Abstract
Sarcoid-like reaction is an immunological reaction that can affect lymph nodes and organs but does not meet the diagnostic criteria for systemic sarcoidosis. Anti-CD20 auto-antibodies have been reported to be responsible for such reactions. There are several reported associations between Chronic lymphocytic leukaemia (CLL), Amyotrophic lateral sclerosis (ALS) and Sarcoid-like reactions (SLR). We report a case of ALS developing in a patient with treated CLL and drug related SLR one day after exposure to Venetoclax and Rituximab. A 60-year-old male presented with lower limb rash, left leg weakness followed by bulbar symptoms which progressed over 12-months. Workup demonstrated a Cerebrospinal fluid (CSF) pleocytosis and inguinal lymphadenopathy. Skin and inguinal lymph node biopsies showed non-necrotising granulomata. Electromyography met diagnostic criteria for ALS. He was treated for presumed neurosarcoidosis mimicking ALS. Despite prednisolone and infliximab treatment, the motor symptoms rapidly progressed; Hence, we made a clinical diagnosis of ALS. We discuss the diagnostic and treatment challenges of this case.
Key points
1. ALS may occur on a background of pre-existing chronic haematological disorders
2. The occasional association of ALS and sarcoidosis has been recognised and there may be shared pathogenic mechanisms
3. Rituximab can induce a SLR
Introduction
Understanding the associations between different diseases is crucial for advancing medical knowledge, improving diagnosis and treatment, and enhancing patient outcomes. Many diseases, despite appearing distinct, often share common risk factors, genetic predispositions, or underlying pathophysiological mechanisms. By exploring these connections, healthcare professionals can uncover new insights into disease aetiology, identify potential therapeutic targets, and develop more effective prevention strategies.
Studies have explored the intriguing association between Amyotrophic lateral sclerosis (ALS) and lymphoproliferative disorders, revealing a correlation between these seemingly disparate entities [1]. Retrospective analyses uncovered a subtle yet significant association between autoimmune diseases such as sarcoidosis and ALS [2, 3]. Interestingly, drug-induced SLR have been rarely described as a potential adverse effect of Rituximab therapy [4].
Here, we describe a case of a 60-year-old male presenting with motor neuron disease, alongside evidence of sarcoid-like reactions (SLR) on skin and inguinal lymph node biopsy. This presentation unfolds after a more than a decade-long history of relapsing chronic lymphocytic leukaemia (CLL), the most recent treatment of which having been with Venetoclax and Rituximab and achieving haematological remission.
Case description
History
A 60- year-old male with a ten-year history of relapsing CLL, which was ultimately treated with Venetoclax and Rituximab for 6 months followed by Venetoclax, as a single-agent, which induced the haematological remission. However, after one day of the re-challenge, he developed a confluent rash sparing his face (Fig. 1). This rash was steroid responsive, and a skin biopsy showed a granulomatous change (Fig. 2).
Fig. 1.
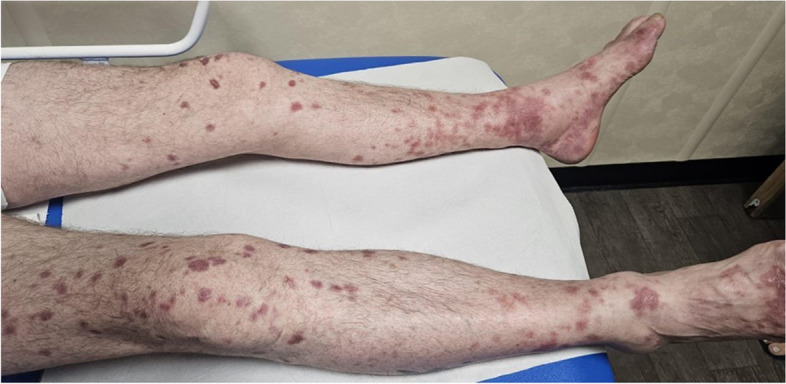
Skin rash after administration of Venetoclax and Rituximab
Fig. 2.
