinases (i.e. creatine and arginine phosphokinases) is small, it is suggested that these reactions are close to equilibrium.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crabtree B., Newsholme E. A. The activities of lipases and carnitine palmitoyltransferase in muscles from vertebrates and invertebrates. Biochem J. 1972 Dec;130(3):697–705. doi: 10.1042/bj1300697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree B., Newsholme E. A. The activities of phosphorylase, hexokinase, phosphofructokinase, lactate dehydrogenase and the glycerol 3-phosphate dehydrogenases in muscles from vertebrates and invertebrates. Biochem J. 1972 Jan;126(1):49–58. doi: 10.1042/bj1260049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree B., Newsholme E. A. The activities of proline dehydrogenase, glutamate dehydrogenase, aspartate-oxoglutarate aminotransferase and alanine-oxoglutarate aminotransferase in some insect flight muscles. Biochem J. 1970 May;117(5):1019–1021. doi: 10.1042/bj1171019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGGLESTON L. V., HEMS R. Separation of adenosine phosphates by paper chromotography and the equilibrium constant of the myokinase system. Biochem J. 1952 Sep;52(1):156–160. doi: 10.1042/bj0520156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevers W., Krebs H. A. The effects of adenine nucleotides on carbohydrate metabolism in pigeon-liver homogenates. Biochem J. 1966 Mar;98(3):720–735. doi: 10.1042/bj0980720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems D. A., Brosnan J. T. Effects of ischaemia on content of metabolites in rat liver and kidney in vivo. Biochem J. 1970 Nov;120(1):105–111. doi: 10.1042/bj1200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAHANA S. E., LOWRY O. H., SCHULZ D. W., PASSONNEAU J. V., CRAWFORD E. J. The kinetics of phosphoglucoisomerase. J Biol Chem. 1960 Aug;235:2178–2184. [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- NODA L., KUBY S. A., LARDY H. A. Adenosinetriphosphate-creatine transphosphorylase. IV. Equilibrium studies. J Biol Chem. 1954 Sep;210(1):83–95. [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B. Metabolic aspects of enzyme activity regulation. Symp Soc Exp Biol. 1973;27:429–460. [PubMed] [Google Scholar]
- Newsholme E. A., Taylor K. Glycerol kinase activities in muscles from vertebrates and invertebrates. Biochem J. 1969 May;112(4):465–474. doi: 10.1042/bj1120465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie L. H., Mansford K. R., Owen P. Effects of increased heart work on glycolysis and adenine nucleotides in the perfused heart of normal and diabetic rats. Biochem J. 1971 Sep;124(3):475–490. doi: 10.1042/bj1240475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolleston F. S., Newsholme E. A. Control of glycolysis in cerebral cortex slices. Biochem J. 1967 Aug;104(2):524–533. doi: 10.1042/bj1040524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüegg J. C. Smooth muscle tone. Physiol Rev. 1971 Jan;51(1):201–248. doi: 10.1152/physrev.1971.51.1.201. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D., Greenbaum A. L. The regulation of triglyceride synthesis and fatty acid synthesis in rat epididymal adipose tissue. Biochem J. 1970 Sep;119(2):193–219. doi: 10.1042/bj1190193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. S. The organization and function of the sarcoplasmic reticulum and T-system of muscle cells. Prog Biophys Mol Biol. 1966;16:107–142. doi: 10.1016/0079-6107(66)90004-6. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON J. R. GLYCOLYTIC CONTROL MECHANISMS. I. INHIBITION OF GLYCOLYSIS BY ACETATE AND PYRUVATE IN THE ISOLATED, PERFUSED RAT HEART. J Biol Chem. 1965 Jun;240:2308–2321. [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]


