Abstract
Background
Wild game meat has over the years gained popularity across the globe as it is considered a food source with high protein content, low fat content, and a balanced composition of fatty acids and minerals, which are requirements for a healthy diet. Despite this popularity, there is a concern over its safety as many species of wildlife are reservoirs of zoonotic diseases including those of bacterial origin, more so antibiotic-resistant bacteria.
Methods
This study aimed to describe the prevalence of antibiotic-resistant bacteria in mammalian wild game, following the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines.
Results
The overall pooled prevalence of antibiotic resistance was established at 59.8% while the prevalence of multidrug resistance (MDR) was 17.2%. Resistance was reported in 32 wild game species and the meta-analysis revealed the highest prevalence of antibiotic resistance in Yersinia spp. (95.5%; CI: 76.8 − 100%) followed by Enterococcus spp. (71%; CI: 44.1 − 92%), Salmonella spp. (69.9%; CI: 44.3 − 90.0%), Staphylococcus spp. (69.3%; CI: 40.3 − 92.3%), and Escherichia coli (39.5%; CI: 23.9 − 56.4%). Most notably, resistance to highest priority, critically important antimicrobials, was recorded in all genera of bacteria studied. Additionally, a significantly higher prevalence of antibiotic resistance was observed in studies conducted in remote settings than those in the vicinity of anthropogenic activities, pointing to extensive contamination of wild habitats.
Conclusion
This review shows the presence of antibiotic resistance and the carriage of antimicrobial resistance (AMR) genes by bacteria isolated from mammalian wild game species. This is a cause for concern if critical steps to prevent transmission to humans from meat and meat products are not applied in the wild game meat production chain. The extensive occurrence of antibiotic resistance in the wild calls for expansion and adaptation of future AMR surveillance plans to include areas with various anthropogenic pressures including in sylvatic habitats.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12917-024-04462-5.
Keywords: Antimicrobial resistance, Food safety, One health, Epidemiology
Introduction
Antimicrobials play a crucial role in prophylaxis and treatment of many diseases in humans and animals. However, the overuse and misuse of antimicrobials have led to the emergence of highly resistant organisms, a phenomenon referred to as AMR that describes the potential of microorganisms including bacteria, viruses, fungi, and parasites to thrive and continue to grow in the presence of drugs designed to stop their growth or kill them. Antimicrobial resistance is a natural response of microorganisms to selection pressure and may be intrinsic or acquired and may be effected through limiting drug uptake, drug target modification, drug inactivation, and active drug efflux [1].
Antimicrobial resistance significantly impacts the world economy, human and animal health [2], and according to the World Health Organization (WHO), it is one of the biggest threats to global health, food security, and development in the 21st century. Globally, infections with MDR pathogens already cause more than 700,000 deaths each year, and it has been predicted that the annual death rate may reach 10 million people per year by 2050, a much higher rate than attributed to cancer [2, 3]. It is well documented that the over-reliance on and use of antibiotics in humans and animals has contributed considerably to the dissemination of antibiotics into environments [4].
Wild animals are widely distributed across the globe, occupying diverse habitats, some near human settlements and livestock farms [5]. As it is unlikely that they are being treated with antibiotics, the presence of AMR genes and/or antibiotic resistant bacteria in wild animals is a sign of anthropic pollution rather than of selection of resistance [6]. However, once AMR is established in the wild, wildlife can facilitate its transmission across different ecosystems through fecal contamination of water sources, soil, and surfaces. Additionally, human exposure to AMR from wildlife may occur directly through consumption of wild game meat contaminated with AMR pathogens [5].
To reduce the risk of human exposure and acquisition of resistant bacteria from wild game, an explicit understanding of the epidemiology of AMR in wild game is required to perform risk analysis. This review evaluates several studies that reported antibiotic resistance in mammalian wild game to assess to what extent AMR occurs in wild game globally, and the potential threat it may pose to public health. This is in line with the five key strategic action plans of the World Health Assembly to combat AMR which include; improving awareness and understanding of antimicrobial resistance, strengthening knowledge through surveillance and research, combating infection through control measures, optimizing the use of antimicrobials in human and animal health, and encouraging sustainable investment in new medicines, diagnostic tools, and vaccines [7].
Materials and methods
Scope of the review
The study reviewed the presence of antibiotic-resistant bacteria in mammalian wild game species, analyzing available data on their prevalence as reported by observational studies or case reports from different geographical areas from the period between the inception of the search database to April 2023.
Literature search strategy
A systematic literature search for articles was conducted in PubMed, Google scholar and Web of Science (‘all databases’ option, which includes MEDLINE, Zoological Records and CAB Abstracts) using the search term, ((“Wild game” OR “wild meat”) AND (“Antibiotic Resistance” OR “Drug Resistance”, Microbial OR “Antibiotic Resistance”, Microbial OR “Antimicrobial Drug Resistance”)). Additional articles not identified from the three databases were obtained from the reference lists of included studies.
Study selection
All study titles and abstracts were exported from the search databases to EndNote™ 20 (Philadelphia, PA, United States of America) by one author. Duplicate records were identified and removed. Studies published in multiple forms were screened by checking for author names associated with multiple publications in the data extraction form.
Study titles and abstracts were jointly reviewed by the authors and discrepancies in opinion among the reviewers were resolved through discussions to reach an agreement. Studies that fulfilled the following PICOs elements were included.
P (population): studies reporting the prevalence of AMR in bacterial isolates from mammalian wild game species.
I (intervention/exposure): resistance of bacteria isolated from mammalian wild game to an antibiotic and/or an antibiotic class.
C (comparator): not applicable.
O (outcomes): [1]: prevalence of phenotypic and/or genotypic resistance to antibiotics /antibiotic classes in bacterial isolates from mammalian wild game species [2]: data on the animal species in which antibiotic resistance is reported [3]: species of bacteria for which resistance is reported and [4]: methods used to detect AMR in the bacteria isolates.
S (study design): cross-sectional studies and case reports.
We excluded studies in domestic animals and non-mammalian wild game species. Furthermore, articles based on experimental studies, clinical trials, and reviews were excluded.
Risk of bias and quality assessment
All authors independently evaluated the quality and methodology of all selected studies using the Joanna Briggs Institute (JBI) Critical Appraisal Tool for prevalence studies [8]. The tool has ‘Yes,’ ‘No,’ ‘Unclear’ or ‘Not applicable’ question types and scores were assigned as 1 for ‘Yes’ and 0 for ‘No’ and “Unclear”. One question (question 9) that was irrelevant to this study was excluded and the number of ‘Yes’ scores were added, and the percentage was computed by dividing by the total number of questions (in this case 8). The studies were classified as: low quality (less than 50% score), moderate quality (50 to 75%), and high quality (> 75%) and scores from all reviewers were compared and discrepancies between the scores resolved through discussion.
Data extraction
Two reviewers independently extracted data from the selected articles to a standardized data extraction form in Microsoft Excel® version 15.0 (Microsoft Corporation, Redmond, WA, United States). Data extracted included geographical location, animal species studied, sample type collected, bacterial species, sample size, detection methods applied, antibiotics for which susceptibility tests were conducted (phenotypic resistance), AMR genes detected (genotypic resistance), number of positive isolates. The prevalence of antibiotic resistance was computed by dividing the number of isolates resistant to at least one antibiotic by the total number of isolates tested. Additional data was collected on names of authors, study title, year of publication, year, and month of studies.
Meta-analysis
The pooled prevalence of AMR for each bacterial species and their 95% confidence intervals were calculated based on the random effects model using the R statistical packages ‘meta’ and ‘metafor’. Heterogeneity across the studies was tested and quantified using I2 statistic (Higgins et al., 2003) and was considered significant if I2 was greater than 50%. The true between-study variance, τ2, and standard deviation, τ were determined using the tau statistic to estimate the amount of heterogeneity (Borenstein et al., 2017). Finally, the heterogeneity between the prevalences and their 95% confidence intervals across studies were visualized using forest plots with proportions and corresponding 95% confidence intervals. Potential sources of heterogeneity were explored through subgroup analysis based on factors that may affect prevalence, including animal species, species of bacteria, geographical region (country and continent) and proximity to human settlements or livestock. Studies were included in the meta-analysis if they reported prevalence of resistance to at least one antibiotic, prevalence in each species of animals studied, prevalence in each species of bacteria studied and antibiotics for which resistance was reported. Additionally, meta-analysis was conducted if at least 5 studies met the selection criteria for each species of bacteria.
Results
Study characteristics
The literature search retrieved 1772 articles from three databases including PubMed, Google scholar and Web of Science (‘all databases’ option, which includes MEDLINE, Zoological Records and CAB Abstracts). After removal of duplicates and title/abstract screening, 1631 were sought for retrieval. Out of the 1631 articles, full texts were obtained for 1629, while 2 were not found. Further screening of full text articles based on the exclusion/inclusion criteria resulted in exclusion of 1549 articles for reasons including studies conducted in other animal species not of interest to the present review (n = 768), inappropriate study design (n = 169), studies providing no relevant data/information to support the review (n = 608) and articles not written in English (n = 4). Finally, 80 studies met the inclusion criteria and were included in the systematic review as shown in Fig. 1. Based on the JBI critical appraisal tool for systematic review of prevalence studies, 38.75% (n = 31) of the selected studies were high quality, 61.25% (n = 49) were moderate and none was of low quality.
Fig. 1.
PRISMA flow diagram for studies selection for the systematic review and meta-analysis of antibiotic resistance in mammalian wild game for study periods up to April 2023
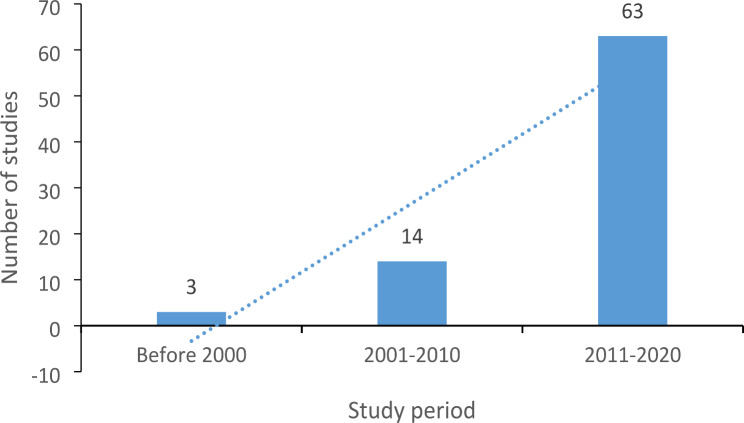
Based on the selected studies, it was observed that most studies on AMR in mammalian wild game were conducted between 2011 and 2020, with an increasing trend observed over the years as shown in Fig. 2.
Fig. 2.
Graphical representation of number of studies conducted on antibiotic resistance in mammalian wild game species up to April 2023
The selected studies (Table 1) included those from Europe (n = 55; 68.75%), Africa (n = 14; 17.5%), Asia (n = 4; 5%), North America (n = 4; 5%), South America (n = 2; 2.5%) and Oceania (n = 1; 1.25%). The highest number of studies were conducted in Spain [18], Italy [15], Portugal [8], Germany [5] and South Africa [4]. The studies were conducted in 32 mammalian wild game species with wild boars (47 studies), red deer (15 studies) and roe deer (10 studies) being the most studied. There were less than ten studies for each of the other species. Most studies (55%; n = 44) applied the disk diffusion method alone for antibiotic susceptibility test, while 15% (n = 12) applied only broth microdilution. Additionally, one study (1.25%) applied disk diffusion and broth microdilution, while 22.5% (n = 18) of the studies combined either disk diffusion or broth microdilution with molecular methods whereas 6.25%; (n = 5) of the studies applied only molecular methods.
