Abstract
Background
Outcomes following surgery to operatively manage extremity fractures are variable, and up to two-thirds of patients report chronic post-surgical pain. Preliminary evidence suggests that psychotherapy directed at improving coping skills and reducing somatic vigilance may improve outcomes among fracture patients. The objective of this pilot study was to test the feasibility and acceptability of a randomized controlled trial comparing an online cognitive behavioural therapy (CBT) program versus usual care in patients with an operatively managed open or closed extremity fracture.
Methods
We conducted a single-centre internal pilot study over a 10-month period in patients with at least one operatively managed open or closed fracture of the appendicular skeleton. Participants were randomized to an online CBT program or usual care and followed for 12 months. The goals of our pilot study were to determine an acceptable rate of recruitment, the degree to which participants randomized to CBT were compliant with treatment, the site investigator’s ability to adhere to study protocol and data collection procedures, and our ability to achieve high follow-up rates. Feasibility criteria were evaluated using a graded “traffic light” approach, in which “green light” indicates moving forward with the definitive trial, “yellow light” indicates proceeding with modifications to the protocol and trial procedures, and “red light” indicates a definitive trial is not feasible without significant protocol and trial procedure modifications.
Results
We enrolled 94 participants over 10 months, which resulted in a “yellow light” for recruitment. Participant compliance with completion of the online CBT program received a “yellow light”, with 60% of participants who were randomized to CBT completing all seven modules. However, 40% of participants in the CBT-arm withdrew from the program, resulting in a “red light”. Adherence with the study protocol activities at baseline was relatively high (88%) which resulted in a “yellow light”. Follow-up was 85% (80 of 94) at 12 months, resulting in a “yellow light”.
Conclusions
These results suggest feasibility of a definitive, multi-centre trial to compare CBT versus usual care in the management of persistent post-operative pain in fracture patients despite the pilot phase identifying some challenges with enrollment timelines, compliance with the CBT program, and participant follow-up. For the definitive trial, we will expand participant recruitment to additional centres and implement strategies to optimize participant engagement and compliance with the CBT program and follow-up.
Trial registration
ClincialTrials.gov (NCT04274530). Registered February 18, 2020, https://classic.clinicaltrials.gov/ct2/show/NCT04274530.
Keywords: Pilot study, Feasibility, Cognitive behavioural therapy, Chronic pain
Key messages regarding feasibility
Emerging evidence suggests that cognitive behavioural therapy (CBT) may improve outcomes after fracture repair. We conducted a pilot study to evaluate the feasibility of a definitive trial of online CBT vs. usual care among patients with operatively managed extremity fractures.
Additionally, with Ontario COVID pandemic regulations requiring research personnel to conduct study measures remotely, our pilot study assessed the practicality of remote recruitment and follow-up methods.
Our internal pilot study identified key issues that might have rendered a definitive trial unfeasible. By modifying our protocol to address these challenges, we have enhanced the feasibility of a definitive trial involving 10 trauma centres in the United States and Canada.
Background
Outcomes following surgery to manage extremity fractures widely vary. While some patients recover quickly and return to their pre-injury levels of activity, many report ongoing disability and persistent pain a year after fracture repair surgery [1, 2]. In a study examining surgical repair of open fractures of the appendicular skeleton, at 1-year post-fixation, 67% of patients reported moderate to severe pain and 38% reported moderate to extreme pain interference [3]. Furthermore, a recent systematic review found an incidence of chronic post-surgical pain of approximately 50% 2 years after extremity fracture surgery [4].
There is a recognized relationship between the development of chronic pain and the presence of stress, distress, anxiety, depression, catastrophizing, fear-avoidance behaviors, and poor coping strategies [5]. The Somatic Pre-Occupation and Coping (SPOC) questionnaire was designed and validated to identify unhelpful illness beliefs among orthopedic trauma patients [6]. Higher somatic pre-occupation and poor coping (as measured by the SPOC questionnaire) have shown a strong association with chronic pain, functional limitations, unemployment, and reduced quality of life 1 year after fracture repair [6, 7]. Further, there is moderate certainty evidence that peri-operative psychotherapy can reduce chronic pain and persistent impairment after surgical procedures [8]. This suggests the possibility that patients with unhelpful illness beliefs undergoing operative fixation of an extremity fracture could be targeted for concurrent therapy designed to modify unhelpful cognitions to improve their prognosis.