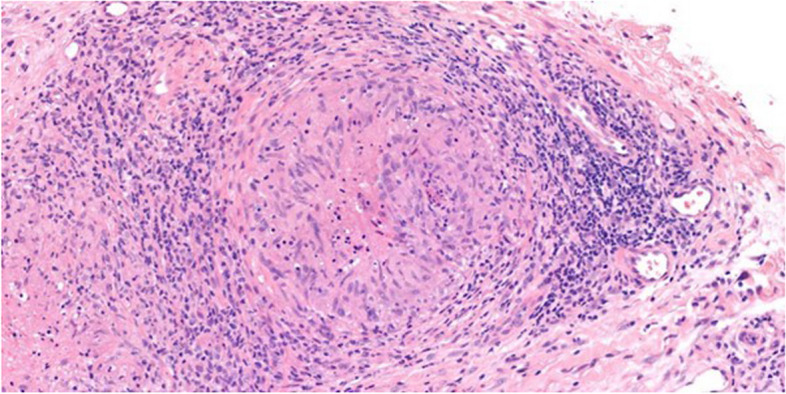
H&E (x200) of skin showing a necrotizing granuloma with central area of necrosis and palisading histiocytes surrounded by a prominent lymphoid inflammatory infiltrate
He subsequently developed progressive left lower limb weakness followed by bulbar symptoms over a 12-month period. Clinically, he was dysarthric with poor palatal elevation and fasciculation was noted in his tongue. His upper limbs motor and sensory examination were normal. The left lower limb examination showed marked distal wasting, with motor power graded as Medical Research Council (MRC) grade 4 proximally and grade 2 distally.
Upper limb reflexes were brisk. Knee and ankle reflexes were absent on the left. Plantar responses were extensor bilaterally.
Initial investigations
The patient was admitted for further investigations. Nerve conduction studies (NCS) showed preserved sensory responses in the lower limbs. The left peroneal and tibial motor responses were significantly reduced in amplitude. The left tibial F response was absent. Responses in the right upper and lower limb were within normal limits. Needle electromyography examination showed neurogenic changes in the left tibialis anterior and to a lesser extent in the left gluteus medius. Occasional fibrillations and fasciculation were also noted in the right tibialis anterior.
All remaining blood tests including creatinine kinase, ACE and serum calcium were normal. Interferon-gamma release assay was negative excluding tuberculosis. Paraneoplastic antibody screen was negative. Magnetic resonance imaging of the head, and whole spine, and lumbosacral plexus were normal. Lumbar puncture showed 29 × 106 mononuclear cells per litre, which were predominantly mixed T cells with no clonal proliferation of B cells. Cerebrospinal fluid (CSF) glucose was normal, and protein was elevated at 890 mg/L. ALS molecular genetic testing was negative (with no family history). PET-CT scan reported bilateral FDG-avid inguinal lymph nodes (Fig. 3). Biopsy of the lymph nodes showed epithelioid cell non-necrotising granulomata and foreign body type giant cells consistent with SLR (Fig. 4).
Fig. 3.
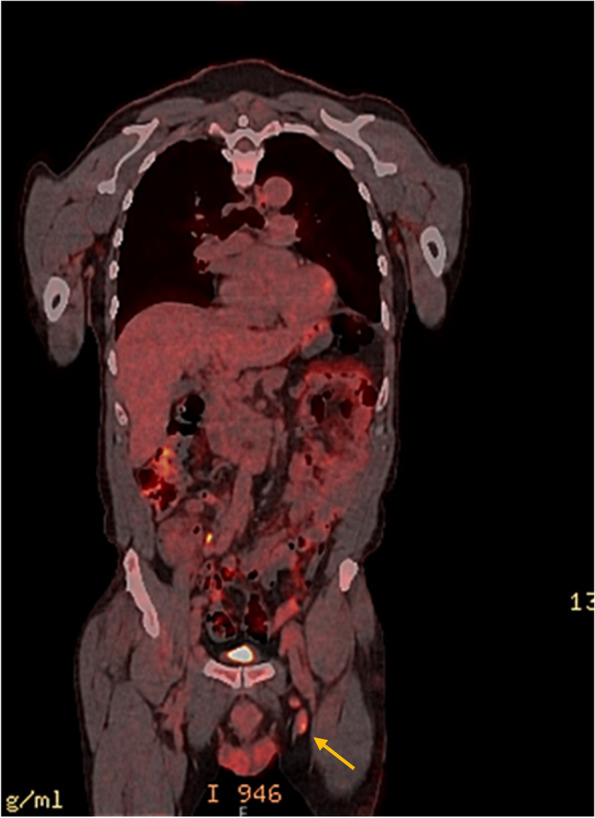
Left FDG avid inguinal lymph nodes are amenable for ultrasound-guided biopsy
Fig. 4.
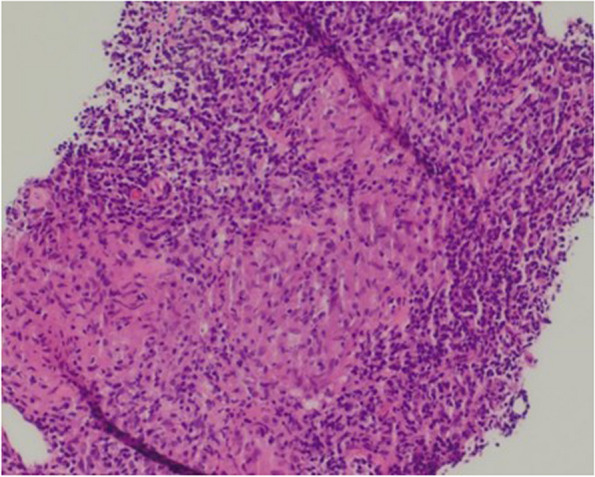
H&E (x200) of lymph node showing epithelioid cell non-necrotising granulomata and foreign body type giant cells
Further progress
Over the following 3 months, his symptoms progressed. Examination demonstrated a prominent pout reflex, a pathologically brisk jaw jerk and a palmomental reflex. There was tongue weakness and wasting. Upper limb examination showed fasciculation in both triceps muscles and mild weakness of left shoulder abduction. Lower limb examination showed bilateral quadriceps fasciculation and a marked distal left sided footdrop with severe weakness of ankle dorsiflexion and plantar flexion. The tendon reflexes were globally brisk.
Differential diagnosis and treatment
In summary, this patient presented with a background of relapsing CLL, albeit now in remission, and a rapidly progressive asymmetrical predominantly motor syndrome in the context of possible sarcoid- like reaction on skin and inguinal lymph node biopsy. The patient was reviewed in a multidisciplinary team with neurology, haematology, and respiratory input, which facilitated the complicated clinical decision making.
The haematologist advised that CLL treatment, in the absence of clear evidence of disease progression, would pose significant toxicity risks. In isolation, the neurological symptoms and signs were consistent with ALS. The neurophysiology report also indicated that motor neuropathy, although rare, can occur in neurosarcoidosis. Additionally, haematological malignancies and sarcoidosis have both been present with an ALS like syndrome. However, it was difficult to determine whether treatment of sarcoidosis would lead to improvement in the motor syndrome. Interestingly, drug-induced SLR have been rarely described as a potential side effect of Rituximab therapy.
The complexity of this case was to ascertain whether these conditions were associated or not. We commenced neurosarcoid therapy based on CSF pleocytosis and biopsy results with prednisolone 60 mg once a day.
However, after four weeks of high dose prednisolone, the symptoms continued to progress with worsening muscle power strength and swallowing difficulties. A repeat CSF examination continued to demonstrate lymphocytosis with a raised protein of 670 mg/L. The patient’s treatment was escalated to infliximab traditionally used for neurosarcoidosis. Despite aggressive treatment, the patient continued to deteriorate, and it was felt that he would no longer benefit from prednisolone or infliximab therapy. It was thus concluded that the biopsy and the CSF findings were likely to be associated with SLR secondary to Rituximab therapy. The patient did however meet the revised El Escorial criteria for ALS and his progression was typical as in ALS patients. The patient was subsequently offered Riluzole and at a 6-months review, he is now requiring two aids, a modified diet and a speech assist device.
Discussion
Diagnosis of ALS remains primarily a clinical diagnosis based on the number of affected body parts (bulbar, cervical, thoracic and lumbosacral) with simultaneous upper motor neuron and lower motor neuron signs. Formally, the patient fulfilled criteria of ALS according to the revised El Escorial criteria [5]. Riluzole is the sole approved medication that has demonstrated in clinical trials its ability to slow ALS progression. However, Riluzole is not a cure, and its impact is limited. In clinical trials, patients treated with Riluzole lived about 3 months longer at the 18-month mark set by the researchers compared to those who received a placebo [6]. ALS remains an incurable progressive disease. It is important that physicians to look for treatable pathology that can mimic the presentation of ALS.
Idiopathic and paraneoplastic association between malignancies and ALS has been previously reported. Gordon et al. reviewed 56 cases of concomitant ALS and lymphoproliferative disorder observed at their centre and reported in the literature [1]. The authors concluded that more than half of the patients with lymphoproliferative disorders including lymphoma, multiple myeloma, chronic lymphocytic leukaemia and macroglobulinemia and ALS had both upper and lower motor neuron signs while the remaining had lower motor neuron signs only. Based on circulating paraproteins and bone marrow biopsies from ALS patients, the authors suggested that the frequency of lymphoproliferative disorders in ALS patients was around 2–5% [1].
Sarcoidosis and ALS occurring comorbidly or as a manifestation of neurosarcoidosis is not common. However, a recent retrospective study revealed that likelihood of both those diseases coinciding might be underestimated. A diagnosis of sarcoid was reported in 6 per 1000 patients with ALS [7]. A literature search yielded twelve cases of ALS and sarcoid. Nine of these twelve cases had a spinal onset ALS, while three had a bulbar onset. Sarcoid and ALS were simultaneously diagnosed in three patients. Sarcoid was diagnosed prior to ALS in two patients and at autopsy of the lymph nodes in one. Four of these cases reported that sarcoid treatment did not change the ALS course, whereas one author reported improvement of symptoms [7].
SLR are characterized by the development of a granulomatous disease on histopathology with or without clinical features of sarcoidosis. SLR may affect a single organ such as the skin, spleen, kidneys, and lungs. Reported medications that have been linked to a sarcoid -like reaction include antiretroviral therapies, tumour necrosis factor-α antagonists, interferons, and immune checkpoint inhibitors [8]. In a study looking at the association of sarcoidosis and lymphoma, the authors described the development of sarcoidosis in 39 patients treated for lymphoma. About one-third of these patients had received rituximab [9], whereas patients receiving chemotherapy alone without rituximab for haematological malignancy did not report sarcoid like reaction as a side effect of treatment [9].
Direct connections between CLL, ALS, and SLR are rare, however, immune dysregulation plays a central role in all three conditions. The chronic inflammation and altered immune responses seen in CLL may theoretically predispose patients to immune-related conditions such as SLR [10]. While CLL, ALS, and SLR are distinct entities, they may share inflammatory and immune pathways, such as cytokine dysregulation (e.g., interleukins, tumour necrosis factor) and abnormal T cell activity. These immune alterations could contribute to the overlap seen in patients who develop both haematological malignancies and neurodegenerative conditions [11]. Finally, in some cases, SLR and neurological disorders can occur as part of a paraneoplastic syndrome, where cancer triggers immune responses that damage tissues unrelated to the tumour, potentially linking CLL and ALS.
Our patient was treated with Venetoclax which selectively binds to BCL/2 inhibitor to supress apoptosis and is commonly used in CLL. No reported SLR have been associated with this drug. Rituximab (anti-CD20) was also used in combination which promotes cell lysis, SLR have been reported in patient receiving rituximab.
Conclusion
This case demonstrates an extremely rare combination of three pathologies: CLL, SLR and ALS. It also highlights both the potential difficulty in diagnosis and treatment of these conditions. Whilst the initial rash, investigations and motor syndrome was suspicious for neurosarcoid, the lack of response to treatment and rapid progression precluded this diagnosis. It became more apparent with time, that the patient’s signs and symptoms progressed in a classical pattern seen in ALS.
Acknowledgements
The authors thank Mathew Flynn and Joseph Yates for providing us with the pathology pictures.
Abbreviations
- CLL
Chronic lymphocytic leukaemia
- ALS
Amyotrophic lateral sclerosis
- SLR
Sarcoid-like reactions
- CSF
Cerebrospinal fluid
- MRC
Medical research council
- NCS
Nerve conduction studies
Author’s contributions
JF gathered all the information and wrote the article. EG contributed and corrected the haematology sections of the paper and was involved in the patient’s care. BM contributed and corrected the respiratory sections of the paper and was involved in the patient’s care. RA contributed and corrected the neurophysiology sections of the paper and was involved in the patient’s care. AP contributed and corrected the neurology sections of the paper and was involved in the patient’s care.CO supervised the finalisation of the paper.
Funding
Not applicable.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This paper did not require any ethical approval from the ethics department. A consent form for participation of the case was signed by the patient.
Consent for publication
A consent form for publication of the case was signed by the patient.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gordon PH, Rowland LP, Younger DS, Sherman WH, Hays AP, Louis ED, et al. Lymphoproliferative disorders and motor neuron disease: an update. Neurology. 1997;48(6):1671–8. [DOI] [PubMed] [Google Scholar]
- 2.Turner MR, Goldacre R, Ramagopalan S, Talbot K, Goldacre MJ. Autoimmune disease preceding amyotrophic lateral sclerosis: an epidemiologic study. Neurology. 2013;81(14):1222–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kutlubaev MA, Hardy TA, Areprintseva DK, Pervushina EV. Comorbid amyotrophic lateral sclerosis and sarcoidosis. Acta Neurol Belgica. 2023;123(2):709–10. [DOI] [PubMed] [Google Scholar]
- 4.Mrabet S, Dahmene R, Fradi A, Jaziri A, Boukadida R, Azzebi A, et al. Sarcoid-like reaction in the kidney following Rituximab for Mantle Lymphoma in a 60-Year-old man. Am J Men’s Health. 2023;17(2):15579883231159344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disorders. 2000;1(5):293–9. [DOI] [PubMed] [Google Scholar]
- 6.Turner MR, Al-Chalabi A, Shaw CE, Leigh PN. Riluzole Motor Neurone Disease. 2003;3(3):160–9. [Google Scholar]
- 7.Canali E, Sola P, Richeldi L, Spagnolo P, Mora G, Georgoulopoulou E, et al. Amyotrophic lateral sclerosis and sarcoidosis: a difficult differential diagnosis. Amyotroph Lateral Scler. 2010;11(4):410–1. [DOI] [PubMed] [Google Scholar]
- 8.Chopra A, Nautiyal A, Kalkanis A, Judson MA. Drug-induced sarcoidosis-like reactions. Chest. 2018;154(3):664–77. [DOI] [PubMed] [Google Scholar]
- 9.London J, Grados A, Fermé C, Charmillon A, Maurier F, Deau B, et al. Sarcoidosis occurring after lymphoma: report of 14 patients and review of the literature. Med (Baltim). 2014;93(21):e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papanikolaou IC, Sharma OP. The relationship between sarcoidosis and lymphoma. Eur Respiratory Soc. 2010;36(5):1207–19. [DOI] [PubMed] [Google Scholar]
- 11.Piccoli T, Castro F, La Bella V, Meraviglia S, Di Simone M, Salemi G, et al. Role of the immune system in amyotrophic lateral sclerosis. Analysis of the natural killer cells and other circulating lymphocytes in a cohort of ALS patients. BMC Neurol. 2023;23(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.


