Abstract
Background
The risk of gastrointestinal (GI) cancer after lung transplantation (LTx) in sarcoidosis patients is not well defined. Given the cancer risks linked to sarcoidosis and organ transplantation, this study investigated the incidence of GI de novo malignancies (DNM), comparing LTx recipients with sarcoidosis or idiopathic pulmonary fibrosis (IPF).
Methods
We analyzed data from the United Network for Organ Sharing registry, including adults with sarcoidosis or IPF who underwent LTx between May 2005 and December 2018. The primary outcome was the incidence of GI DNM by March 2023.
Results
Of 7996 lung transplant recipients, 108 (1.35%) developed GI malignancies post-transplantation. Among these, 662 patients (9%) had sarcoidosis and 7334 (91%) had IPF. Sarcoidosis patients showed a non-significant trend toward a higher risk of GI malignancies compared to those with IPF (subhazard ratio 1.72, 95% confidence interval 0.90-3.29; P=0.099), with no observed difference in the risk of non-GI cancers.
Conclusions
The overall incidence of GI DNM following LTx is low, and sarcoidosis does not appear to increase the risk of GI cancers compared to IPF. This finding suggests that enhanced GI cancer screening beyond standard guidelines may not be warranted in this population, allowing for targeted surveillance of more prevalent malignancies in sarcoidosis patients post-LTx.
Keywords: Gastrointestinal cancer, de novo malignancy, idiopathic pulmonary fibrosis, immunosuppression, lung transplantation
Introduction
Gastrointestinal (GI) malignancies are a potential but underrecognized risk in patients with systemic inflammatory diseases, including sarcoidosis. Sarcoidosis, characterized by granuloma formation, is a chronic condition that can affect multiple organs, including the GI tract [1]. Although pulmonary involvement is most common, sarcoidosis can also lead to inflammation and malignancy at upper gastrointestinal, colorectal and hepatic sites [1]. The systemic inflammatory milieu of sarcoidosis is thought to contribute to an increased risk of malignancies, including GI cancers [2].
Lung transplantation (LTx) is a life-saving intervention for patients with severe pulmonary sarcoidosis, representing 2.4% of all LTx procedures between 1998 and 2018 [3]. However, patients undergoing LTx are already at a heightened risk for cancer, due to the need for prolonged immunosuppression and potential oncogenic viral infections [4]. In sarcoidosis patients, these post-transplantation cancer risks may be compounded by the preexisting inflammatory state, potentially elevating the risk of GI malignancies beyond that seen in LTx recipients with idiopathic pulmonary fibrosis (IPF) [5].
While previous research has examined the general cancer risk in sarcoidosis, the specific risk of GI de novo malignancies (DNM) post-LTx remains unclear [6]. This study aimed to compare the incidence of these malignancies in patients undergoing LTx for sarcoidosis vs. IPF. Understanding these risks is crucial for developing appropriate cancer screening and surveillance strategies in this unique patient population [7,8].
Patients and methods
Adult patients age 18 years or older, who underwent single or double lung transplantation between May 4, 2005, and December 31, 2018, for an indication of sarcoidosis or IPF were identified using the United Network for Organ Sharing (UNOS) registry, which includes data on donor and recipient demographics, medical characteristics, organ match information, transplant outcomes and longitudinal follow-up, for solid organ transplants performed in the United States of America [9]. A de-identified version of the registry was obtained for analysis, and the use of these de-identified data did not constitute human subjects research, as determined by the local Institutional Review Board.
Patients undergoing multi-organ transplantation or re-transplantation (including LTx after any previous solid organ transplantation) were excluded. We also excluded patients with any history of pre-transplantation malignancy, and cases with missing data, though data regarding malignancy at the time of listing were not available from 2015 onward. Eligible patients were tracked until development of GI malignancy, development of any other malignancy, graft failure, death, or censoring. Follow-up data were available through March 24, 2023.
The primary outcome was GI DNM, defined as the earliest date of diagnosis of new esophageal, stomach, small intestinal, pancreatic, colorectal or primary liver cancer, as recorded in the UNOS follow-up records. Routine post-transplantation follow-up data collection was scheduled for 6 months and 1 year post-transplantation and annually thereafter [10]. Competing risks considered in our analysis included: (a) death; (b) graft failure or re-transplantation; or (c) diagnosis of any other type of malignancy.
All patients who did not experience the primary outcome or one of the competing risks were considered to have been censored at the time of the latest follow-up record available for each patient.
The primary exposure was indication for LTx (sarcoidosis as compared to IPF). Covariates were measured at the time of transplantation, and included patient age, sex, race and ethnicity (White, Black, Hispanic, or none of the above); hepatitis B virus (HBV), and hepatitis C virus (HCV) status (positive or negative for each virus); the final calculated Lung Allocation Score (LAS), a measure of priority for LTx and expected post-transplantation survival; the type of transplantation (single or bilateral LTx); and the year of transplantation.
As in a prior study [11], maintenance immunosuppression recorded at the time of transplantation was classified based on use of tacrolimus, cyclosporine without tacrolimus, or neither, and use of mycophenolic acid, azathioprine without mycophenolic acid, or neither.
Statistical analysis
Study variables were summarized using medians with interquartile ranges (IQRs) or counts and percentages. Patient characteristics were compared by indication for LTx using chi-square, Fisher’s exact, or Mann-Whitney U tests, as appropriate. The cumulative incidence function (CIF) was used to describe the probability of developing a GI malignancy. The subhazard ratio (SHR) of developing GI malignancy was compared between sarcoidosis and IPF cohorts using Fine-Gray competing risks regression, adjusting for any covariates found to be statistically significantly different between the groups on bivariate analysis. Analyses were performed using Stata/SE version 18.0, and P<0.05 was considered statistically significant.
Results
We identified 9408 patients undergoing LTx for sarcoidosis or IPF during the study period, of whom we excluded 25 secondary to undergoing multiorgan transplantation, 73 for undergoing re-transplantation, 801 diagnosed with malignancy pre-transplantation, 29 diagnosed with malignancy post-transplantation but missing data on the date of diagnosis, and 484 missing data on study covariates for HBV and HCV status.
The final sample included 7996 patients (median age 62 years, 72% male, 8% undergoing LTx for sarcoidosis), of whom 108 (1%) developed GI malignancy. The most common competing risk events in the sample included death (43%), development of any non-GI malignancy (27%), and graft failure (1%).
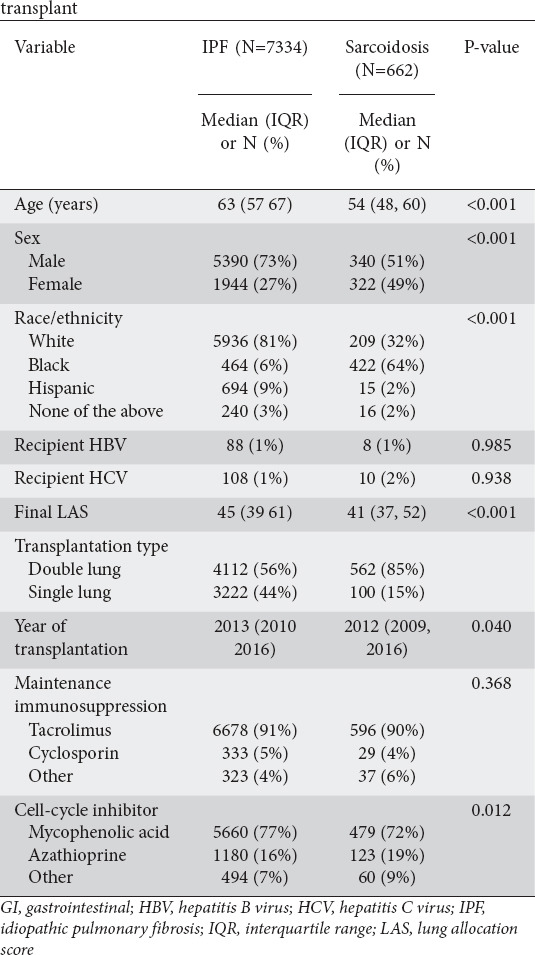
The CIF of each study endpoint is shown in Fig. 1, and patient characteristics are compared between the sarcoidosis and IPF groups in Table 1. Sarcoidosis patients were younger than IPF patients, and more likely to be female or black. The sarcoidosis group also had a lower final LAS and were more likely to receive a double lung transplant. Most subjects from both groups were administered tacrolimus. Variations in the application of other maintenance immunosuppressive regimens were not statistically significant. However, IPF patients were more likely to be treated with mycophenolic acid, while patients with sarcoidosis were more likely to receive azathioprine.
Figure 1.
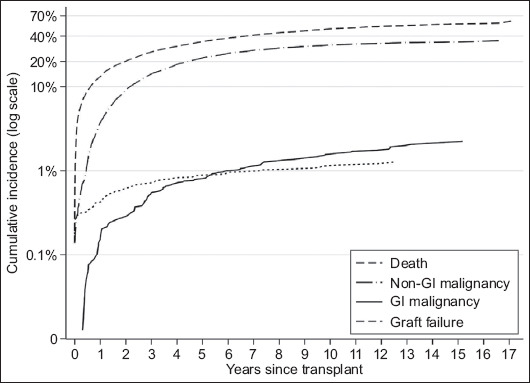
Cumulative incidence plot of gastrointestinal (GI) de novo malignancy and competing risk events after lung transplantation for sarcoidosis or idiopathic pulmonary fibrosis
Table 1.
Comparison of patient characteristics by indication for lung transplant
On multivariate analysis, LTx for sarcoidosis conferred a SHR of 1.72 for GI malignancy, though not statistically significant (P=0.099, 95% confidence interval [CI] 0.90-3.29). For non-GI malignancy, sarcoidosis patients had a SHR of 1.04, also not statistically significant (P=0.747, 95%CI 0.84-1.28) (Table 2).
Table 2.
Multivariate competing-risks regression of gastrointestinal (GI) and non-GI malignancy after lung transplantation
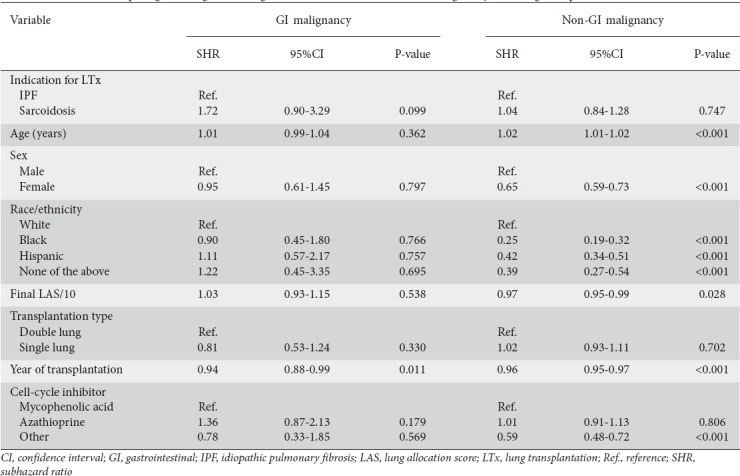
The timing of transplantation was the only factor associated with GI malignancy, with a notable decrease in subhazard ratio observed as the follow-up period approached, indicating that the risk of GI malignancy decreased over time after transplantation. Factors including female sex, non-White ethnicity, closer proximity of LTx to the follow-up period, a higher final LAS at follow up, and the use of antimetabolites—particularly mycophenolic acid or azathioprine—instead of alternative cell cycle inhibitor therapies, were associated with a lower subhazard ratio for non-GI malignancy. Advanced age was associated with a slightly higher subhazard ratio for non-GI malignancy.
Discussion
This study investigated the incidence of GI malignancy in patients who underwent LTx for an indication of sarcoidosis, compared to patients with an indication of IPF. Cancer risk in LTx recipients is 4-fold higher than in the general population [12], and LTx is specifically associated with an elevated risk of GI malignancy [13]. The GI tract is rich in mucosa-associated lymphoid tissue, which is a hub for immune activity. This dense concentration of lymphoid tissue increases the risk of gastrointestinal carcinogenesis in patients with sarcoidosis, because of the inflammatory response’s predilection for lymphoid tissue and lymph nodes [14,15]. Although LTx and sarcoidosis were found to be independent risk factors for GI malignancy in prior studies, our findings suggest that sarcoidosis as an indication for LTx does not appear to confer a further risk of GI cancer.
The life expectancy of patients with sarcoidosis who are LTx recipients is similar to that of patients who have other indications, their median survival being roughly 5.8 years [16]. However, it is important to note that, in our study, LTx recipients were more likely to die from other causes than to develop GI malignancy, with a cumulative incidence of approximately 1% for GI malignancy at 6 years post-transplantation, and a 10% cumulative incidence of non-GI malignancy at 2 years post LTx (Fig. 1). Post-transplantation malignancy is the second most common long-term cause of death after LTx, with mortality estimates as high as 15.6% [17]. Screening LTx recipients for cancer is crucial, so as to optimize patient outcomes and the use of scarce donor organs [18]. Moreover, the occurrence of post-transplantation de novo malignancy incurs substantial healthcare costs, driven by a surge in outpatient visits and procedural expenses [19], underscoring the importance of proactive measures to mitigate this risk.
Sarcoid patients with extra-thoracic involvement are at higher risk of malignancy compared to patients without extra-thoracic disease [20]. Extrapulmonary involvement often contraindicates LTx, and the discovery of extrapulmonary or mediastinal lymph node disease during surgery may result in the termination of the lung transplantation procedure [21]. The exclusion of sarcoidosis patients with extrapulmonary disease from transplantation waitlisting may have masked a possible association between sarcoidosis and GI DNM after LTx in our study. Additionally, our study’s exclusion of patients who had a history of cancer before their transplantation may have led to an underestimation of the cancer risk in our analysis. LTx recipients with a previous cancer diagnosis are over 3 times more likely to develop post-transplantation malignancy [22], and patients with sarcoidosis are particularly likely to have been diagnosed with cancer before their LTx. However, our findings did not show a significantly greater GI malignancy risk for sarcoidosis patients compared to those with IPF, despite these considerations.
The comparison of malignancy outcomes in sarcoidosis patients undergoing LTx with an IPF cohort was another limitation. Although there is no perfect comparator, IPF was selected as it is the most common disease leading to lung transplantation [3]. While smoking is a risk factor for both IPF and malignancy, its direct causal relationship with IPF is not as clear, as it is with chronic obstructive pulmonary disease (COPD) [23,24], making IPF a more appropriate comparator than COPD for this study. Additionally, cystic fibrosis (CF) was not considered because of its specific link to gastrointestinal cancers [25], and the much younger ages at which LTx is usually performed for CF as compared to IPF. Other limitations of our study include the exclusion of cases with missing data, multi-organ transplantation or re-transplantation, potentially omitting cases where the risk of GI DNM was higher overall, or more sensitive to risks associated with sarcoidosis.
Given the procedural risks associated with GI cancer screening and its relatively low incidence among LTx recipients, our results do not suggest the need for further screening in this patient group, beyond existing population-level guidelines. This finding is crucial, because it implies that the routine implementation of additional GI cancer screening protocols for LTx recipients may not be necessary and could expose patients to unnecessary risks and complications. Instead, prioritizing surveillance for more prevalent malignancies, such as skin cancers, lymphomas, and non-GI solid tumors, which have a higher incidence in LTx recipients, may optimize resource utilization and improve outcomes within these patients’ expected lifespans. By focusing on the most common and impactful health risks, healthcare providers can better allocate resources and enhance the overall care of LTx recipients.
Summary Box
What is already known:
Gastrointestinal (GI) malignancies are a potential but underrecognized risk in patients with systemic inflammatory diseases such as sarcoidosis
Sarcoidosis is associated with an increased risk of GI cancers, possibly due to its systemic inflammatory environment
Lung transplantation (LTx) increases cancer risk as a result of prolonged immunosuppression, particularly in patients with preexisting inflammatory conditions
The specific risk of developing de novo GI malignancies after LTx in sarcoidosis patients, compared to those with idiopathic pulmonary fibrosis (IPF), remains unclear
What the new findings are:
This study found a 1.72-fold greater subhazard ratio for GI malignancies in sarcoidosis patients post-LTx compared to IPF patients, although this was not statistically significant
The risk of GI malignancies appears to decrease over time following LTx, emphasizing the importance of early post-transplantation surveillance
Certain demographic and clinical factors, such as the timing of transplantation and the choice of immunosuppressive therapy, influence the risk of GI malignancies in this population
The findings suggest a need for specialized cancer screening strategies tailored to sarcoidosis patients following LTx
Acknowledgments
This study was based on OPTN data as of 24 March 2023, which was supported in part by the Health Resources and Services Administration contract 234-2005-370011C. The content is the responsibility of the authors alone and does not reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Biography
East Carolina University Brody School of Medicine; ECU Health, Greenville, NC; Porter Adventist Hospital, Centura Health, Denver, Colorado, USA
Footnotes
Conflict of Interest: DT discloses salary support from Kate B. Reynolds Charitable Trust and Lilly Grant Office for unrelated research and quality improvement projects. D.G. Adler: Consultant for Boston Scientific. Other authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest. The authors declare that they have no conflicts of interest.
Data availability statement: The datasets used and analyzed during the current study are freely available from the United Network for Organ Sharing (UNOS) registry Database (https://unos.org/data/)
References
- 1.Arkema EV, Cozier YC. Epidemiology of sarcoidosis:current findings and future directions. Ther Adv Chronic Dis. 2018;9:227–240. doi: 10.1177/2040622318790197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyer KC. Lung transplantation for pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2019;36:92–107. doi: 10.36141/svdld.v36i2.7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapnadak SG, Raghu G. Lung transplantation for interstitial lung disease. Eur Respir Rev. 2021;30:210017. doi: 10.1183/16000617.0017-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franzen DP, Brutsche M, Nilsson J, et al. Sarcoidosis - a multisystem disease. Swiss Med Wkly. 2022;152:w30049. doi: 10.4414/smw.2022.w30049. [DOI] [PubMed] [Google Scholar]
- 5.Jameson A, Revels J, Wang LL, Wang DT, Wang SS. Sarcoidosis, the master mimicker. Curr Probl Diagn Radiol. 2022;51:60–72. doi: 10.1067/j.cpradiol.2020.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Blank N, Lorenz HM, Ho AD, Witzens-Harig M. Sarcoidosis and the occurrence of malignant diseases. Rheumatol Int. 2014;34:1433–1439. doi: 10.1007/s00296-014-2983-5. [DOI] [PubMed] [Google Scholar]
- 7.Bonifazi M, Bravi F, Gasparini S, et al. Sarcoidosis and cancer risk:systematic review and meta-analysis of observational studies. Chest. 2015;147:778–791. doi: 10.1378/chest.14-1475. [DOI] [PubMed] [Google Scholar]
- 8.Engels EA, Pfeiffer RM, Fraumeni JF, Jr, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891–1901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Organ Procurement and Transplantation Network (OPTN) Request Data. U. S. Department of Health and Human Services. [[Accessed 27 November 2024]]. Available from: https://optn.transplant.hrsa.gov/data/view-data-reports/request-data/
- 10.Organ Procurement and Transplantation Network (OPTN) OPTN Policies. U. S. Department of Health and Human Services. [[Accessed 27 November 2024]]. Available from: https://optn.transplant.hrsa.gov/media/eavh5bf3/optn_policies.pdf .
- 11.Todd JL, Neely ML, Kopetskie H, et al. Risk factors for acute rejection in the first year after lung transplant. A multicenter study. Am J Respir Crit Care Med. 2020;202:576–585. doi: 10.1164/rccm.201910-1915OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spetsotaki K, Koch A, Taube C, Theegarten D, Kamler M, Pizanis N. Incidence of malignancies after lung transplantation and their effect on the outcome. 26 years'experience. Heliyon. 2023;9:e20592. doi: 10.1016/j.heliyon.2023.e20592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magruder JT, Crawford TC, Grimm JC, et al. Risk factors for de novo malignancy following lung transplantation. Am J Transplant. 2017;17:227–238. doi: 10.1111/ajt.13925. [DOI] [PubMed] [Google Scholar]
- 14.Ji J, Shu X, Li X, Sundquist K, Sundquist J, Hemminki K. Cancer risk in hospitalized sarcoidosis patients:a follow-up study in Sweden. Ann Oncol. 2009;20:1121–1126. doi: 10.1093/annonc/mdn767. [DOI] [PubMed] [Google Scholar]
- 15.McGhee JR, Fujihashi K. Inside the mucosal immune system. PLoS Biol. 2012;10:e1001397. doi: 10.1371/journal.pbio.1001397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taimeh Z, Hertz MI, Shumway S, Pritzker M. Lung transplantation for pulmonary sarcoidosis. Twenty-five years of experience in the USA. Thorax. 2016;71:378–379. doi: 10.1136/thoraxjnl-2015-207497. [DOI] [PubMed] [Google Scholar]
- 17.Raskin J, Vanstapel A, Verbeken EK, et al. Leuven Lung Transplant Group. Mortality after lung transplantation:a single-centre cohort analysis. Transpl Int. 2020;33:130–141. doi: 10.1111/tri.13540. [DOI] [PubMed] [Google Scholar]
- 18.Valapour M, Lehr CJ, Wey A, Skeans MA, Miller J, Lease ED. Expected effect of the lung Composite Allocation Score system on US lung transplantation. Am J Transplant. 2022;22:2971–2980. doi: 10.1111/ajt.17160. [DOI] [PubMed] [Google Scholar]
- 19.Gordon LG, Hopkins PM, Chambers DC, Green AC. Contribution of skin cancer to overall healthcare costs of lung transplantation in Queensland, Australia. J Heart Lung Transplant. 2023;42:1437–1444. doi: 10.1016/j.healun.2023.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Ungprasert P, Crowson CS, Matteson EL. Risk of malignancy among patients with sarcoidosis:a population-based cohort study. Arthritis Care Res (Hoboken) 2017;69:46–50. doi: 10.1002/acr.22941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leard LE, Holm AM, Valapour M, et al. Consensus document for the selection of lung transplant candidates:An update from the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2021;40:1349–1379. doi: 10.1016/j.healun.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekowski V, Jackson K, Halloran K, et al. Pre-transplant malignancy is associated with increased risk of de novo malignancy post-lung transplantation. Respir Med. 2022;197:106855. doi: 10.1016/j.rmed.2022.106855. [DOI] [PubMed] [Google Scholar]
- 23.Samara KD, Margaritopoulos G, Wells AU, Siafakas NM, Antoniou KM. Smoking and pulmonary fibrosis:novel insights. Pulm Med. 2011;2011:461439. doi: 10.1155/2011/461439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forey BA, Thornton AJ, Lee PN. Systematic review with meta-analysis of the epidemiological evidence relating smoking to COPD, chronic bronchitis and emphysema. BMC Pulm Med. 2011;11:36. doi: 10.1186/1471-2466-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada A, Komaki Y, Komaki F, Micic D, Zullow S, Sakuraba A. Risk of gastrointestinal cancers in patients with cystic fibrosis:a systematic review and meta-analysis. Lancet Oncol. 2018;19:758–767. doi: 10.1016/S1470-2045(18)30188-8. [DOI] [PubMed] [Google Scholar]