Psychological treatments such as cognitive behavioural therapy (CBT) can potentially help to address unhealthy illness beliefs and behaviours and could reduce persistent post-surgical pain and its associated effects among orthopaedic fracture patients [3]. To date, there has only been one randomized trial of CBT compared to usual care for older people who underwent surgery for hip fracture, with the aim to reduce depressive symptoms [9]. No randomized trial has explored the effectiveness of CBT for reducing chronic pain after fracture repair surgery [8]. Therefore, we designed a trial assessing CBT compared to usual care for reducing chronic pain after operatively managed extremity fractures. We elected to provide CBT delivered remotely by an online service provider due to the mobility and transportation challenges involved in getting fracture patients to access in-person CBT in a previous in-person pilot study [10]. This service provider delivered CBT via online modules that participants completed with asynchronous support and feedback from licensed therapists. In a recent systematic review and meta-analysis of randomized controlled trials, high certainty evidence showed no difference in effectiveness if CBT was delivered in-person or remotely with therapist guidance, across a range of clinical conditions [11].
The purpose of this internal pilot study was to assess the feasibility of a definitive randomized trial comparing online CBT to usual care for patients with operatively managed extremity fractures. Specific feasibility objectives were centered around adequate participant enrollment, CBT program compliance, and overall participant follow-up and compliance with the study protocol.
Methods
Study overview
We conducted a local internal pilot study to determine the feasibility of the Cognitive Behavioural Therapy to Optimize Post-Operative Recovery (COPE) trial before expanding enrollment to additional trauma centres in Canada and the United States. The COPE trial is coordinated by the Surgery Methods Centre at McMaster University. One local site (Hamilton Health Sciences–General Site), which is affiliated with McMaster University, participated in the pilot phase. The trial was registered with ClinicalTrials.gov (NCT04274530) and was approved by the Hamilton Integrated Research Ethics Board (#4336) prior to participant enrollment. The pilot phase was conducted over a period of 10 months from January to October 2021. At the time of this pilot study initiation, COVID-19 pandemic restrictions prevented research personnel from performing in-person study procedures. Therefore, the protocol was designed to allow all research activities, including recruitment, CBT registration, and follow-up visits, to be completed remotely.
Participant identification and screening
Clinical staff within the patient’s circle of care, including surgeons, nurses, and surgical residents, were the first to approach eligible patients about the possibility of participating in the COPE trial. The research team verified the eligibility of patients who consented to be contacted and called each patient to give a brief introduction to the study and schedule the informed consent discussion if desired. This initial contact via phone provided an opportunity to assess each patient’s willingness and availability to be contacted remotely for data collection, which was essential to their successful participation in the year-long trial.
Eligibility criteria
Eligible patients were men and women ages 18 or older who had received definitive operative treatment with internal fixation for an open or closed fracture of the appendicular skeleton. Patients were required to have access to a Wi-Fi-enabled smartphone, tablet, or computer and possess the English language skills and cognitive ability required to independently participate in an online CBT program. Among those patients who were fully weight-bearing, those not experiencing any pain in the fracture region were ineligible to participate in the trial. All patients were required to provide informed consent and be randomized to a treatment arm between 2 and 12 weeks after their fracture.
Informed consent
Study personnel called eligible patients to discuss the study and obtained informed consent, either verbally with another study coordinator acting as phone witness, or through an electronically signed informed consent form. The study coordinator then provided participants with contact information for the research team and offered them a choice between an electronic or mailed copy of the informed consent form.
Randomization
After providing informed consent, participants were randomized using REDCap Cloud electronic data capture (EDC) software to either the online CBT (intervention) group or to the usual care (control) group. Treatment allocation was stratified based on the following factors: (1) sex; (2) at least one open fracture versus no open fractures; (3) military, veteran, or first responder status; and (4) illness beliefs as defined by SPOC score (greater illness beliefs defined as SPOC score ≥ 48 versus lesser illness beliefs defined as SPOC score < 48).
Treatment groups
Participants randomized to the CBT group were provided with access to an online CBT program offered by a private company, LifeWorks (recently transitioned to Telus Health; telushealth.com), that specializes in treating patients using online psychotherapy. The program was developed specifically for study participants and focused on managing pain after fracture surgery through seven self-led modules [12]. All parts of the program were accessible using a mobile app on an internet-enabled smartphone or tablet, or via an internet browser on a personal laptop or computer. Participants completed an initial assessment and were then scheduled to meet with their assigned therapist via telephone to ensure they were not experiencing active psychosis or suicidal ideation, in which case they were ineligible for the trial and referred to emergency services in their area. A full list of eligibility criteria is reported in the COPE trial protocol [13]. Eligible patients who consented to participate and were randomized to the intervention arm were instructed to complete the seven CBT modules at their own pace which was expected to take approximately 1–2 h per week for 8 weeks. Each module provided education and exercises to help participants reflect on their beliefs related to pain and recovery and improve their coping skills for pain management. Topics included changing negative thoughts, goal setting, mindfulness, and sleep habits. The participant’s therapist monitored their progress, made modules available, and provided feedback and support as needed. In-app messaging was also available to allow participants to reach out to their therapist with questions at any time.
Participants randomized to the usual care group did not have access to the online CBT program and received standard and routine care as determined by their surgeon.
Outcome measures
The goals of our pilot study were to determine the rate of recruitment, adherence to study protocol and data collection procedures, our ability to achieve close to 100% follow-up rates, and the degree to which patients randomized to CBT complied with treatment.
The primary clinical outcome of the definitive trial is the prevalence of moderate to severe persistent post-surgical pain at 12 months post-fracture. Secondary clinical outcomes of the definitive trial include physical function, mental function, return to function, pain severity, and pain interference over 12 months post-fracture, and the proportion of participants prescribed opioid class medications (and average dose) at 6 and 12 months post-fracture. Clinical outcomes were assessed at 3, 6, 9, and 12 months post-fracture.
Data collection and participant follow-up
At enrollment, study coordinators sent private links to the baseline surveys to participants via email. Research staff stayed on the phone with participants during survey completion to provide support and answer questions as participants completed the surveys for the first time. Research personnel emailed the registration link for the CBT program to participants who were randomized into the online CBT program and guided them through the registration process. Links to the follow-up questionnaires were sent via email with research staff calling or emailing participants to complete the required study follow-up visits at 3, 6, 9, and 12 months post-fracture.
To further encourage participant compliance with the study, research personnel also emailed participants with details of their next study follow-up visit. They also provided participants enrolled into the CBT program with a “User’s Guide” that listed common questions and answers related to the use of the program [14].
Success criteria for pilot study outcomes
Feasibility outcomes were selected to determine what modifications, if any, would be required before proceeding to a larger, definitive trial. Since our outcomes did not lend to a traditional quantitative analysis, we established thresholds of feasibility using a “traffic light” set of criteria, which are outlined in detail in Table 1. Using this approach, a “green light” indicated moving forward as is with the definitive trial, a “yellow light” indicated proceeding with some modifications, and a “red light” indicated the objective was not feasible without significant modifications [15]. In addition to the traffic light criteria, the research team reviewed the common reasons for exclusion, including why patients declined to participate, as well as reasons for early withdrawal. This approach allowed us to frequently monitor our feasibility measures and evaluate the need for adjustments. Criteria for feasibility and threshold details for each of the traffic light categories are outlined in Table 1. Success thresholds were determined based on prior orthopaedic trauma pilot studies while taking into consideration the timelines and methodological standards required for efficiently conducting a high-quality clinical trial [16, 17].
Table 1.
Traffic light criteria
| Criteria | Traffic light criteria | ||
|---|---|---|---|
| Green light | Yellow light | Red light | |
| 1. Participant enrollment | |||
| a. Proportion of eligible patients who were missed or unable to be contacted between 2 and 12 weeks (remote enrollment/COVID) | Less than 10% were missed or unable to be contacted | 10 to 30% were missed or unable to be contacted | Greater than 30% were missed or unable to be contacted |
| b. Proportion of eligible patients who provided informed consent | Greater than 50% provided informed consent | 25 to 50% provided informed consent | Less than 25% provided informed consent |
| c. Number of participants enrolled per month | Greater than 10 participants enrolled per month | 5–10 participants enrolled per month | Less than 5 participants enrolled per month |
| 2. Acceptability of online, self-led CBT intervention to patients | |||
| a. Proportion of participants who were randomized to CBT and completed the program | Greater than 75% completed the program | 50 to 75% completed the program | Less than 50% completed the program |
| b. Proportion of CBT-arm participants who withdrew from CBT | Less than 15% withdrew from the program | 15 to 35% withdrew from the program | Greater than 35% withdrew from the program |
| 3. Compliance with the protocol | |||
| a. Proportion of randomization errors | Fewer than 5% errors | 5 to 10% errors | Greater than 10% errors |
| b. Proportion of participants who completed all required baseline activities (initial surveys, CBT registration, and assessment) | Greater than 90% completed the baseline activities | 80 to 90% completed the baseline activities | Less than 80% completed the baseline activities |
| 4. Complete follow-up | |||
| a. Proportion of participants who completed each follow-up visit with study staff | Greater than 90% complete each visit | 80 to 90% complete each visit | Less than 80% complete each visit |
| b. Proportion of participants who completed the primary outcome measure (chronic post surgical pain [CPSP]) at each follow-up visit | Greater than 90% completed the CPSP | 80 to 90% completed the CPSP | Less than 80% completed the CPSP |
| c. Overall proportion of participants who withdrew from the study | Less than 10% withdrew from the study | 10 to 30% withdrew from the study | Greater than 30% withdrew from the study |
Sample size
Since feasibility objectives in our pilot trial did not lend themselves to traditional quantitative sample size calculations, we selected an enrollment period of 10 months and a minimum sample size of 75 patients to assess the feasibility of a definitive trial. Additionally, other orthopaedic fracture trials conducted through our methods centre using a traditional randomized controlled trial design have successfully conducted pilot studies using sample sizes ranging from 50 to 100 participants [18].
Statistical analysis
Descriptive statistics were used to analyze the baseline characteristics reported by treatment groups as count (percent) for categorical variables and mean (and standard deviation) for continuous variables when normally distributed or median (and interquartile range) when not. Descriptive statistics were used to summarize feasibility outcomes. Using the results of the feasibility analyses, the investigators determined which traffic light criteria were met for each feasibility endpoint.
Results
Feasibility category 1: participant enrollment
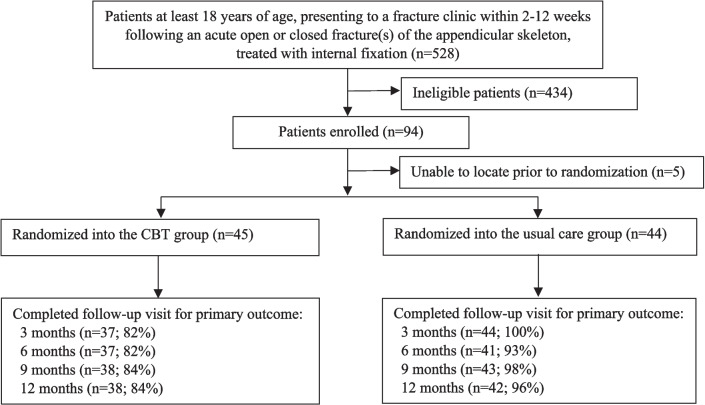
In this pilot study, a total of 528 patients were screened between January and October 2021. Of these, 290 did not meet inclusion/exclusion criteria (55%), an additional 132 (25%) declined participation, and 12 potential participants were missed (not screened) (23%) (Fig. 1). The most common reasons for exclusion were anticipated problems with follow-up (n = 118, 41%) and patients not having consistent internet access (n = 44, 15%) (Table 2). In total, 94 patients were enrolled and of these, 89 were randomized. The remaining five participants could not be reached by research personnel after the enrollment visit (n = 5) (Table 3). Overall, 11 (12%) of 94 participants withdrew prior to or at the baseline visit, resulting in 83 active participants.
Fig. 1.
Study flow diagram of enrollment
Table 2.
Reasons for exclusion
| Reasons for exclusion | Number of patients excluded n = 290 Number (%) |
|---|---|
| Anticipated problems with follow-up | 118 (41%) |
| Does not have required language skills or cognitive skills | 44 (15%) |
| Does not have consistent online access | 44 (15%) |
| Not experiencing pain | 40 (14%) |
| Not suitable for CBT program (active psychosis, suicidality, incarceration, substance use disorder or already participating in mental health therapy) | 35 (12%) |
| Concomitant injury | 9 (3%) |
Table 3.
Early withdrawals
| Reasons for withdrawal and visit of withdrawal | Number of participants n = 14 Number (%) |
|---|---|
| Withdrew consent after randomization to the CBT program and prior to initiation of the CBT program | 6 (43%) |
| Unable to locate prior to randomization | 5 (36%) |
| Unable to locate by the end of the follow-up period | 3 (21%) |
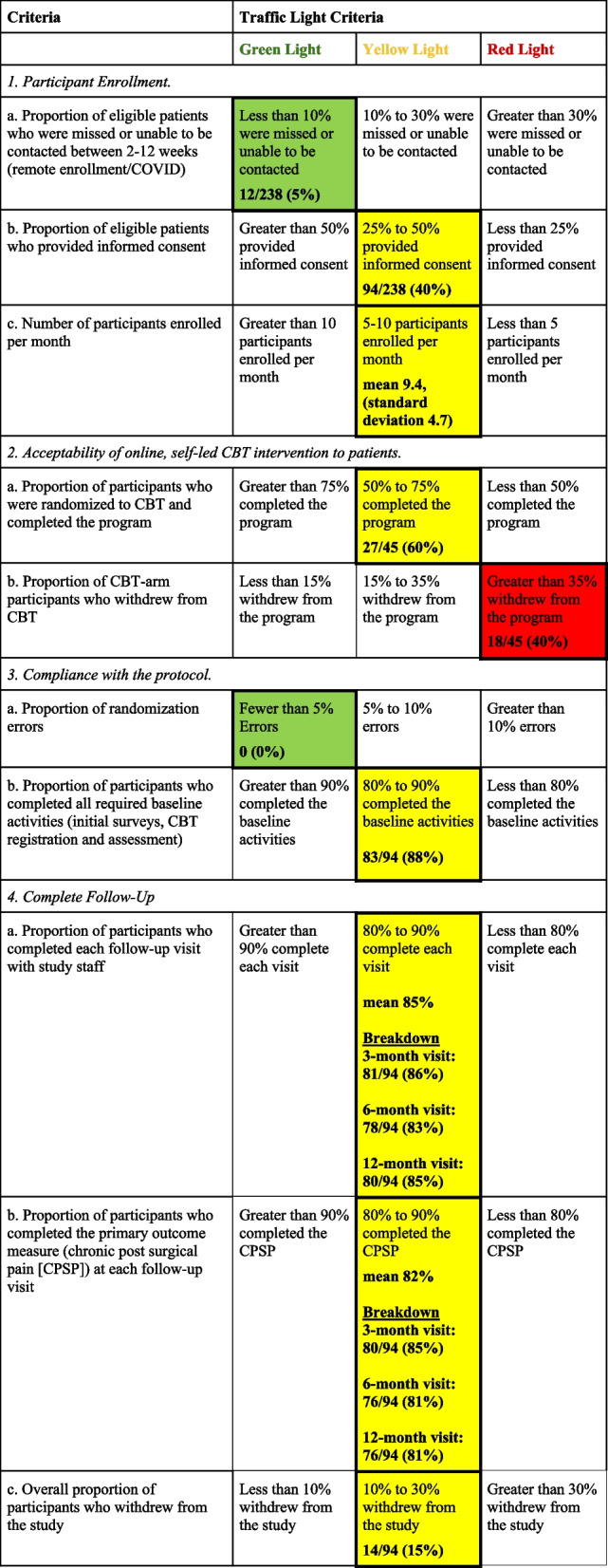
Examining the first of the traffic light criteria for enrollment, 12 of the 238 participants who met the eligibility criteria (5%) were missed, resulting in a “green light” (threshold for the greenlight was less than 10% missed) (Table 4). Of the 238 eligible participants, 94 consented to participate (40%), resulting in a “yellow light” (threshold for the yellow light was 25 to 50% of eligible participants providing informed consent). The mean number of participants enrolled per month was 9.4 which yielded a “yellow light” (threshold for the “yellow light” was 5 to 10 participants per month).
Table 4.
Traffic light criteria results
Feasibility category 2: acceptability of online, self-led CBT intervention to participants
Of the 45 participants randomized to CBT, 27 participants completed all CBT modules (60%), resulting in a “yellow light” (Table 4). Eighteen of 45 participants (40%) withdrew from the CBT program after having completed anywhere from 0 to 5 modules, resulting in a “red light”.
Feasibility category 3: compliance with the protocol
Throughout the randomization process, there were 0 (0%) randomization errors, and all active participants (83/94 (88%)) completed their baseline activities (initial surveys, CBT registration, and assessment), which resulted in a “yellow light”.
Feasibility category 4: complete follow-up
When accounting for all 94 enrolled participants, participant follow-up was 86% (81/94) at 3 months, 83% (78/94) at 6 months, 86% (81/94) at 9 months, and 85% (80/94) at 12 months (Fig. 1). The mean completion rate across all visits was 85% resulting in a “yellow light” for overall participant follow-up. Research personnel obtained the primary outcome for 80 of 94 (85%) of participants at 3 months and 76 of 94 (81%) at 6 months, 9 months, and 12 months (mean completion rate across all visits: 82%), resulting in a “yellow light”. Of the 94 patients initially enrolled in the study, 11 (12%) withdrew early (6 withdrew consent and 5 participants could not be contacted). Three (3) additional withdrawals occurred during the 12-month follow-up period because the participants could not be located. Therefore, a total of 14 of 94 (15%) participants withdrew from the study, resulting in a “yellow light” (Table 4).
Discussion
This pilot study identified the multiple challenges posed by conducting a trial evaluating an online CBT program in fracture patients. Most of the feasibility criteria were met with a “yellow light” and one feasibility endpoint relating to the proportion of CBT-arm participants who withdrew from CBT received a “red light”. These results indicate that changes to procedures, especially related to CBT compliance, are necessary for a successful definitive trial.
Participant enrollment was slower than anticipated (9.4 patients per month), and we did not meet the green light criteria of enrolling greater than 10 patients per month. COVID-19 pandemic restrictions required participant enrollment for this pilot trial to occur remotely via telephone. While this process worked well, we believe that in-person visits may personalize the explanation of the trial, help study coordinators minimize the number of patients missed for screening, and improve the consent process. To address this issue, we plan to enroll our 94 pilot participants into our definitive trial and invite at least 10 additional trauma centres to participate in the trial. This should allow us to enroll our target of 1000 patients within 18 months, which would require a minimum of 4.6 patients per month per site.
Participant compliance with the CBT program received a yellow light in the category relating to completion of the CBT program and a red light in the category relating to the withdrawal from the CBT program. To successfully conduct a definitive trial, it is necessary to optimize the online CBT experience to more effectively engage participants and ensure completion of the program modules. Since the study coordinators at each clinical site have the most contact and rapport with participants, we will implement several strategies to support them in their efforts to maximize CBT participant engagement. We will provide access to the CBT platform to study coordinators at each clinical site so that they can view the program for themselves, accurately describe it to participants, and assist them while they complete their modules. In addition, the study coordinators from the pilot clinical site will attend site initiation visits to give practical advice to new centres on how to effectively engage participants and help them navigate the online CBT platform. Finally, we will provide reimbursement to participants to offset their time and effort for completing the CBT program.
The criteria relating to compliance with the protocol were met with a “green light” for randomization errors and a “yellow light” for completion of all required baseline activities. There were no randomization errors, and although all 83 active participants completed the baseline activities, the 11 participants who withdrew prior to or at the baseline visit did not complete their baseline activities. Of these 11 participants, five participants withdrew prior to randomization and another six participants withdrew immediately after being randomized to the CBT program. To address this issue, study coordinators will prioritize anticipated compliance with CBT over rapid enrollment when screening potentially eligible patients.
The follow-up rates were met with a “yellow light”. Study coordinators will revise their enrollment procedures to better explain the trial to potential participants with the goal of reducing the rate of early withdrawals from the trial. They will emphasize the importance of completing follow-up and advise that uncertainty in meeting this requirement is an acceptable reason to decline participation in the study. To maximize completion of all required baseline activities, we will request for participants to complete these activities at the time of enrollment, but prior to randomization. We will also continue to provide funding to participants to offset their time for study participation after completion of each of the baseline, 6-month, and 12-month follow-up visits.
There were small differences in the completion of the follow-up visits and the collection of the primary endpoint (persistent post-surgical pain). This was a result of how links to the follow-up data collection forms were sent to participants. Since some participants struggled to find all the individual email links to the study questionnaires, we will combine them into one email for each study visit. We also anticipate that the removal of restrictions on in-person research at participating fracture clinics will provide study coordinators with the opportunity to complete study visits while participants are attending their scheduled clinic follow-up visits.
This pilot study is strengthened by using a Traffic Light approach with clear thresholds for achieving feasibility outcomes that facilitated modification of the protocol based on findings of the pilot phase. This pilot study is limited by being a single-centre initiative that was conducted at the principal investigator’s clinical site. The pilot phase was active while COVID-19 pandemic restrictions were in place, necessitating telephone consent and follow-up, as opposed to in-person consent and follow-up.
The results of our pilot study suggest that a definitive, multi-centre trial to compare CBT versus usual care to reduce chronic post-surgical pain among fracture patients may be feasible if we modify our protocol. Specifically, we will enlist a sufficient number of trauma centres to complete enrollment of 1000 patients over 18 months, focus on enrollment of patients who are more likely to complete the intervention modules and all follow-up visits, provide research coordinators with tools to better engage CBT participants, and continue to work closely with the CBT provider to ensure a patient-friendly experience. Our results illustrate the value of pilot studies before embarking on definitive orthopedic trials.
Acknowledgements
The COPE Investigators
Steering Committee: Jason W. Busse (Principal Investigator, McMaster University, Hamilton, ON), Sheila Sprague (Principal Investigator, McMaster University, Hamilton, ON), Mohit Bhandari (McMaster University, Hamilton, ON), Gerard Slobogean (University of Maryland School of Medicine, Baltimore, MD), Lehana Thabane (McMaster University, Hamilton, ON), Randi E. McCabe (McMaster University, Hamilton, ON), Emil H. Schemitsch (University of Western Ontario, London, ON).
Research Methodology Advisory Core: Gordon H. Guyatt (McMaster University, Hamilton, ON), PJ Devereaux (McMaster University, Hamilton, ON), Lehana Thabane (McMaster University, Hamilton, ON), Mohit Bhandari (McMaster University, Hamilton, ON).
Orthopaedic Surgery Advisory Core: I. Leah Gitajn (Dartmouth University, Hanover, NH), Gerard Slobogean (University of Maryland School of Medicine, Baltimore, MD), Emil H. Schemitsch (University of Western Ontario, London, ON).
Psychology Advisory Core: Randi E. McCabe (McMaster University, Hamilton, ON), Matilda Nowakowski (St. Joseph’s Healthcare, Hamilton, ON), Eleni Hapidou (McMaster University, Hamilton, ON), Delia Chiaramonte (Greater Baltimore Medical Center, Baltimore, MD).
Medical/Pain Advisory Core: Henrick Kehlet (Copenhagen University, Denmark), James Khan (Stanford University, Stanford, CA).
Adjudication Committee: Matilda Nowakowski (St. Joseph’s Healthcare, Hamilton, ON), Aresh Sepehri (University of Maryland, Baltimore, MD).
Methods Centre, McMaster University, Hamilton, ON: Paula McKay, Gina Del Fabbro, Natalie Fleming, Christy Shibu, Diane Heels-Ansdell, Sofia Bzovsky.
Hamilton Health Sciences—General Site, Hamilton, ON: Brad A. Petrisor, Dale Williams, Bill Ristevski, Jamal Al-Asiri, Herman Johal, Matthew Denkers, Kris Rajaratnam, Jodi L. Gallant, Sarah MacRae, Kaitlyn Pusztai, Sara Renaud, Nicki Johal.
The Ottawa Hospital, Ottawa, ON: Steven Papp, Karl-Andre Lalonde, Bradley Meulenkamp, Allan Liew, Manisha Mistry, Braden Gammon, Wade Gofton, Geoffrey Wilkin, Melanie Dodd-Moher.
Foothills Medical Centre, University of Calgary, Calgary, AB: Prism S. Schneider, Tosin Ogunleye, Tanya Cherppukaran, Karin Lienhard.
Memorial University, St. John’s, NFLD: Nicholas Smith, Sarah Anthony, Krista Butt.
University of Maryland, R Adams Cowley Shock Trauma Center, Baltimore, MD: Gerard Slobogean, LaShann Selby, Murali Kovvur, Joshua Lawrence, Skyler Sampson, Kristin Turner.
University of Maryland Capital Region Health, Baltimore, MD: Todd Jaeblon, Haley K. Demyanovich, Sneh Talwar, Caroline Benzel.
Dartmouth-Hitchcock Medical Center, Lebanon, NH: I. Leah Gitajn, Theresa Chockbengboun, Devin Mullin.
Beth Israel Deaconess Medical Center, Boston, MA: Paul J. Appleton, John J. Wixted, Edward K. Rodriguez, Michael F. McTague, Katiri Wagner, Kristina Brackpool, Kate Hegermiller, Nhi Nguyen.
Indiana University School of Medicine, Indianapolis, IN: Roman M. Natoli, Courteney Fentz, Maricela Diaz, Jill Niceley, Tammy Garrett, Jena Robin.
Prisma Health – Upstate, Greenville, SC: Kyle J. Jeray, Thomas M. Schaller, Michael S. Sridhar, John D. Adams, Richard W. Gurich Jr., Stephanie L. Tanner, Kyle Adams, Michelle Donohue, Emily Bray, Calleigh Brignull, Harper Sprouse.
Abbreviations
- CBT
Cognitive behavioural therapy
- EDC
Electronic data capture
- SPOC
Somatic Pre-Occupation and Coping
Authors’ contributions
All authors listed under the Acknowledgments section reviewed the manuscript and provided critical input regarding its intellectual content. All authors read and approved the final manuscript.
Funding
This study was funded by a grant from the Orthopaedic Trauma Association. The funding body played no role in the design of the study, nor in the collection, analysis, and interpretation of data, nor in writing the manuscript.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The trial was approved by the Hamilton Integrated Research Ethics Board (#4336) and by all participating clinical sites’ research ethics boards/institutional review boards. Informed consent was obtained from every enrolled participant.
Consent for publication
Not applicable.
Competing interests
SS reports editorial or governing board for BMC Women’s Health, employment from Global Research Solutions Inc., and employment from McMaster University, outside the submitted work. HJ reports paid presenter or speaker for DePuy, A Johnson & Johnson Company, and board or committee member for the Orthopaedic Research Society, outside the submitted work. JWB is supported, in part, by a Canadian Institutes of Health Research Canada Research Chair in the prevention and management of chronic pain. All other authors have nothing to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sheila Sprague, Email: sprags@mcmaster.ca.
Jason W. Busse, Email: bussejw@mcmaster.ca
on behalf of the COPE Investigators:
Sheila Sprague, Jodi L. Gallant, Sarah MacRae, Gina Del Fabbro, Sofia Bzovsky, Paula McKay, Herman Johal, Jason W. Busse, Mohit Bhandari, Gerard Slobogean, Lehana Thabane, Randi E. McCabe, Emil H. Schemitsch, Gordon H. Guyatt, PJ Devereaux, Lehana Thabane, Mohit Bhandari, I. Leah Gitajn, Gerard Slobogean, Emil H. Schemitsch, Randi E. McCabe, Matilda Nowakowski, Eleni Hapidou, Delia Chiaramonte, Henrick Kehlet, James Khan, Matilda Nowakowski, Aresh Sepehri, Natalie Fleming, Christy Shibu, Diane Heels-Ansdell, Brad A. Petrisor, Dale Williams, Bill Ristevski, Jamal Al-Asiri, Matthew Denkers, Kris Rajaratnam, Kaitlyn Pusztai, Sara Renaud, Nicki Johal, Steven Papp, Karl-Andre Lalonde, Bradley Meulenkamp, Allan Liew, Manisha Mistry, Braden Gammon, Wade Gofton, Geoffrey Wilkin, Melanie Dodd-Moher, Prism S. Schneider, Tosin Ogunleye, Tanya Cherppukaran, Karin Lienhard, Nicholas Smith, Sarah Anthony, Krista Butt, Gerard Slobogean, LaShann Selby, Murali Kovvur, Joshua Lawrence, Skyler Sampson, Kristin Turner, Todd Jaeblon, Haley K. Demyanovich, Sneh Talwar, Caroline Benzel, I. Leah Gitajn, Theresa Chockbengboun, Devin Mullin, Paul J. Appleton, John J. Wixted, Edward K. Rodriguez, Michael F. McTague, Katiri Wagner, Kristina Brackpool, Kate Hegermiller, Nhi Nguyen, Roman M. Natoli, Courteney Fentz, Maricela Diaz, Jill Niceley, Tammy Garrett, Jena Robin, Kyle J. Jeray, Thomas M. Schaller, Michael S. Sridhar, John D. Adams, Richard W. Gurich , Jr, Stephanie L. Tanner, Kyle Adams, Michelle Donohue, Emily Bray, Calleigh Brignull, and Harper Sprouse
References
- 1.Khan JS, Devereaux PJ, LeManach Y, Busse JW. Patient coping and expectations about recovery predict the development of chronic post-surgical pain after traumatic tibial fracture repair. Br J Anaesth. 2016;117(3):365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin CA, Swiontkowski M, Bhandari M, et al. Reaming does not affect functional outcomes after open and closed tibial shaft fractures: the results of a randomized controlled trial. J Orthop Trauma. 2016;30(3):142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busse JW, Heels-Ansdell D, Makosso-Kallyth S, et al. Patient coping and expectations predict recovery after major orthopaedic trauma. Br J Anaesth. 2019;122(1):51–9. 10.1016/j.bja.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 4.Katsoulis E, Court-Brown C, Giannoudis PV. Incidence and aetiology of anterior knee pain after intramedullary nailing of the femur and tibia. J Bone Joint Surg Br. 2006;88(5):576–80. [DOI] [PubMed] [Google Scholar]
- 5.Linton SJ. A review of psychological risk factors in back and neck pain. Spine (Phila Pa 1976). 2000;25(9):1148–56. [DOI] [PubMed] [Google Scholar]
- 6.Busse JW, Bhandari M, Guyatt GH, et al. Development and validation of an instrument to predict functional recovery in tibial fracture patients: the Somatic Pre-Occupation and Coping (SPOC) questionnaire. J Orthop Trauma. 2012;26(6):370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reininga IHF, Brouwer S, Dijkstra A, et al. Measuring illness beliefs in patients with lower extremity injuries: reliability and validity of the Dutch version of the Somatic Pre-Occupation and Coping questionnaire (SPOC-NL). Injury. 2015;46(2):308–14. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Chang Y, Kennedy SA, et al. Perioperative psychotherapy for persistent post-surgical pain and physical impairment: a meta-analysis of randomised trials. Br J Anaesth. 2018;120(6):1304–14. [DOI] [PubMed] [Google Scholar]
- 9.Burns A, Banerjee S, Morris J, et al. Treatment and prevention of depression after surgery for hip fracture in older people: randomized, controlled trials. J Am Geriatr Soc. 2007;55(1):75–80. [DOI] [PubMed] [Google Scholar]
- 10.Gouveia K, Sprague S, Gallant J, et al. In-person cognitive behavioural therapy vs. usual care after surgical management of extremity fractures: an unsuccessful feasibility trial. Pilot Feasibility Stud. 2024;10(1):2. 10.1186/s40814-023-01430-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zandieh S, Abdollahzadeh SM, Sadeghirad B, et al. Therapist-guided remote versus in-person cognitive behavioural therapy: a systematic review and meta-analysis of randomized controlled trials. CMAJ. 2024;196(10):E327-40. 10.1503/cmaj.230274. [DOI] [PMC free article] [PubMed]
- 12.Nowakowski ME, McCabe RE, Busse JW. Cognitive behavioral therapy to reduce persistent postsurgical pain following internal fixation of extremity fractures (COPE): rationale for a randomized controlled trial. Can J Pain. 2019;3(2):59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Investigators COPE. Cognitive Behavioural Therapy to Optimize Post-Operative Fracture Recovery (COPE): protocol for a randomized controlled trial. Trials. 2022;23(1):894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pogorzelski D, McKay P, Weaver MJ, et al. The impact of COVID-19 restrictions on participant enrollment in the PREPARE trial. Contemp Clin Trials Commun. 2022;29: 100973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avery KNL, Williamson PR, Gamble C, et al. Informing efficient randomised controlled trials: exploration of challenges in developing progression criteria for internal pilot studies. BMJ Open. 2017;7(2):e013537–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sprague S, Guyatt P, Bzovsky S, et al. Pragmatic randomized trial evaluating pre-operative aqueous antiseptic skin solution in open fractures (Aqueous-PREP): the feasibility of a cluster randomized crossover study. Pilot Feasibility Stud. 2021;7(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sprague S, Bhandari M, Bzovsky S, et al. Fixation using alternative implants for the treatment of hip fractures: the feasibility of a multicenter 2 × 2 factorial randomized controlled trial evaluating surgical treatment and vitamin D supplementation in young femoral neck fracture patients. OTA International. 2020;3(2):e66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.