Table 1.
Characteristics of selected studies on antibiotic resistance in mammalian wild game species from three databases from the time of inception to April 2023
| Study | Resistant bacteria reported | Country | Continent | Animal species studied | Sample type | Assay used |
|---|---|---|---|---|---|---|
| Alonso, Alcala [9] | E. coli | Spain | Europe | Roe deer | Fecal | Disk diffusion + PCR |
| Alonso, Gonzalez-Barrio [10] | E. coli | Spain | Europe | Roe deer, Mouflon, Wild boar | Fecal | Disk diffusion + PCR |
| Asai, Usui [11] | E. coli | Japan | Asia | Sika deer, Wild boar | Facal | Broth microdilution |
| Bachiri, Bakour [12] | E. coli and Klebsiella | Algeria | Africa | Wild boar | Fecal | Disk diffusion |
| Ben Said, Jouini [13] | E. coli and Enterococcus | Tunisia | Africa | Wild rabbits | Fecal | Disk diffusion |
| Bertelloni, Cilia [14] | E. coli | Italy | Europe | Wild boar | Fecal | Disk diffusion + PCR |
| Bonardi, Cabassi [15] | E. coli | Italy | Europe | Wild boar | Mesenteric lymph nodes | Disk diffusion |
| Brisson, Caron [16] | E. coli | France | Europe | Impala, Greater kudu, Zebra | Fecal | Disk diffusion |
| Caleja, Toro [17] | Salmonella | Portugal | Europe | Wild boar | Fecal | Disk diffusion + PCR |
| Caprioli, Donelli [18] | E. coli | Italy | Europe | Red deer, Roe deer, Chamois | Fecal | Disk diffusion |
| Carbonero, Paniagua [19] | Campylobacter | Spain | Europe | Wild boar, Red deer, Mouflon, Fallow deer | Fecal | Broth microdilution |
| Carella, Romano [20] | Yersinia | Italy | Europe | Alpine ibex, Red deer, Roe deer | Fecal | Disk diffusion |
| Carraro, Barbosa [21] | Salmonella | Brazil | South America | Wild boar | Fecal | Disk diffusion |
| Carrillo-Del Valle, De la Garza-García [22] | E. coli | Mexico | North America | Red deer | Fecal | Disk diffusion |
| Castillo-Contreras, Marin [23] | Campylobacter and Salmonella | Spain | Europe | Wild boar | Fecal | Broth microdilution |
| Cummings, Rodriguez-Rivera [24] | Salmonella | USA | North America | Wild boar | Fecal | Disk diffusion |
| Darwich, Seminati [25] | E. coli | Spain | Europe | Wild boar | Fecal | PCR |
| Dias, Fonseca [26] | E. coli and Enterococcus | Portugal | Europe | Red deer | Fecal | Disk diffusion |
| Dias, Fonseca [27] | E. coli and Enterococcus | Portugal | Europe | Wild boar | Fecal | Disk diffusion |
| Dias, Cruz [28] | Yersinia | Portugal | Europe | Red deer | Fecal | PCR |
| Donazzolo, Turchetto [29] | Salmonella | Italy | Europe | Wild boar | Fecal | Disk diffusion + Broth microdilution |
| Duangurai, Rungruengkitkul [30] | E. coli | Thailand | Asia | Wild deer | Fecal | Disk diffusion |
| Elsby, Zadoks [31] | E. coli | Scotland | Europe | Red deer, Roe deer, Fallow deer, Sika deer | Fecal | Disk diffusion |
| Ferreira [32] | E. coli | Portugal | Europe | Wild boar, Red deer, Fallow deer | Fecal | Disk diffusion |
| Formenti, Calo [33] | E. coli | Italy | Europe | Wild boar | Fecal | PCR |
| Frank, Justice [34] | E. coli and Enterococcus | United Kingdom | Europe | Arabian oryx, Addax, Eastern bongo, Dwarf forest buffalo, Zebra, Dorcus gazelle, Nyala, Roan antelope, Sable antelope, Sitatunga, Sable antelope, Waterbuck, Warthog | Fecal | Disk diffusion |
| García, Torres [35] | Enterococcus | Spain | Europe | Iberian ibex, Mouflon, Red deer, Roe deer, Wild boar, Wild rabbits, Grenada hare | Fecal | Disk diffusion |
| Gil Molino, Risco Perez [36] | Salmonella | Spain | Europe | Wild boar | Mixed | Disk diffusion + PCR |
| Gil-Molino, Goncalves [37] | Salmonella | Spain | Europe | Wild boar | Fecal | Disk diffusion + PCR |
| Gómez, Lozano [38] | Staphylococcus | Spain | Europe | Red deer | Nasal swabs | Disk diffusion + PCR |
| Guerrero-Ramos, Cordero [39] | Enterococcus | Spain | Europe | Wild boar, Wild rabbits, Roe deer | Meat | Disk diffusion |
| Holtmann, Meemken [40] | E. coli | Germany | Europe | Wild boar | Nasal swabs + Fecal | Broth microdilution + PCR |
| Jobbins and Alexander [41] | E. coli | Botswana | Africa | Bushbuck, African buffalo, Giraffe, Greater kudu, Hippopotamus, Impala, Sable antelope, Warthog, Waterbuck | Fecal | Disk diffusion |
| Kabali, Pandey [42] | E. coli | Zambia | Africa | Impala, African buffalo | Fecal | Disk diffusion |
| Katakweba, Moller [43] | E. coli | Tanzania | Africa | African buffalo, Wildebeest, Zebra | Fecal | Broth microdilution |
| King and Schmidt [44] | E. coli | South Africa | Africa | Giraffe, Zebra, Wildebeest | Fecal | Disk diffusion |
| Kraushaar and Fetsch [45] | Staphylococcus | Germany | Europe | Wild boar | Meat | Broth microdilution |
| Laatu, Rautelin [46] | Campylobacter | Finland | Europe | Reindeer | Fecal | Disk diffusion |
| Lillehaug, Bergsjo [47] | Campylobacter., Salmonella, Escherichia coli | Norway | Europe | Red deer, Roe deer, Moose, Reindeer | Fecal | Broth microdilution |
| Maguina-Molina, Pons [48] | Salmonella | Peru | South America | Paca | Meat swabs | Disk diffusion |
| Mama, Ruiz-Ripa [49] | Staphylococcus | Spain | Europe | Wild boar | Nasal swabs | Disk diffusion + PCR |
| Marotta, Di Marcantonio [50] | Campylobacter | Italy | Europe | Wild boar | Mixed | Broth micro dilution + PCR |
| Mateus-Vargas, Atanassova [51] | E. coli | Germany | Europe | Red deer, Roe deer, Wild boar | Meat | Broth dilution + PCR |
| Mateus-Vargas, Lienen [52] | Staphylococcus | Germany | Europe | Fallow deer, Red deer, Roe deer, Wild boar | Nasal swabs | Broth microdilution |
| Mercato, Cortimiglia [53] | E. coli | Italy | Europe | Wild boar | Mesenteric lymph nodes + Fecal | Disk diffusion |
| Modesto, De Ciucis [54] | Yersinia | Italy | Europe | Wild boar | Fecal | Disk diffusion |
| Molino, Garcia [55] | Salmonella | Iberia | Europe | Wild boar | Fecal | Disk diffusion + PCR |
| Morita, Sato [56] | E. coli | Japan | Asia | Wild boar, Wild deer | Fecal | Disk diffusion |
| Mubita, Muma [57] | Salmonella | Zambia | Africa | Sable antelope, Impala | Fecal | Disk diffusion |
| Navarro-Gonzalez, Mentaberre [58] | Salmonella | Spain | Europe | Wild boar | Fecal | Disk diffusion |
| Navarro-Gonzalez, Casas-Diaz [59] | Salmonella, campylobacter, E. coli, Enterococcus | Spain | Europe | Wild boar, Iberian ibex | Broth microdilution | |
| Navarro-Gonzalez, Porrero [60] | E. coli | Spain | Europe | Wild boar | Fecal | Disk diffusion |
| Navarro-Gonzalez, Porrero [61] | E. coli | Spain | Europe | Wild boar, Iberian ibex | Fecal | Disk diffusion |
| Navarro-Gonzalez, Castillo-Contreras [62] | E. coli and Enterococcus | Spain | Europe | Wild boar | Fecal | Disk diffusion |
| Nevins [63] | Salmonella, E. coli and Listeria | USA | North America | Wild boar | Fecal | Broth microdilution |
| Nishino, Shimojima [64] | E. coli | Japan | Asia | Wild boar | Meat | Disk diffusion |
| Nocera, Ferrara [65] | Staphylococcus and Enterococcus | Italy | Europe | Wild boar | Nasal swabs | Disk diffusion |
| Odyniec and Bancerz-Kisiel [66] | Yersinia | Poland | Europe | Fallow deer | Fecal | Disk diffusion |
| Ojo, Ogunjobi [67] | Salmonella and Yersinia | Nigeria | Africa | Cane rat, Royal antelope, Water buck | Fecal | Disk diffusion |
| Ojo, Ogunjobi [67] | Salmonella and Yersinia | Nigeria | Africa | Cane rat, African giant rat, Royal antelope | Fecal | Disk diffusion |
| Ojo, Amosun [68] | E. coli | Nigeria | Africa | Cane rat, Royal antelope, African giant rats, Water buck | Fecal | Disk diffusion |
| Pérez, Rosa [69] | Staphylococcus | Spain | Europe | Wild boar, Red deer | Nasal swabs | Disk diffusion + PCR |
| Pipova, Jevinova [70] | Staphylococcus | Slovakia | Europe | Wild rabbits | Meat | Broth microdilution |
| Piras, Spanu [71] | Salmonella | Italy | Europe | Wild boar | Colon + Lymph nodes | Disk diffusion + PCR |
| Porrero, Mentaberre [72] | Staphylococcus | Spain | Europe | Red deer, Iberian ibex, Wild boar | Nasal and skin swabs | Disk diffusion |
| Razzuoli, Listorti [73] | Salmonella | Italy | Europe | Wild boar | Liver | Disk diffusion |
| Rega, Carmosino [74] | E. coli | Italy | Europe | Wild boar | Meat | Disk diffusion |
| Rega, Andriani [75] | E. coli | Italy | Europe | Wild boar | Meat | Disk diffusion |
| Shaffer and Rogers [76] | Campylobacter, Salmonella, E. coli | USA | North America | White tailed deer | Fecal | PCR |
| Shin, Mduma [77] | Enterococcus | Tanzania | Africa | African buffalo | Nasal and Fecal swabs | Broth microdilution |
| Silva, Igrejas [78] | E. coli and Enterococcus | Portugal | Europe | Wild rabbits | Fecal | Disk diffusion |
| Silva, Pereira [79] | Staphylococcus | Portugal | Europe | Wild rabbits | Oral, nasal, and skin swabs | Disk diffusion + PCR |
| Sousa, Silva [80] | Staphylococcus | Portugal | Europe | Wild boar | Oral and nasal swabs | PCR |
| Van Breda and Ward [81] | E. coli | Australia | Oceania | Wild boar | Fecal | Broth microdilution |
| Van den Honert, Gouws [82] | E. coli and enterococcus | south Africa | Africa | Wildebeest, African buffalo, Impala | Fecal | Disk diffusion + PCR |
| Van den Honert, Gouws [83] | E. coli | South Africa | Africa | Springbok, Fallow deer, Eland, Wildebeest | Fecal | Disk diffusion + PCR |
| Van den Honert, Gouws [84] | E. coli and Staphylococcus | south Africa | Africa | Impala, Bontebok, Springbok | Meat and Fecal | Disk diffusion |
| Velhner, Todorovic [85] | E. coli | Serbia | Europe | Hare, Roe deer, Wild boars, “Other deer species” | Fecal | Broth microdilution + PCR |
| Von Altrock, Seinige [86] | Yersinia | Germany | Europe | Wild boar | Tonsils | Disk diffusion |
| Zottola, Montagnaro [87] | Salmonella | Italy | Europe | Wild boar | Fecal | Disk diffusion |
Overall prevalence of antibiotic resistance
Seventy-three out of the 80 selected studies were included in the meta-analysis. Based on the random effects model, the overall pooled global prevalence of antibiotic resistance in microorganisms isolated from mammalian wild game was 59.8% (CI: 48.4 − 70.7%) as shown in the forest plot in supplementary file 1. The prevalence of MDR was 17.2% (CI: 10 − 25.7%). Subgroup analysis by continent showed antibiotic resistance prevalence of 100% in Oceania, 88% in Africa, 58% in Europe, 36% in North America, 33% in South America and 30% in Asia.
Study settings significantly influenced the prevalence of AMR (QM = 3.9287, P = 0.0475), with higher prevalence (66%) recorded in samples from animals living in remote locations than in those collected in settings of close to human / livestock establishments (41%). Significant differences (P = 0.0475) were observed in prevalence of AMR among different genera of bacteria : Campylobacter spp. (98%; CI: 47 -100%) followed by Yersinia spp. (96%; CI: 62 − 100%), Listeria spp. (90%; CI: 73 -100%), Enterococcus spp. (71%; CI: 42 − 93%), Staphylococcus spp. (69%; CI: 40 − 92%), Salmonella spp. (67%; CI: 42 − 89%), and Escherichia coli (40%; CI: 25 − 55%). Differences in prevalence of antibiotic-resistant isolates were not significant (P > 0.05) based on study period, study location (country and continent) and animal species studied. The predictor analyses are shown in Table 2.
Table 2.
Predictors for prevalence of antibiotic resistance in wild game, analysis by mixed-effects Model (k = 73; tau^2 estimator: REML
| Variable | Level | No. studies | Pooled prevalence based on the random effects model | Test for moderator association with resistance to antibiotics | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Strains tested | No. resistant | Prevalence (95% CI) | QM (df) | P-value | tau2 | H2 | R2% | |||
| Study period | 1.3130 (2) | 0.5187 | 0.2226 | 82.09 | 0.00 | |||||
| Before 2000 | 2 | 74 | 39 | 0.73 (0.11 -1.00) | ||||||
| 2000–2010 | 15 | 1067 | 471 | 0.47 (0.24–0.72) | ||||||
| 2011–2020 | 56 | 5887 | 1812 | 0.63 (0.50–0.75) | ||||||
| Location | 8.4122 (5) | 0.1349 | 0.2092 | 72.32 | 4.85 | |||||
| Europe | 53 | 5370 | 1826 | 0.58 (0.45–0.71) | ||||||
| Africa | 9 | 497 | 263 | 0.88 (0.58 -1.00) | ||||||
| Asia | 4 | 730 | 68 | 0.30 (0.01–0.76) | ||||||
| North America | 5 | 310 | 106 | 0.36 (0.05–0.76) | ||||||
| South America | 1 | 6 | 2 | 0.33 (0.04–0.78) | ||||||
| Oceania | 1 | 115 | 115 | 1.00 (0.97–1.00) | ||||||
| Study setting | 3.9287 (1) | 0.0475* | 0.2113 | 76.32 | 3.9 | |||||
| Remote | 53 | 5405 | 1916 | 0.66 (0.54–0.78) | ||||||
| Close to humans/livestock | 20 | 1623 | 438 | 0.41 (0.21–0.63) | ||||||
| Animal species | 9.1823 (4) | 0.0567 | 0.1997 | 44.81 | 5.70 | |||||
| Cervidae | 28 | 1389 | 302 | 0.43 (0.28–0.64) | ||||||
| Suidae | 51 | 4443 | 1507 | 0.56 (0.43–0.69) | ||||||
| Bovidae | 16 | 402 | 85 | 0.68 (0.35–0.95) | ||||||
| Leporidae | 7 | 103 | 103 | 1.00 (0.69 -1.00) | ||||||
| Muridae | 5 | 308 | 222 | 0.80 (0.44 -1.00) | ||||||
| Bacteria species | 3.9287 (1) | 0.0475* | 0.2113 | 76.32 | 3.90 | |||||
| Staphylococcus | 10 | 673 | 246 | 0.69 (0.40–0.92) | ||||||
| Salmonella | 14 | 790 | 511 | 0.67 (0.42–0.89) | ||||||
| E. coli | 32 | 4710 | 1080 | 0.40 (0.25–0.55) | ||||||
| Enterococcus | 9 | 610 | 292 | 0.71 (0.42–0.93) | ||||||
| Campylobacter | 2 | 44 | 43 | 0.98 (0.47–1.00) | ||||||
| Yersinia | 5 | 172 | 124 | 0.96 (0.62–1.00) | ||||||
| Listeria | 1 | 29 | 26 | 0.90 (0.73–1.00) | ||||||
CI = Confidence interval, QM = Coefficient of test for heterogeneity between subgroups, df = degrees of freedom, tau2 = estimated amount of residual heterogeneity, H2 = unaccounted variability / sampling variability, R2 = amount of heterogeneity accounted for, * = significant P-value
Antibiotic resistance in staphylococcus spp
Resistance to antibiotics in Staphylococcus spp. isolates was reported by eleven studies, ten from Europe and one from Africa. All but one, were included in the meta-analysis for phenotypic resistance, while 6 were included for genotypic resistance (Supplementary file 2).
The overall pooled prevalence of phenotypic antibiotic resistance in Staphylococcus spp. was 69.3% (CI: 40.3 − 92.3%) and prevalence of MDR was 7.6% (CI: 0.0 − 32.6%). Animal species from which resistant Staphylococcus spp. were isolated included Iberian ibex (100%; CI: 16 − 100%), European wild rabbits (99%; CI: 57 − 100%), red deer (68%: CI: 11 -100%), wild boars (52%; CI: 21 − 82%), fallow deer (22%; CI:14 − 32%) and roe deer (11%; CI: 6 -19%).
Antibiotic resistance was reported in 15 Staphylococcus species including S. aureus, S. saprophyticus, S.epidermidis, S. succinus, S.sciuri, S. chromogenes, S. xylosus, S. simulans, S.hyicus, S.vitulinus, S.fleurette, S. warneri, S. lentus, S. cohnii. subsp. urealyticus and S.pseudintermedius. However, one study did not specify the Staphylococcus species.
Antibiotic resistance in Staphylococcus spp. was reported for 16 classes of antibiotics including; penicillins (67%; CI: 46 − 85%), cephalosporins (48%; CI: 20 − 76%), macrolides (40%; CI: 11 − 73%), streptogramins ( 32%; CI: 0.0 − 92%), fluoroquinolones (24%;CI: 1 − 59%), tetracyclines (23%; CI: 2 − 54%), aminocoumarins (23%; CI: 16 − 32%), lincosamides (22%; CI: 0.0 − 56%), steroid esters (16%; CI: 0.0 − 58%), macrolactams (14%; CI: 0.0 − 75%), carbapenems (13%; CI: 2- 40%), polymyxins (12%; CI: 9 − 16%), aminoglycosides (10%; CI: 0.0 − 42%), glycopeptides (7%; CI: 0.0 − 62%), phenicols (6%; CI: 0.0 − 52%) and oxazolidinones (1%; CI: 0.0 − 36%). Additionally, resistance was reported for drug combinations including penicillin – cephalosporin (3%; CI: 0.0 − 17%) and penicillin -aminoglycoside (4%; CI: 1 − 13%).
Regarding specific antibiotics, the Staphylococcus isolates were fully susceptible to mupirocin, sulfamethoxazole, sulphamethoxazole-trimethoprim, and tobramycin, while less than 10% resistance was reported for vancomycin, linezolid, penicillin-streptomycin, oxacillin-cefoxitin, chloramphenicol and streptomycin. A prevalence between 10% and 20% was registered for rifampin, imipenem, enrofloxacin, cefotaxime, amoxicillin-clavulanate, colistin, fusidic acid, gentamycin, and kanamycin, while between 20% and 50% prevalence was recorded for novobiocin, cepalothin, ciprofloxacin, clindamycin, tetracycline, erythromycin and quinupristin-dalfopristin. Moreover, the highest prevalence of resistance was observed for cefoxitin (55%), oxacillin (65%) and penicillin (71%).
Antibiotic resistance genes were detected in 29.2% (CI: 12.9 − 48.2%) of the Staphylococcus isolates tested, and these included those encoding for macrolide resistance (ermC, ermT and mphC), tetracycline resistance (tetL, tetK and tetM), vancomycin resistance (vanA, vanB) streptomycin resistance (str), methicillin resistance (mecA and aacA-AphD), aminoglycoside resistance (aac(6´)-Ie-aph(2´´)-Ia) and beta-lactamase production (blaZ).
Antibiotic resistance in salmonella
Antibiotic resistance in Salmonella spp. isolates was reported by seventeen studies: from Europe [10], Africa [2], North America [3] and South America [2], and 14 were included in the meta-analysis for phenotypic resistance, while 5 were included for genotypic resistance (supplementary file 3).
Overall pooled prevalence of antibiotic resistance in Salmonella isolates was 69.9% (CI: 44.3 − 90.9%) and prevalence of MDR was 14.9% (CI: 3 − 31.8%). Animal species from which resistant Salmonella spp. were isolated included sable antelopes (100%; CI: 2 − 100%), impalas (100%; CI: 2 -100%), royal antelopes (100%; CI:48 − 100%), African giant rats (100%; CI: 2 − 100%), cane rats (100%; CI: 66 − 100%) and wild boars (68%: CI: 42 -89%).
All studies reported antibiotic resistance in Salmonella enterica. The subspecies reported included S. enterica, S. diarizonae and S. salamae. Salmonella enterica subsp enterica serovars in which antibiotic resistance was reported included: S. Typhimurium, S. Muenster, S. Bardo, S. Enteritidis, S. Pomona, S. Roan, and S. Mbandaka.
Antibiotic resistance in Salmonella spp. was reported for 11 classes of antibiotics including; nitrofurans (50%; CI: 1 − 99%), macrolides (48%; CI: 12 − 86%), sulphonamides (35%; CI: 17 − 54%), tetracyclines (27%; CI: 9 − 50%), polymyxins (20%; CI: 0 − 57%), penicillins (14%; CI: 3 − 31%), organic phosphonic acid (12%; CI: 5 − 24%), cephalosporins (9%; CI: 1 − 20%), aminoglycosides (7%; CI: 1 − 18%), phenicols (7%; CI: 0 − 27%) and fluoroquinolones (5%; CI: 0 − 17%).
Salmonella isolates were fully susceptible to aztreonam, imipenem, gentamycin, tobramycin, amikacin, ciprofloxacin, kanamycin, florfenicol, trimethoprim, azithromycin, and ceftriaxone. However, less than 10% resistance was registered for amoxicillin-clavulanic acid, cefotaxime, ceftazidime, nalidixic acid, chloramphenicol, sulfisoxazole, neomycin, norfloxacin, cephalothin and cefazolin. Additionally, prevalence of between 10% and 20% was registered for ampicillin, sulphamethoxazole-trimethoprim, enrofloxacin, colistin- and Fosfomycin while between 20 and 50% prevalence of resistance was enumerated for cefoxitin, streptomycin, tetracycline, ceftiofur, doxycycline, erythromycin, nitrofurantoin and penicillin G. Prevalence greater than 50% was observed for resistance to cephalexin (53%), sulphamethoxazole (75%), sulfadiazine-sulfamerazine-sulfamethazine combination (96%), chloramphenicol (100%), spiramycin (100%) and tilmicosin (100%).
Antibiotic resistance genes were detected in 27.4% (CI: 16.4 − 39.7%) of the isolates, and these included those encoding for streptomycin resistance (strB and strA), tetracycline resistance (tetA, tetB and tetQ), quinolone resistance (gyrA), aminoglycoside resistance (aadA1 and aac(6′)-Iaa), sulphonamide resistance (sul1, sul3) fosfomycin resistance (fosA7), vancomycin resistance (vanA), macrolide resistance (ermB) and beta-lactamase production (blaTEM).
Antibiotic resistance in escherichia coli
Antibiotic resistance in Escherichia coli isolates was reported by 44 studies of which 32 were included in the meta-analysis for phenotypic resistance, while 20 were included for genotypic resistance. Thirty-one studies reported MDR strains (supplementary file 4).
The overall pooled prevalence of phenotypic antibiotic resistance in E. coli was 39.5% (CI: 23.9 − 56.4%) and prevalence of MDR was 12.5% (CI: 4.5 − 23.2%). Animal species from which resistant E. coli was isolated included; waterbuck (100%; CI: 16 -100%), royal antelope (100%; CI: 91 − 100%), African giant rat (100%; CI: 16 − 100%), cane rat (100%; CI: 19 − 100%), African buffalo (75% ; CI: 35 − 97%), impala (73% ; CI: 45-92%), reindeer (67% ; CI: 38 − 88%), mouflon (67%; CI: 0.0 − 99%), European wild rabbits (56%; CI: 3 − 100%), wild boar (37%; CI: 19 -57%), red deer (18%; CI: 0.0 − 57%), fallow deer (11% ; CI: 1 − 33%), roe deer (8%: CI: 0.0 − 65%) and sika deer (5%; CI:4 − 8%).
Antibiotic resistance in E.coli was reported for 11 classes of antibiotics including; macrolides (19%; CI: 0.0 − 58%), monobactams (17%;CI: 0.0 − 48%), penicillins (13%; CI: 6 − 21%), cephalosporins (13%; CI: 7 − 20%), thiamphenicols (8%; CI: 0.0 − 49%), tetracyclines (13%; CI: 5 − 24%), sulphonamides (11%; CI: 5 − 20%), fluoroquinolones (5%; CI: 0.0 − 11%), aminoglycosides (3%; CI:0.0 − 7%), amphenicols (2%; CI: 0.0 − 10%) and organic phosphonic acids (1%; CI: 0.0 − 26%).
E. coli isolates were fully susceptible to meropenem, kanamycin, colistin, amikacin, cefoxitin, imipenem, enrofloxacin, florfenicol, neomycin, tigecycline, apramycin, tulathromycin, danofloxacin, spectinomycin and tylosin. Less than 10% resistance was registered for nalidixic acid, sulphamethoxazole, cefazolin, gentamycin, ciprofloxacin, fosfomycin, streptomycin, amoxicillin-clavulanic acid, tobramycin, sulfisomidine, cephalothin, piperacillin, ampicillin-sulbactam, cotrimazole, cefpodoxime and florfenicol. In contrast, prevalence of between 10% and 20% was recorded for aztreonam, ampicillin, ceftazidime, cefotaxime, tetracycline, trimethoprim-sulphamethoxazole, trimethoprim, ceftriaxone, ceftiofur and norfloxacin. Moreover, 21–50% prevalence of resistance was enumerated for amoxicillin, sulphathiazole, levofloxacin, sulfisoxazole and sulfadimethoxin, while prevalence greater than 50% was observed for resistance to cefepime (70%), cephalexin (87%) and erythromycin (100%).
Antibiotic resistance genes were detected in 31.2% (CI: 25.1% − 37.6%) of E. coli isolates tested. Genes detected included those encoding for aminoglycoside resistance (aadA1, aadA2 and aac [3]-II), sulphonamide resistance (sul1, sul2 and sul3), tetracycline resistance (tetA, tetB, tetG and tetR), aminoglycoside resistance (aac [3]-IId, aph(3”)-Ib, aph [6]-Id, aadA1 and aadA2), phenicol resistance (floR and cmlA1), trimethoprim resistance (dfrA1, dfrA5, dfrA7, dfrA12, dfrA14 and dfrA17), macrolide resistance (mphA), gentamycin resistance (qacG2), quinolone resistance (qnrB19, qnrS, gyrA and gyrA87), ampicillin resistance (AmpC, AmpH and blaCMY-2), streptomycin resistance (strA, strB), colistin resistance (mcr1), multidrug resistance ( mdfA) and beta-lactamase production (blaSHV-12, blaSHV-28, blaTEM-1a, blaTEM-1b, blaCTX-M-1, blaCTX-M2, blaCTX-M-14, blaCTX-M-15, blaCTX-M-55 and blaCTX − M−245).
Antibiotic resistance in enterococcus
Twelve [12] studies reported antibiotic resistance in Enterococcus spp. isolates and 9 were included in the meta-analysis for phenotypic resistance, while 4 were included for genotypic resistance. Nine [9] studies reported MDR strains (supplementary file 5).
The overall pooled prevalence of phenotypic antibiotic resistance in Enterococcus spp. was 71% (CI: 44.1 − 92%) and prevalence of MDR was 26.7% (CI: 5.4 − 56%). Animal species from which resistant Enterococcus strains were isolated included roe deer (100%; CI: 90 − 100%), wild boar (84%; CI: 60 − 98%), red deer (74%; CI: 62 − 83%), European wild rabbits (70%: CI: 30 − 99%) and African buffalo (1%; CI: 0.0 − 3%).
Antibiotic resistance was reported in seven Enterococcus species including E. faecium, E. faecalis, E. durans, E. casseliflavus, E. avium, E. hirae, E. mundtii. One study from South Africa did not specify the Enterococcus species for which antibiotic resistance was reported and this was noted as “Enterococcus sp.”
Antibiotic resistance in Enterococcus spp. was reported for 14 classes of antibiotics including; nitrofurans (67%; CI: 52 − 79%), carbapenems (44%; CI: 20 − 70%), organic phosphonic acids (43%; CI: 29 − 58%), macrolactams (39%; CI: 26 − 54%), streptogramins (36%; CI: 15 − 59%), tetracyclines (32%; CI: 16 − 51%), macrolides (22%; CI: 8 − 41%), penicillins (19%; CI: 5 − 39%), fluoroquinolones (15%;CI: 3 − 35%), aminoglycosides (9%; CI: 2 − 20%), sulphonamides (8%; CI: 0.0 − 39%), glycopeptides (4%; CI: 0.0 − 16%), oxazolidinones (4%; CI: 0.0 − 40%) and amphenicols (3%; CI: 0.0 − 18%).
Enterococcus spp. isolates were fully susceptible to sulphamethoxazole-trimethoprim, cefoxitin and pristinamycin, while less than 10% resistance was registered for vancomycin, teicoplanin, gentamycin, chloramphenicol, florfenicol, linezolid and norfloxacin. In contrast, prevalence of between 10% and 20% was recorded for ampicillin, streptomycin, ciprofloxacin, kanamycin, and trimethoprim, while 21–50% prevalence of resistance was enumerated for tetracycline, erythromycin, quinupristin-dalfopristin, tigecycline, rifampicin, fosfomycin, penicillin and imipenem. Highest prevalence of resistance was recorded for nitrofurantoin (67%) and amoxicillin-clavulanic acid (75%).
Genes encoding antibiotic resistance were detected in 52.2% (CI: 33.4 − 70.7%) of Enterococcus isolates tested. Genes detected included those encoding for tetracycline resistance (tetM, tetL, tetK and Tn916), macrolide resistance (ermB and ermC), gentamycin resistance (acc (6’)-aph (2’)), kanamycin resistance (aph(3’)-IIIa), aminoglycoside resistance (ant [6]-Ia [3] and aac (6′)-aph (2″)), streptogramins resistance (vatD) and vancomycin resistance (vanA and vanB).
Antibiotic resistance in yersinia
Six studies reported antibiotic resistance in Yersinia spp. isolates and five were included in the meta-analysis for phenotypic resistance, while none was included for genotypic resistance. Five reported MDR strains (supplementary file 6).
The pooled prevalence of phenotypic antibiotic resistance in Yersinia spp. was 95.5% (CI: 76.8 − 100%) and prevalence of MDR was 67% (CI: 12.1 − 100%). Animal species from which resistant Yersinia strains were isolated included alpine ibex, red deer, roe deer, wild boar, fallow deer, cane rats, royal antelope, and waterbuck. As each animal species was reported by only one study, meta-analysis with animal species as moderator was not performed.
Antibiotic resistance was reported in four Yersinia species including Yersinia enterocolitica, Yersinia pseudotuberculosis, Yersinia aldovae and Yersinia federiksenii.
Antibiotic resistance in Yersinia spp. was reported for eight classes of antibiotics including; macrolides (100%; CI: 48 − 100%), penicillins (85%; CI: 61 − 100%), cephalosporins (42%; CI: 20 − 66%), aminoglycosides (32%; CI: 8 − 61%), sulphonamides (25%; CI: 2 − 58%), fluoroquinolones (10%; CI: 0.0 − 34%), amphenicols (6%; CI: 0.0 − 46%) and tetracyclines (6%; CI: 0.0 − 43%).
Yersinia spp. isolates were fully susceptible to enrofloxacin and norfloxacin, while less than 10% resistance was registered for chloramphenicol and tetracycline. In contrast, prevalence of between 10% and 20% was recorded for cefotaxime, ciprofloxacin, cefuroxime, triple-sulfa, sulfisoxazole and ceftiofur, while 21–50% prevalence of resistance was enumerated for streptomycin, sulphamethoxazole-trimethoprim, nalidixic acid, gentamycin, and ceftazidime. Highest prevalence of resistance was recorded for kanamycin (56%), amoxicillin-clavulanic acid (56%), cephalexin (80%), ampicillin (96%), erythromycin (100%) and cephalothin (100%).
Antibiotic resistance in other bacteria species
Resistance to antibiotics was also reported for Campylobacter spp. and Listeria spp. However, there were not enough studies to allow performance of meta-analysis for these species. Antibiotic resistance in Campylobacter was reported in four studies in wild boars, red deer, mouflon, fallow deer, and reindeer from Europe. Species studied included C. lanienae, C. coli, C. jejuni, C. hyointestinalis. Similarly, one study reported 89.7% antibiotic resistance in Listeria spp. isolated from wild boars in the United States of America.
Discussion
The present study reports the occurrence and estimated prevalence of antibiotic resistance in bacteria isolated from mammalian wild game from various parts of the world.
Prior to the 2000s, there were fewer studies reporting antibiotic resistance in these game species. However, an upward trend in the number of publications of such studies was observed. This points to an increase in scientific interest in the subject. Moreover, the evolution of detection methodologies over the years could have enhanced detection hence an increase in studies of AMR in different settings including in wildlife.
There were differences in the number of publications on AMR in mammalian wild game by countries and continents, with most originating from Europe. Antimicrobial resistance prevalence data are limited, particularly in low- and middle-income countries. Torres, Carvalho [88] observed that 72% of research outputs on AMR in wildlife were from European countries, more specifically Spain, Portugal, United Kingdom, Sweden, Czech Republic, and Italy. Differences in research priorities at country or continental level directly impact the kind of research conducted within a country /territory [89]. Europe as a continent has alongside other research areas, prioritized AMR research in wildlife, most importantly the mammalian game species and with this it has developed a research fund portfolio and plans to provide support, along with the required technology, as compared to other parts of the world [90].
It was observed that wild boars, red deer, and roe deer were the most studied animal species while very few studies on antibiotic resistance focused on other wild game species. This trend is mostly attributed to governments prioritizing control of wild boar populations across Europe and the Americas, encouraging hunting and elimination of these animals, as well as research into possible public health impacts of such invasive animal species [91].
All studies applied at least one of the methods recommended by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) for detection of antibiotic resistance, with most (55%) applying the disk diffusion method. Disk diffusion is however only suitable for screening, while confirmation requires detection of resistance genes by molecular methods [92]. In this review, 15% of the studies applied broth microdilution while 22.5% combined either disk diffusion or broth microdilution with molecular methods, while 6.25% applied only molecular methods to detect resistance genes. It was noted that resistance to certain antibiotics were not detected phenotypically but genes conferring resistance to the same antibiotics were detected in isolates. Differences in test results using different detection methods may arise due to heterogeneous expression of resistance as reported by [93]. This confirms the importance of applying confirmatory detection methods and using multiple detection methods in AMR surveillance.
The results of the meta-analysis showed that 59.8% of the bacteria isolates from a wide array of mammalian wild game species were resistant to at least one antibiotic and 17.2% were MDR, indicating widespread occurrence of AMR among mammalian wild game. Multidrug resistant organisms are associated with high morbidity and high mortality rates among human patients, requiring longer treatment regimens and hospitalizations [94]. Moreover, selection pressure imposed by antibiotics can lead to the survival, proliferation, and dominance of more virulent strains of bacteria within a population. The same effect may be amplified because of co-selection of resistance and virulence genes [95, 96]. Some of the most critical MDR organisms were reported in mammalian wild game species, a great public health concern for consumers and handlers of game meat. The AMR prevalences reported in the review figures are in agreement with the report on AMR in wildlife by [94]. Even though this may be interpreted as high, the prevalence is much lower than prevalences in domestic animals as highlighted by [97] and [98].
All the bacteria species for which antibiotic resistance was reported are known to potentially cause serious illnesses in humans [99]. However, in this review it was observed that majority of the studies focused on Escherichia coli (44 studies) and Salmonella spp. (17 studies) while there were barely any studies conducted on Listeria spp. (1 study) and Campylobacter spp. (4 studies), and no studies on other emerging food borne bacteria. This therefore leaves a knowledge gap on the epidemiology of important emerging food borne bacteria that can potentially pose a public health risk in consumers and handlers of mammalian wild game.
Escherichia coli is an important bacterium that resides in the lower intestines of warm-blooded animals and humans. Even though most serovars are considered commensal and harmless, some have acquired pathogenic or toxigenic virulent factors, causing illnesses ranging from mild diarrhea to severe colitis that can be life threatening. Moreover, it has also been reported to cause extraintestinal illnesses including urinary tract infections that lead to acute cystitis and pyelonephritis, requiring urgent treatment with antibiotics [100]. Generally, E. coli is intrinsically susceptible to most antibiotics but with a great capacity to amass resistance genes through horizontal gene transfer. Of greatest concern is the ability of E. coli to acquire genes coding for extended-spectrum β-lactamases, carbapenemases, 16 S rRNA methylases, plasmid-mediated quinolone resistance (PMQR) genes, and mcr genes, resulting in resistance to cephalosporins, penicillins, monobactams, carbapenems, aminoglycosides, quinolones and fluoroquinolones, and polymyxins, most of which are clinically important antibiotics and may serve as last line treatment choices [101]. A 39.5% overall prevalence of antibiotic resistance and 12.5% prevalence of MDR in mammalian wild game species is reported in this review, with several resistance genes including those coding for resistance to aminoglycosides, sulphonamides, tetracyclines, aminoglycosides, phenicols, trimethoprim, macrolides, quinolones, ampicillin and polymyxins. Additionally, genes coding for MDR and beta-lactamase production were also reported. The presence of a wide array of resistance genes in wild game shows that wild game may play a vital role in dissemination of resistant organisms to the environment and humans.
Salmonella was the second most studied genera of bacteria, and all studies reported antibiotic resistance in Salmonella enterica with a 69.9% pooled prevalence of resistance to at least one antibiotic and 14.9% MDR. Salmonella enterica infections are often self-limiting and do not require treatment, but systemic infections, mostly in immunocompromised individuals, can be fatal [102]. As Salmonella has increasingly exhibited resistance to common antibiotics like chloramphenicol, ampicillin and trimethoprim-sulphamethoxazole, its treatment is now dependent on more extended spectrum antibiotics such as cephalosporins and fluoroquinolones [103]. However, the emergence of MDR S. enterica serovars, including those resistant to quinolones (fluoroquinolones) and the later generation cephalosporins has become a serious public health concern globally. The findings of the present study are coherent with scientific literature regarding resistance of Salmonella to antibiotics. Several resistance determinants in Salmonella isolates from wild game, including those that confer resistance to fluoroquinolones, extended spectrum β-lactams, aminoglycosides, tetracyclines, and chloramphenicol, macrolides, colistin, and almost all common antibiotics were reported in wild game species.
Staphylococcus spp, especially S. aureus, is a potential human pathogen commonly implicated in a variety of infectious diseases, such as skin and soft tissue infections, endocarditis, osteomyelitis, bacteremia, and lethal pneumonia. These diseases can be life-threatening, and urgent and effective treatment is paramount [104]. However, the emergence of highly resistant strains like the methicillin resistant Staphylococcus aureus (MRSA) poses a challenge to treatment. This strain is almost always resistant to penicillins, cephalosporins, chloramphenicol, lincomycin, aminoglycosides, tetracyclines, macrolides, quinolones, sulfonamides, and rifampicin [105]. On a positive note, none of the studies included in this review reported MRSA. Currently, the most important drugs used in the treatment of Staphylococcus infections include vancomycin, daptomycin, linezolid [106, 107]. In this review, a 69.3% pooled prevalence of antibiotic resistance was reported for 15 species of Staphylococcus isolated from wild game. Highest resistance was registered for penicillin (71%), oxacillin (65%) and cefoxitin (55%) while the least was against oxazolidinones (Linezolid) (1%), Phenicols (6%), glycopeptides and (vancomycin) (7%). Although the prevalence of resistance to linezolid and vancomycin in this case is low, it is a cause for concern as they are some of the few available effective therapies for Staphylococcal infections. It is worthwhile to note that even though resistance was high for penicillins, there was less resistance to penicillin combinations with other drug classes, for example there was less than 10% resistance for penicillin-streptomycin, oxacillin-cefoxitin. This suggests that combination therapies can be one of the ways to address the AMR problem in Staphylococcus.
Antibiotic resistance was reported in seven species of Enterococcus, including the two most clinically important species, E. faecalis and E. feacium. Enterococcus spp. are known to exhibit high level resistance to most of the commonly used antibiotics including penicillins and aminoglycosides. They are also known to be tolerant to vancomycin and β-lactam antibiotics [108, 109]. Recognizing the problem of vancomycin resistance in enterococci, newer agents including quinupristin-dalfopristin, linezolid, daptomycin and tigecycline that have activity against vancomycin-resistant enterococci have been developed. Unfortunately, just like the earlier antibiotics, resistance to these agents is already being reported [110]. Studies included in our review reported resistance of Enterococcus spp to vancomycin and up to 50% resistance to all the new antibiotics that are supposed to act against vancomycin resistant Enterococcus. On a positive note, research showed that Trimethoprim-sulfamethoxazole seems active against enterococci. Also, treatment may be achievable with a combination of aminoglycoside and β-lactam antibiotics [109]. In our review, Enterococcus spp were fully susceptible to Trimethoprim-sulfamethoxazole. We however cannot confirm the activity of combination treatments as none of the included studies performed susceptibility tests for drug combinations combined drugs.
Yersinia, Campylobacter and Listeria are some of the most important food-borne bacterial pathogens and illnesses due to these pathogens are the topmost frequently reported globally [111, 112]. Antibiotic resistance in these pathogens have extensively been studied and prevalences ranging between 50 and 100% [113], up to 83.6% [114] and up to 100% [115], have been reported for Campylobacter, Listeria and Yersinia respectively, in livestock. This review reports similar prevalences of antibiotic resistance in Campylobacter, Listeria and Yersinia isolated from mammalian wild game.
The occurrence of AMR in wildlife is often interpreted as a consequence of anthropogenic impact in the areas described or of inadequate management of antibiotic residues of human and/or animal origin [116, 117]. In this review, a significantly higher prevalence of antibiotic resistance was recorded in isolates from mammalian wild game in remote settings than those from areas close to human/livestock activities. A similar report was made by Cristóbal-Azkarate, Dunn [118] in a study on non-human primates in Mexico, as well as by [119]. The AMR patterns may be an indicator of specific resistance traits in the environment, and a continuous monitoring of the presence of these bacteria in wild mammals and their role as a reservoir and in the dispersal of resistant bacteria even far from areas of high anthropogenic activity could be useful, given their ability to move over long distances. Notwithstanding, studies including that of Miller, Gammon [120] have also established that certain resistant bacteria are ubiquitous in natural ecosystems, with species like MDR E. coli being isolated from Antarctic waters, thus it is possible that wildlife can also acquire resistant strains- of bacteria from their environment through horizontal gene transfers, even in the absence of anthropic activities.
The world health organization advisory group on integrated surveillance of antimicrobial resistance (AGISAR) classified antimicrobials as “critically important”, “highly important” and “important” based on two criteria: (1) They are the sole or one of the limited available therapies for treatment of serious bacterial infections in humans, and (2) are used to treat infections caused by bacteria that are possibly transmitted from non-human sources or with resistance genes from non-human sources. Critically important antibiotics meet criteria 1 and 2, highly important antibiotics meet either criteria 1 or 2, while important antimicrobials do not meet either criterion but are useful for treatment of human infections [121]. From this review, majority of the drugs for which resistance was reported fall under the critically important and highly important group. The presence of resistance to critically important and highly important antibiotics like cephalosporins, vancomycin and fluoroquinolones in wild game is a cause for concern as they could potentially pose a dilemma in treatment of infections for which they are the sole available treatment once humans acquire such resistant strains through consumption of contaminated wild game meat. Moreover, in all species of bacteria studied, substantial resistance to carbapenems, the treatment of choice for serious infections with ESBL-producing bacteria was observed. This has a serious implication on future management of such infections as selection pressure could accelerate the development and amplification of carbapenemase resistance in the wild and onward transmission through the wild game food chain.
Limitations
This systematic review and meta-analysis aimed at estimating the global prevalence of antibiotic resistance in mammalian wild game. However, it may have overestimated /underestimated the prevalence due to lack of representation of studies from certain countries. Also, the lack of uniformity and differences in test parameters of the susceptibility test methods applied by the different studies, as well as studies with small sample sizes can introduce bias and increase variability in a meta-analysis leading to over / under estimation of prevalence.
Conclusion and recommendations
This systematic review and meta-analysis established the occurrence of antibiotic-resistant bacteria, including MDR strains in mammalian wild game species. However, the source of the resistant organisms can only be speculated. To this end, it is imperative that a combined effort of environmental, veterinary, and medical sciences be applied in surveillance and epidemiological/ecological studies of antibiotic resistance in a One-Health approach. Along with this, effective detection mechanisms ought to be applied in surveillance of AMR. As noted in this review, in some studies, susceptibility tests did not detect resistance to certain antibiotics but resistance genes to the same were later identified by molecular methods. Adoption of more advanced approaches therefore greatly enhances AMR surveillance. The carriage of resistant bacteria by mammalian wild game species does not inevitably point to their role in transmission of resistant bacteria to humans or livestock. This still requires more in-depth studies to understand their role in the AMR transmission pathway, and to this effect, it would be worthwhile to undertake studies aimed at assessing the risk of AMR transmission and its linkage to hunting and trapping practices, as well as handling and consumption of wild game meat.
As existing studies focused only on a few species of bacteria, we recommend that studies be conducted to cover a wider diversity of bacteria, including commensals as they may still carry and spread resistance genes that could be acquired by pathogenic bacteria in domestic animals and humans.
Finally, this review highlighted the possible contamination of wildlife habitats with resistant bacteria based on the high prevalence of antibiotic resistance in mammalian wild game living in remote locations. Based on this result, more emphasis should be placed on the disclosure of environmental issues related to anthropogenic waste management to minimize the contamination and spread of resistant bacteria in wild habitats. Along with this, surveillance for AMR should be expanded to cover more ecosystems including sylvatic habitats. Mapping and tracking movements of wild mammals can enable a more detailed understanding of the sources /origins of resistant bacteria in the wild. There would be a need for continuous monitoring of the presence of these bacteria in wild mammals and their role as reservoirs and in the dispersal of resistant bacteria even far from areas of high anthropogenic activity, given their ability to move over long distances.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- AGISAR
Advisory Group on Integrated Surveillance of Antimicrobial Resistance
- AMR
Antimicrobial resistance
- CI
Confidence interval
- ESBL
Extended spectrum beta lactamase
- EUCAST
European Committee on Antimicrobial Susceptibility Testing
- JBI
Joanna Briggs Institute
- MDR
Multi-drug resistance
- MRSA
Methicillin-resistant Staphylococcus aureus
- PCR
Polymerase chain reaction
- PRISMA
Preferred Reporting Items for Systematic reviews and Meta-Analyses
- PMQR
Plasmid-mediated quinolone resistance
- WHO
World Health Organization
Author contributions
All authors contributed to the article and have approved its publication in the current form. Specifically, CJA was responsible for Conceptualization, methodology, investigation, data curation and formal analysis, writing original draft, as well as reviewing and editing the final paper. MFP supported conceptualization, methodology, reviewing and editing final paper, while KF, LB, AF and NM supported conceptualization, methodology, supervision, validation, funding acquisition and reviewing and editing the final paper.
Funding
This work is part of the CRESCENDO Doctoral Programme funded by the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie programme (MSCA-COFUND-2020) with Grant Agreement No. 101034245.
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Reygaert WC. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018;4(3):482–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Aguilar GR, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boucher HW. Bad bugs, no drugs 2002–2020: progress, challenges, and call to action. Trans Am Clin Climatol Assoc. 2020;131:65. [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmad I, Malak HA, Abulreesh HH. Environmental antimicrobial resistance and its drivers: a potential threat to public health. J Global Antimicrob Resist. 2021;27:101–11. [DOI] [PubMed] [Google Scholar]
- 5.Dolejska M, Literak I. Wildlife is overlooked in the epidemiology of medically important antibiotic-resistant bacteria. Antimicrob Agents Chemother. 2019;63(8):01167–19. 10.1128/aac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S, Mir RA, Park SH, Kim D, Kim H-Y, Boughton RK, et al. Prevalence of extended-spectrum β-lactamases in the local farm environment and livestock: challenges to mitigate antimicrobial resistance. Crit Rev Microbiol. 2020;46(1):1–14. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organisation. Global Action Plan on Antimicrobial Resistance. Geneva, Switzerland2015.
- 8.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. In: Aromataris EMZ, editor. Systematic reviews of prevalence and incidence. JBI Manual for Evidence Synthesis: JBI; 2020. [Google Scholar]
- 9.Alonso CA, Alcala L, Simon C, Torres C. Novel sequence types of extended-spectrum and acquired AmpC beta-lactamase producing Escherichia coli and Escherichia clade V isolated from wild mammals. FEMS Microbiol Ecol. 2017;93(8). [DOI] [PubMed]
- 10.Alonso CA, Gonzalez-Barrio D, Ruiz-Fons F, Ruiz-Ripa L, Torres C. High frequency of B2 phylogroup among non-clonally related fecal Escherichia coli isolates from wild boars, including the lineage ST131. FEMS Microbiol Ecol. 2017;93(3). [DOI] [PubMed]
- 11.Asai T, Usui M, Sugiyama M, Andoh M. A survey of antimicrobial-resistant Escherichia coli prevalence in wild mammals in Japan using antimicrobial-containing media. J Vet Med Sci. 2022;84(12):1645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachiri T, Bakour S, Ladjouzi R, Thongpan L, Rolain JM, Touati A. High rates of CTX-M-15-producing Escherichia coli and Klebsiella pneumoniae in wild boars and barbary macaques in Algeria. J Global Antimicrob Resist. 2017;8:35–40. [DOI] [PubMed] [Google Scholar]
- 13.Ben Said L, Jouini A, Fliss I, Torres C, Klibi N. Antimicrobial resistance genes and virulence gene encoding intimin in Escherichia coli and Enterococcus isolated from wild rabbits (Oryctolagus cuniculus) in Tunisia. Acta Vet Hung. 2019;67(4):477–88. [DOI] [PubMed] [Google Scholar]
- 14.Bertelloni F, Cilia G, Bogi S, Ebani VV, Turini L, Nuvoloni R et al. Pathotypes and Antimicrobial susceptibility of Escherichia Coli isolated from wild boar (Sus scrofa) in Tuscany. Anim (Basel). 2020;10(4). [DOI] [PMC free article] [PubMed]
- 15.Bonardi S, Cabassi C, Longhi S, Pia F. … Detection of extended-spectrum beta-lactamase producing Escherichia coli from mesenteric lymph nodes of wild boars (Sus scrofa): ncbi.nlm.nih.gov; 2018. [DOI] [PMC free article] [PubMed]
- 16.Brisson L, Caron A, Mazuy-Cruchadet C, Gilot-Fromont E, Lécu A, Mathieu B et al. COMPARING ANTIBIOTIC RESISTANCE IN FREE-RANGING VS. CAPTIVE AFRICAN WILD HERBIVORES. J Wildl Dis. 2023. [DOI] [PubMed]
- 17.Caleja C, Toro Md, Gonçalves A, Themudo P. Antimicrobial resistance and class I integrons in Salmonella enterica isolates from wild boars and Bísaro pigs. core.ac.uk; 2011. [DOI] [PubMed]
- 18.Caprioli A, Donelli G, Falbo V, Passi C, Pagano A, Mantovani A. Antimicrobial resistance and production of toxins in Escherichia coli strains from wild ruminants and the alpine marmot. J Wildl Dis. 1991;27(2):324–7. [DOI] [PubMed] [Google Scholar]
- 19.Carbonero A, Paniagua J, Torralbo A, Arenas-Montes A, Borge C, Garcia-Bocanegra I. Campylobacter infection in wild artiodactyl species from southern Spain: occurrence, risk factors and antimicrobial susceptibility. Comp Immunol Microbiol Infect Dis. 2014;37(2):115–21. [DOI] [PubMed] [Google Scholar]
- 20.Carella E, Romano A, Domenis L, Robetto S, Spedicato R, Guidetti C, et al. Characterisation of Yersinia Enterocolitica Strains Isolated from Wildlife in the Northwestern Italian Alps. J Vet Res. 2022;66(2):141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carraro PE, Barbosa FD, Benevides VP, Casas MRT, Berchieri A, Burger KP. Prevalence and antimicrobial resistance of Salmonella spp. isolated from free ranging wild boars in the state of Sao Paulo, Brazil. Ciencia Rural. 2022;52(8).
- 22.Carrillo-Del Valle MD, De la Garza-García JA, Díaz-Aparicio E, Valdivia-Flores AG, Cisneros-Guzmán LF, Rosario C, et al. Characterization of Escherichia coli strains from red deer (Cervus elaphus) faeces in a Mexican protected natural area. Eur J Wildl Res. 2016;62(4):415–21. [Google Scholar]
- 23.Castillo-Contreras R, Marin M, Lopez-Olvera JR, Ayats T, Fernandez Aguilar X, Lavin S, et al. Zoonotic Campylobacter spp. and Salmonella spp. carried by wild boars in a metropolitan area: occurrence, antimicrobial susceptibility and public health relevance. Sci Total Environ. 2022;822:153444. [DOI] [PubMed] [Google Scholar]
- 24.Cummings KJ, Rodriguez-Rivera LD, Grigar MK, Rankin SC, Mesenbrink BT, Leland BR, et al. Prevalence and characterization of Salmonella isolated from feral pigs throughout Texas. Zoonoses Public Health. 2016;63(6):436–41. [DOI] [PubMed] [Google Scholar]
- 25.Darwich L, Seminati C, Lopez-Olvera JR, Vidal A, Aguirre L, Cerda M et al. Detection of Beta-Lactam-Resistant Escherichia coli and Toxigenic Clostridioides difficile strains in wild boars foraging in an anthropization gradient. Anim (Basel). 2021;11(6). [DOI] [PMC free article] [PubMed]
- 26.Dias D, Fonseca C, Caetano T, Mendo S, Oh. Deer! How worried should we be about the diversity and abundance of the faecal resistome of red deer? Sci Total Environ. 2022;825:153831. [DOI] [PubMed] [Google Scholar]
- 27.Dias D, Fonseca C, Mendo S, Caetano T. A closer look on the variety and abundance of the faecal resistome of wild boar. Environ Pollut. 2022;292:118406. Pt B). [DOI] [PubMed] [Google Scholar]
- 28.Dias D, Cruz A, Fonseca C, Mendo S, Caetano TS. Antibiotic resistance and potential bacterial pathogens identified in red deer’s faecal DNA. Transbound Emerg Dis. 2022;69(5):e3425–9. [DOI] [PubMed] [Google Scholar]
- 29.Donazzolo C, Turchetto S, Ustulin M, Citterio C, Conedera G, Vio D et al. Antimicrobial susceptibility of Salmonella enterica subsp. enterica serovar Choleraesuis strains from wild boar (Sus scrofa) in Italy. Paulsen P, Bauer A, Smulders FJM, editors2017. 121-7 p.
- 30.Duangurai T, Rungruengkitkul A, Kong-Ngoen T, Tunyong W, Kosoltanapiwat N, Adisakwattana P, et al. Phylogenetic analysis and antibiotic resistance of Escherichia coli isolated from wild and domestic animals at an agricultural land interface area of Salaphra wildlife sanctuary, Thailand. Vet World. 2022;15(12):2800–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elsby DT, Zadoks RN, Boyd K, Silva N, Chase-Topping M, Mitchel MC, et al. Antimicrobial resistant Escherichia coli in Scottish wild deer: prevalence and risk factors. Environ Pollut. 2022;314:120129. [DOI] [PubMed] [Google Scholar]
- 32.Ferreira A. Antimicrobial resistance at the livestock-wildlife-human interface using wild boar, fallow deer and red deer as models. repositorio.ul.pt; 2019.
- 33.Formenti N, Calo S, Parisio G, Guarneri F, Birbes L, Pitozzi A et al. ESBL/AmpC-Producing Escherichia coli in Wild Boar: Epidemiology and Risk Factors. Animals (Basel). 2021;11(7). [DOI] [PMC free article] [PubMed]
- 34.Frank M-A, Justice WS, La Ragione R, Chambers MA. Antibiotic resistance in escherichia coli and enterococcus spp. isolated from ungulates at a zoological collection in the United Kingdom. J Zoo Wildl Med. 2021;51(4):761–70. [DOI] [PubMed] [Google Scholar]
- 35.García LA, Torres C, López AR, Rodríguez CO, Valencia CS. Antimicrobial resistance of species isolated from wild mammals in Aragón, Spain. J Veterinary Res. 2022;66(2):151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gil Molino M, Risco Perez D, Goncalves Blanco P, Fernandez Llario P, Quesada Molina A, Garcia Sanchez A, et al. Outbreaks of antimicrobial resistant Salmonella Choleraesuis in wild boars piglets from central-western Spain. Transbound Emerg Dis. 2019;66(1):225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gil-Molino M, Goncalves P, Risco D, Martin-Cano FE, Garcia A, Rey J, et al. Dissemination of antimicrobial-resistant isolates of Salmonella spp. in wild boars and its relationship with management practices. Transbound Emerg Dis. 2022;69(5):e1488–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gómez P, Lozano C, González-Barrio D, Zarazaga M, Ruiz-Fons F, Torres C. High prevalence of methicillin-resistant Staphylococcus aureus (MRSA) carrying the mecC gene in a semi-extensive red deer (Cervus elaphus hispanicus) farm in Southern Spain. Vet Microbiol. 2015;177(3–4):326–31. [DOI] [PubMed] [Google Scholar]
- 39.Guerrero-Ramos E, Cordero J, Molina-Gonzalez D, Poeta P, Igrejas G, Alonso-Calleja C, et al. Antimicrobial resistance and virulence genes in enterococci from wild game meat in Spain. Food Microbiol. 2016;53(Pt B):156–64. [DOI] [PubMed] [Google Scholar]
- 40.Holtmann AR, Meemken D, Muller A, Seinige D, Buttner K, Failing K et al. Wild boars carry extended-spectrum beta-lactamase- and AmpC-Producing Escherichia coli. Microorganisms. 2021;9(2). [DOI] [PMC free article] [PubMed]
- 41.Jobbins SE, Alexander KA. From whence they came—antibiotic-resistant Escherichia coli in African wildlife. J Wildl Dis. 2015;51(4):811–20. [DOI] [PubMed] [Google Scholar]
- 42.Kabali E, Pandey GS, Munyeme M, Kapila P, Mukubesa AN, Ndebe J et al. Identification of Escherichia coli and related Enterobacteriaceae and examination of their phenotypic antimicrobial resistance patterns: a Pilot Study at A Wildlife-Livestock Interface in Lusaka, Zambia. Antibiot (Basel). 2021;10(3). [DOI] [PMC free article] [PubMed]
- 43.Katakweba AA, Moller KS, Muumba J, Muhairwa AP, Damborg P, Rosenkrantz JT, et al. Antimicrobial resistance in faecal samples from buffalo, wildebeest and zebra grazing together with and without cattle in Tanzania. J Appl Microbiol. 2015;118(4):966–75. [DOI] [PubMed] [Google Scholar]
- 44.King TLB, Schmidt S. Assessment of three indigenous South African herbivores as potential reservoirs and vectors of antibiotic-resistant Escherichia coli. Eur J Wildl Res. 2017;63(3).
- 45.Kraushaar B, Fetsch A. First description of PVL-positive methicillin-resistant Staphylococcus aureus (MRSA) in wild boar meat. Int J Food Microbiol. 2014;186:68–73. [DOI] [PubMed] [Google Scholar]
- 46.Laatu M, Rautelin H, Hänninen M-L. Susceptibility of Campylobacter hyointestinalis subsp. hyointestinalis to antimicrobial agents and characterization of quinolone-resistant strains. J Antimicrob Chemother. 2005;55(2):182–7. [DOI] [PubMed] [Google Scholar]
- 47.Lillehaug A, Bergsjo B, Schau J, Bruheim T, Vikoren T, Handeland K. Campylobacter spp., Salmonella spp., verocytotoxic Escherichia coli, and antibiotic resistance in indicator organisms in wild cervids. Acta Vet Scand. 2005;46(1–2):23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maguina-Molina C, Pons MJ, Beltran MJ, Morales-Cauti S. Multidrug-resistant Salmonella enterica isolated in Paca (Cuniculus paca) carcasses from the Belen Market, Iquitos, Peru. Foodborne Pathog Dis. 2021;18(2):131–8. [DOI] [PubMed] [Google Scholar]
- 49.Mama OM, Ruiz-Ripa L, Fernandez-Fernandez R, Gonzalez-Barrio D, Ruiz-Fons JF, Torres C. High frequency of coagulase-positive staphylococci carriage in healthy wild boar with detection of MRSA of lineage ST398-t011. FEMS Microbiol Lett. 2019;366(4). [DOI] [PubMed]
- 50.Marotta F, Di Marcantonio L, Janowicz A, Pedonese F, Di Donato G, Ardelean A, et al. Genotyping and Antibiotic Resistance traits in Campylobacter jejuni and Coli from pigs and Wild boars in Italy. Front Cell Infect Microbiol. 2020;10:592512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mateus-Vargas RH, Atanassova V, Reich F, Klein G. Antimicrobial susceptibility and genetic characterization of Escherichia coli recovered from frozen game meat. Food Microbiol. 2017;63:164–9. [DOI] [PubMed] [Google Scholar]
- 52.Mateus-Vargas RH, Lienen T, Maaz D, Richter M, Maurischat S, Steinhoff-Wagner J. Evaluation of the occurrence of Staphylococcaceae with reduced susceptibility to Cefoxitin in Wild ungulates in Brandenburg, Germany, based on Land Use-related factors. Microbiol Spectr. 2022;10(5):e0256022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mercato A, Cortimiglia C, Abualsha’ar A, Piazza A, Marchesini F, Milani G, et al. Wild boars as an Indicator of Environmental Spread of ESbetaL-Producing Escherichia coli. Front Microbiol. 2022;13:838383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Modesto P, De Ciucis CG, Vencia W, Pugliano MC, Mignone W, Berio E et al. Evidence of Antimicrobial Resistance and Presence of pathogenicity genes in Yersinia enterocolitica isolate from wild boars. Pathogens. 2021;10(4). [DOI] [PMC free article] [PubMed]
- 55.Molino MG, Garcia A, Zurita SG, Martin-Cano FE, Garcia-Jimenez W, Risco D et al. Spread of Antimicrobial Resistance by Salmonella enterica Serovar Choleraesuis between Close Domestic and Wild environments. Antibiotics-Basel. 2020;9(11). [DOI] [PMC free article] [PubMed]
- 56.Morita S, Sato S, Maruyama S, Nagasaka M, Murakami K, Inada K, et al. Whole-genome sequence analysis of Shiga toxin-producing Escherichia coli O157 strains isolated from wild deer and boar in Japan. J Vet Med Sci. 2021;83(12):1860–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mubita C, Muma B, Nalubamba K, Pandey G, … Characterization of non-typhoid Salmonellae isolated from domestic animals and wildlife from selected areas of Zambia: Elsevier;2020.
- 58.Navarro-Gonzalez N, Mentaberre G, Porrero CM, Serrano E, Mateos A, Lopez-Martin JM, et al. Effect of cattle on Salmonella carriage, diversity and antimicrobial resistance in free-ranging wild boar (Sus scrofa) in northeastern Spain. PLoS ONE. 2012;7(12):e51614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Navarro-Gonzalez N, Casas-Diaz E, Porrero CM, Mateos A, Dominguez L, Lavin S, et al. Food-borne zoonotic pathogens and antimicrobial resistance of indicator bacteria in urban wild boars in Barcelona, Spain. Vet Microbiol. 2013;167(3–4):686–9. [DOI] [PubMed] [Google Scholar]
- 60.Navarro-Gonzalez N, Porrero MC, Mentaberre G, Serrano E, Mateos A, Dominguez L, et al. Antimicrobial resistance in indicator Escherichia Coli isolates from free-ranging livestock and sympatric wild ungulates in a natural environment (Northeastern Spain). Appl Environ Microbiol. 2013;79(19):6184–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Navarro-Gonzalez N, Porrero MC, Mentaberre G, Serrano E, Mateos A, Cabal A, et al. Escherichia coli O157:H7 in wild boars (Sus scrofa) and Iberian ibex (Capra pyrenaica) sharing pastures with free-ranging livestock in a natural environment in Spain. Vet Q. 2015;35(2):102–6. [DOI] [PubMed] [Google Scholar]
- 62.Navarro-Gonzalez N, Castillo-Contreras R, Casas-Diaz E, Morellet N, Porrero MC, Molina-Vacas G et al. Carriage of antibiotic-resistant bacteria in urban versus rural wild boars. Eur J Wildl Res. 2018;64(5).
- 63.Nevins JW. Prevalence of antibiotic resistant pathogens in feral hogs of Texas. Angelo State University; 2011.
- 64.Nishino Y, Shimojima Y, Morita K, Ida M, Fukui R, Kuroda S, et al. [Prevalence of Antimicrobial Resistance in Escherichia coli isolated from Retail Meat in Tokyo, Japan]. Shokuhin Eiseigaku Zasshi. 2019;60(3):45–51. [DOI] [PubMed] [Google Scholar]
- 65.Nocera FP, Ferrara G, Scandura E, Ambrosio M, Fiorito F, De Martino L. A preliminary study on Antimicrobial susceptibility of Staphylococcus spp. and Enterococcus spp. Grown on Mannitol Salt Agar in European Wild Boar (Sus scrofa) hunted in Campania Region-Italy. Anim (Basel). 2021;12(1). [DOI] [PMC free article] [PubMed]
- 66.Odyniec M, Bancerz-Kisiel A. Assessment of the role of free-living and farmed fallow deer (Dama dama) as a potential source of human infection with multiple-drug-resistant strains of Yersinia enterocolitica and Yersinia pseudotuberculosis. Pathogens. 2022;11(11). [DOI] [PMC free article] [PubMed]
- 67.Ojo O, Ogunjobi O, Oyekunle M, … Prevalence and antimicrobial resistance of Salmonella and Yersinia in the feces of hunted wildlife in Abeokuta, Nigeria. Revue d’élevage et de …. 2019.
- 68.Ojo OE, Amosun EA, Opebiyi OO, Oyekunle MA, Dipeolu MA, Otesile EB. Multidrug resistant enterohaemorrhagic Escherichia coli serogroups in the faeces of hunted Wildlife, Abeokuta, Nigeria. Vet Ital. 2022;58(2). [DOI] [PubMed]
- 69.Pérez JR, Rosa LZ, Sánchez AG, … Multiple Antimicrobial Resistance in Methicillin-Resistant Staphylococcus sciuri Group Isolates from Wild Ungulates in Spain. Antibiotics. 2021. [DOI] [PMC free article] [PubMed]
- 70.Pipova M, Jevinova P, Kmet’ V, Regecova I, Maruskova K. Antimicrobial resistance and species identification of staphylococci isolated from the meat of wild rabbits (Oryctolagus cuniculus) in Slovakia. Eur J Wildl Res. 2012;58(1):157–65. [Google Scholar]
- 71.Piras F, Spanu V, Siddi G, Gymoese P, Spanu C, Cibin V et al. Whole-genome sequencing analysis of highly prevalent Salmonella serovars in wild boars from a national park in Sardinia. Food Control. 2021;130.
- 72.Porrero MC, Mentaberre G, Sanchez S, Fernandez-Llario P, Gomez-Barrero S, Navarro-Gonzalez N, et al. Methicillin resistant Staphylococcus aureus (MRSA) carriage in different free-living wild animal species in Spain. Vet J. 2013;198(1):127–30. [DOI] [PubMed] [Google Scholar]
- 73.Razzuoli E, Listorti V, Martini I, Migone L, Decastelli L, Mignone W et al. Prevalence and Antimicrobial resistances of Salmonella Spp. Isolated from wild boars in Liguria Region, Italy. Pathogens. 2021;10(5). [DOI] [PMC free article] [PubMed]
- 74.Rega M, Carmosino I, Bonilauri P, Frascolla V, Vismarra A, Bacci C. Prevalence of ESbetaL, AmpC and Colistin-Resistant E. Coli in Meat: a comparison between pork and wild boar. Microorganisms. 2021;9(2). [DOI] [PMC free article] [PubMed]
- 75.Rega M, Andriani L, Cavallo S, Bonilauri P, Bonardi S, Conter M et al. Antimicrobial resistant E. coli in pork and wild boar meat: a risk to consumers. Foods. 2022;11(22). [DOI] [PMC free article] [PubMed]
- 76.Shaffer CE, Rogers SW, editors. Wild Deer as reservoirs of manure pathogens and resistance genes. International Symposium on Air Quality and Manure Management for Agriculture 2010; Dallas, Texas.
- 77.Shin E, Mduma S, Keyyu J, Fyumagwa R, Lee Y. An investigation of Enterococcus species isolated from the African buffalo (Syncerus caffer) in Serengeti National Park, Tanzania. Microbes Environ. 2017;32(4):402–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Silva N, Igrejas G, Figueiredo N, Gonçalves A, Radhouani H, Rodrigues J, et al. Molecular characterization of antimicrobial resistance in enterococci and Escherichia coli isolates from European wild rabbit (Oryctolagus cuniculus). Sci Total Environ. 2010;408(20):4871–6. [DOI] [PubMed] [Google Scholar]
- 79.Silva V, Pereira JE, Maltez L, Ferreira E, Manageiro V, Canica M et al. Diversity of methicillin-resistant staphylococci among wild Lepus granatensis: first detection of mecA-MRSA in hares. FEMS Microbiol Ecol. 2020;96(1). [DOI] [PubMed]
- 80.Sousa M, Silva N, Manageiro V, Ramos S, Coelho A, Goncalves D, et al. First report on MRSA CC398 recovered from wild boars in the north of Portugal. Are we facing a problem? Sci Total Environ. 2017;596–597:26–31. [DOI] [PubMed] [Google Scholar]
- 81.Van Breda LK, Ward MP. Evidence of antimicrobial and disinfectant resistance in a remote, isolated wild pig population. Prev Vet Med. 2017;147:209–12. [DOI] [PubMed] [Google Scholar]
- 82.Van den Honert MS, Gouws PA, Hoffman LC. A preliminary study: antibiotic resistance patterns of Escherichia coli and Enterococcus species from Wildlife Species subjected to supplementary feeding on various South African farms. Anim (Basel). 2020;10(3). [DOI] [PMC free article] [PubMed]
- 83.Van den Honert MS, Gouws PA, Hoffman LC. Escherichia coli Antibiotic Resistance patterns from Co-grazing and Non-co-grazing Livestock and Wildlife species from two farms in the Western Cape, South Africa. Antibiot (Basel). 2021;10(6). [DOI] [PMC free article] [PubMed]
- 84.Van den Honert MS, Gouws PA, Hoffman LC. A preliminary study: Antibiotic Resistance of Escherichia coli and Staphylococcus aureus from the meat and feces of various South African Wildlife species. Food Sci Anim Resour. 2021;41(1):135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Velhner M, Todorovic D, Grego E, Jovcic B, Prunic B, Stojanov I, et al. Fluoroquinolone-resistant and extended-spectrum beta-lactamase producing Escherichia coli isolates from free-living wild animals. Vet Microbiol. 2018;223:168–72. [DOI] [PubMed] [Google Scholar]
- 86.Von Altrock A, Seinige D, Kehrenberg C. Yersinia enterocolitica isolates from wild boars hunted in Lower Saxony, Germany. Appl Environ Microbiol. 2015;81(14):4835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zottola T, Montagnaro S, Magnapera C, Sasso S, … Prevalence and antimicrobial susceptibility of Salmonella in European wild boar (Sus scrofa); Latium Region–Italy: Elsevier; 2013. [DOI] [PubMed]
- 88.Torres RT, Carvalho J, Cunha MV, Serrano E, Palmeira JD, Fonseca C. Temporal and geographical research trends of antimicrobial resistance in wildlife-a bibliometric analysis. One Health. 2020;11:100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Klavans R, Boyack KW. The Research Focus of Nations: economic vs. altruistic motivations. PLoS ONE. 2017;12(1):e0169383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.EU. CORDIS EU research results: European Commission. 2023 [Available from: https://cordis.europa.eu/
- 91.Jori F, Massei G, Licoppe A, Ruiz-Fons F, Linden A, Václavek P, et al. Management of wild boar populations in the European Union before and during the ASF crisis. Understanding and combatting African swine fever: a European perspective. Wageningen Academic; 2021. pp. 263–71.
- 92.Burnham C-AD, Leeds J, Nordmann P, O’Grady J, Patel J. Diagnosing antimicrobial resistance. Nat Rev Microbiol. 2017;15(11):697–703. [DOI] [PubMed] [Google Scholar]
- 93.Ghosh D, Veeraraghavan B, Elangovan R, Vivekanandan P. Antibiotic resistance and epigenetics: more to it than meets the eye. Antimicrob Agents Chemother. 2020;64(2):02225–19. 10.1128/aac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Breijyeh Z, Jubeh B, Karaman R. Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules. 2020;25(6):1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yazdanpour Z, Tadjrobehkar O, Shahkhah M. Significant association between genes encoding virulence factors with antibiotic resistance and phylogenetic groups in community acquired uropathogenic Escherichia coli isolates. BMC Microbiol. 2020;20(1):241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hussain A, Mazumder R, Asadulghani M, Clark TG, Mondal D. Chapter 8 - combination of virulence and antibiotic resistance: a successful bacterial strategy to survive under hostile environments. In: Kumar A, Tenguria S, editors. Bacterial survival in the hostile. Environment: Academic; 2023. pp. 101–17. [Google Scholar]
- 97.Van Boeckel TP, Pires J, Silvester R, Zhao C, Song J, Criscuolo NG, et al. Global trends in antimicrobial resistance in animals in low-and middle-income countries. Science. 2019;365(6459):eaaw1944. [DOI] [PubMed] [Google Scholar]
- 98.Xu C, Kong L, Gao H, Cheng X, Wang X. A review of current bacterial resistance to Antibiotics in Food animals. Front Microbiol. 2022;13. [DOI] [PMC free article] [PubMed]
- 99.Abebe E, Gugsa G, Ahmed M. Review on major food-borne zoonotic bacterial pathogens. Journal of tropical medicine. 2020;2020. [DOI] [PMC free article] [PubMed]
- 100.MacKinnon M, Sargeant J, Pearl D, Reid-Smith R, Carson C, Parmley E, et al. Evaluation of the health and healthcare system burden due to antimicrobial-resistant Escherichia coli infections in humans: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2020;9:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Poirel L, Madec JY, Lupo A, Schink AK, Kieffer N, Nordmann P et al. Antimicrobial Resistance in Escherichia coli. Microbiol Spectr. 2018;6(4). [DOI] [PMC free article] [PubMed]
- 102.Havelaar AH, Kirk MD, Torgerson PR, Gibb HJ, Hald T, Lake RJ, et al. World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015;12(12):e1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dawan J, Ahn J. Assessment of cross-resistance potential to serial antibiotic treatments in antibiotic-resistant Salmonella Typhimurium. Microb Pathog. 2020;148:104478. [DOI] [PubMed] [Google Scholar]
- 104.Linz MS, Mattappallil A, Finkel D, Parker D. Clinical impact of Staphylococcus aureus skin and soft tissue infections. Antibiotics. 2023;12(3):557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vestergaard M, Frees D, Ingmer H. Antibiotic resistance and the MRSA problem. Microbiol Spectr. 2019;7(2). 10.1128/microbiolspec.gpp3-0057-2018. [DOI] [PMC free article] [PubMed]
- 106.Bassetti M, Russo A, Carnelutti A, Wilcox M. Emerging drugs for treating methicillin-resistant Staphylococcus aureus. Expert Opin Emerg Drugs. 2019;24(3):191–204. [DOI] [PubMed] [Google Scholar]
- 107.Douglas EJ, Wulandari SW, Lovell SD, Laabei M. Novel antimicrobial strategies to treat multi-drug resistant Staphylococcus aureus infections. Microb Biotechnol. 2023. [DOI] [PMC free article] [PubMed]
- 108.Kristich CJ, Rice LB, Arias CA. Enterococcal infection—treatment and antibiotic resistance. Enterococci: from commensals to leading causes of drug resistant infection [Internet]. 2014.
- 109.Rello J, Campogiani L, Eshwara VK. Understanding resistance in enterococcal infections. Intensive Care Med. 2020;46:353–6. [DOI] [PubMed] [Google Scholar]
- 110.Cairns KA, Udy AA, Peel TN, Abbott IJ, Dooley MJ, Peleg AY. Therapeutics for vancomycin-resistant Enterococcal Bloodstream infections. Clin Microbiol Rev. 2023:e00059–22. [DOI] [PMC free article] [PubMed]
- 111.Lee H, Yoon Y. Etiological Agents Implicated in Foodborne Illness World wide. Food Sci Anim Resour. 2021;41(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chlebicz A, Śliżewska K. Campylobacteriosis, salmonellosis, yersiniosis, and listeriosis as zoonotic foodborne diseases: a review. Int J Environ Res Public Health. 2018;15(5):863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lazou TP, Chaintoutis SC. Comparison of disk diffusion and broth microdilution methods for antimicrobial susceptibility testing of Campylobacter isolates of meat origin. J Microbiol Methods. 2023;204:106649. [DOI] [PubMed] [Google Scholar]
- 114.Grudlewska-Buda K, Bauza-Kaszewska J, Wiktorczyk-Kapischke N, Budzyńska A, Gospodarek-Komkowska E, Skowron K. Antibiotic Resistance in selected emerging bacterial foodborne Pathogens-An issue of concern? Antibiot (Basel). 2023;12(5). [DOI] [PMC free article] [PubMed]
- 115.Seakamela EM, Diseko L, Malatji D, Makhado L, Motau M, