Abstract
1. The nature of the acetyl-CoA hydrolase (EC 3.1.2.1) reaction in rat and sheep liver homogenates was investigated. 2. The activity determined in an incubated system was 5.10 and 3.28nmol/min per mg of protein for rat and sheep liver homogenate respectively. This activity was not affected by the addition of l-carnitine, but was decreased by the addition of d-carnitine. 3. No acetyl-CoA hydrolase activity could be detected in rat or sheep liver homogenates first treated with Sephadex G-25. This treatment decreased the carnitine concentrations of the homogenates to about one-twentieth. Subsequent addition of l-carnitine, but not d-carnitine, restored the apparent acetyl-CoA hydrolase activity. 4. Sephadex treatment did not affect acetyl-carnitine hydrolase activity of the homogenates, which was 5.8 and 8.1nmol/min per mg of protein respectively for rat and sheep liver. 5. Direct spectrophotometric assay of acetyl-CoA hydrolase, based on the reaction of CoA released with 5,5′-dithiobis-(2-nitrobenzoic acid), clearly demonstrated that after Sephadex treatment no activity could be measured. 6. Carnitine acetyltransferase (EC 2.3.1.7) activity measured in the same assay system in response to added l-carnitine was very low in normal rat liver homogenates, owing to the apparent high acetyl-CoA hydrolase activity, but was increased markedly after Sephadex treatment. The Vmax. for this enzyme in rat liver homogenates was increased from 3.4 to 14.8nmol/min per mg of protein whereas the Km for l-carnitine was decreased from 936 to 32μm after Sephadex treatment. 7. Acetyl-CoA hydrolase activity could be demonstrated in disrupted rat liver mitochondria but not in separated outer or inner mitochondrial membrane fractions. Activity could be demonstrated after recombination of outer and inner mitochondrial membrane fractions. The outer mitochondrial membrane fraction showed acetylcarnitine hydrolase activity and the inner mitochondrial membrane fraction showed carnitine acetyltransferase activity. 8. The results presented here demonstrate that acetyl-CoA hydrolase activity in rat and sheep liver is an artifact and the activity is due to the combined activity of carnitine acetyltransferase and acetylcarnitine hydrolase.
Full text
PDF

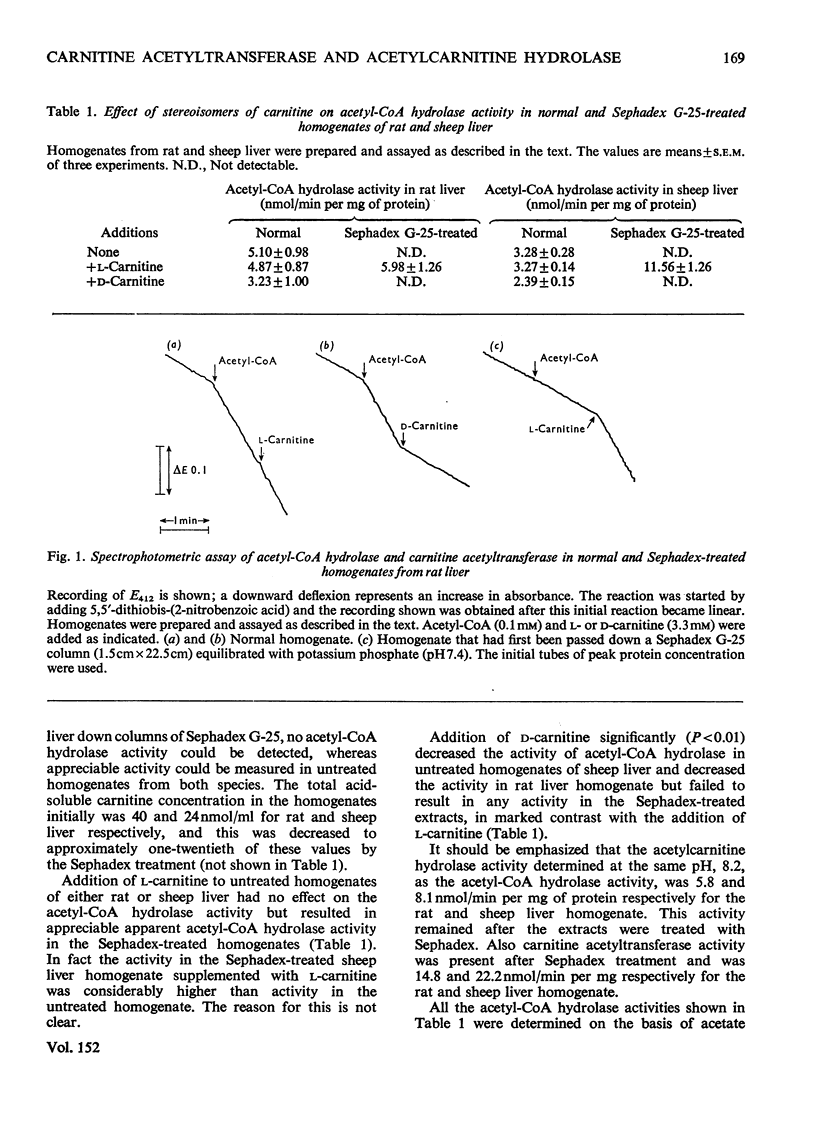
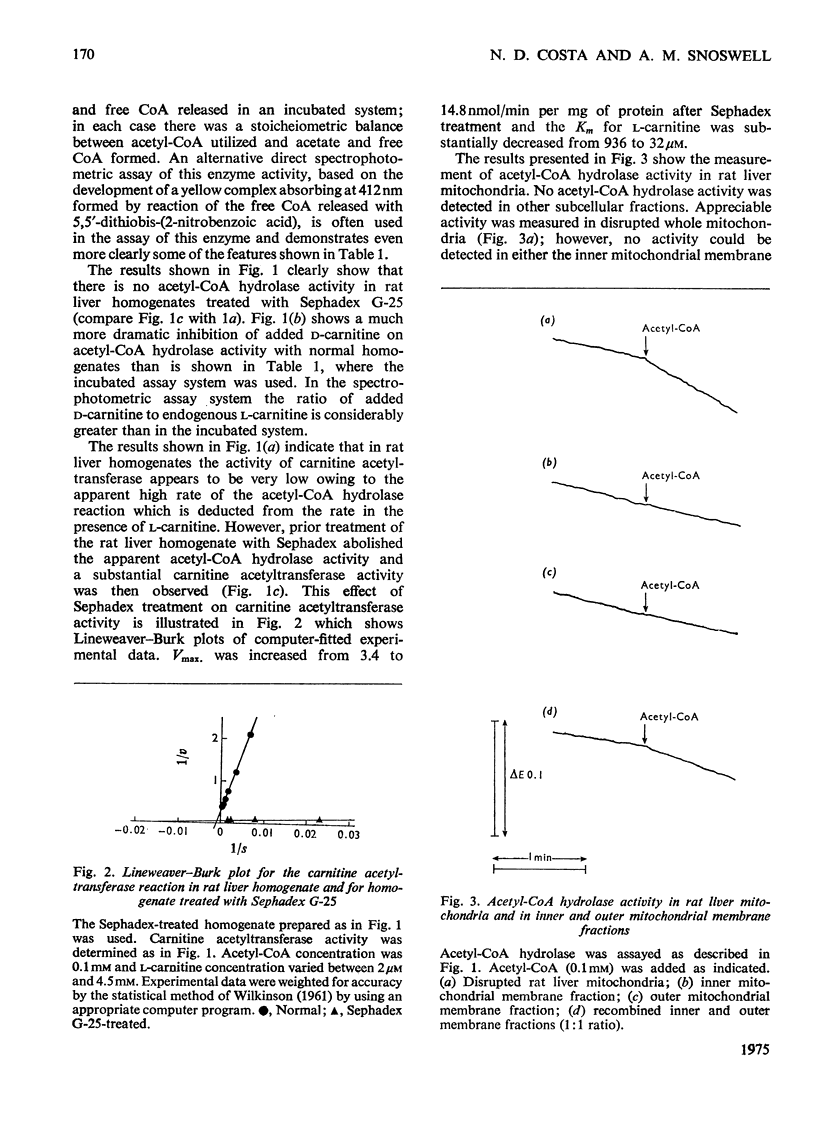


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allred J. B., Guy D. G. Determination of coenzyme A and acetyl CoA in tissue extracts. Anal Biochem. 1969 May;29(2):293–299. doi: 10.1016/0003-2697(69)90312-1. [DOI] [PubMed] [Google Scholar]
- Barker P. J., Fincham N. J., Hardwick D. C. The availability of carnitine acetyltransferase in mitochondria from guinea-pig liver and other tissues. Biochem J. 1968 Dec;110(4):739–746. doi: 10.1042/bj1100739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brdiczka D., Gerbitz K., Pette D. Localization and function of external and internal carnitine acetyltransferases in mitochondria of rat liver and pig kidney. Eur J Biochem. 1969 Dec;11(2):234–240. doi: 10.1111/j.1432-1033.1969.tb00765.x. [DOI] [PubMed] [Google Scholar]
- Chase J. F. The substrate specificity of carnitine acetyltransferase. Biochem J. 1967 Aug;104(2):510–518. doi: 10.1042/bj1040510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. F., Tubbs P. K. Conditions for the self-catalysed inactivation of carnitine acetyltransferase. A novel form of enzyme inhibition. Biochem J. 1969 Jan;111(2):225–235. doi: 10.1042/bj1110225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa N. D., Snoswell A. M. Enzymic hydrolysis of acetylcarnitine in liver from rats, sheep and cows. Biochem J. 1975 Nov;152(2):161–166. doi: 10.1042/bj1520161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards Y. H., Chase J. F., Edwards M. R., Tubbs P. K. Carnitine acetyltransferase: the question of multiple forms. Eur J Biochem. 1974 Jul 1;46(1):209–215. doi: 10.1111/j.1432-1033.1974.tb03613.x. [DOI] [PubMed] [Google Scholar]
- FRITZ I. B., SCHULTZ S. K. CARNITINE ACETYLTRANSFERASE. II. INHIBIITON BY CARNITINE ANALOGUES AND BY SULFHYDRYL REAGENTS. J Biol Chem. 1965 May;240:2188–2192. [PubMed] [Google Scholar]
- FRITZ I. B., SCHULTZ S. K., SRERE P. A. Properties of partially purified carnitine acetyltransferase. J Biol Chem. 1963 Jul;238:2509–2517. [PubMed] [Google Scholar]
- GERGELY J., HELE P., RAMAKRISHNAN C. V. Succinyl and acetyl coenzyme a deacylases. J Biol Chem. 1952 Sep;198(1):324–334. [PubMed] [Google Scholar]
- Hoppel C. L., Tomec R. J. Carnitine palmityltransferase. Location of two enzymatic activities in rat liver mitochondria. J Biol Chem. 1972 Feb 10;247(3):832–841. [PubMed] [Google Scholar]
- Knowles S. E., Jarrett I. G., Filsell O. H., Ballard F. J. Production and utilization of acetate in mammals. Biochem J. 1974 Aug;142(2):401–411. doi: 10.1042/bj1420401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARQUIS N. R., FRITZ I. B. ENZYMOLOGICAL DETERMINATION OF FREE CARNITINE CONCENTRATIONS IN RAT TISSUES. J Lipid Res. 1964 Apr;5:184–187. [PubMed] [Google Scholar]
- Murthy V. K., Steiner G. Hepatic acetate levels in relation to altered lipid metabolism. Metabolism. 1973 Jan;22(1):81–84. doi: 10.1016/0026-0495(73)90032-2. [DOI] [PubMed] [Google Scholar]
- PEARSON D. J., TUBBS P. K. ACETYL-CARNITINE IN HEART AND LIVER. Nature. 1964 Apr 4;202:91–91. doi: 10.1038/202091a0. [DOI] [PubMed] [Google Scholar]
- Quraishi S., Cook R. M. Utilization of volatile fatty acids in ruminants. IV. Relative activities of acetyl CoA synthetase and acetyl CoA hydrolase in mitochondria and intracellular localization of acetyl CoA synthetase. J Agric Food Chem. 1972 Jan-Feb;20(1):91–95. doi: 10.1021/jf60179a003. [DOI] [PubMed] [Google Scholar]
- Snoswell A. M., Henderson G. D. Aspects of carnitine ester metabolism in sheep liver. Biochem J. 1970 Aug;119(1):59–65. doi: 10.1042/bj1190059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoswell A. M., Koundakjian P. P. Relationships between carnitine and coenzyme A esters in tissues of normal and alloxan-diabetic sheep. Biochem J. 1972 Mar;127(1):133–141. doi: 10.1042/bj1270133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg H. E., Aas M., Daae L. N. The activity of the different carnitine acyltransferases in the liver of clofibrate-fed rats. Biochim Biophys Acta. 1972 Nov 30;280(3):434–439. doi: 10.1016/0005-2760(72)90249-4. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]


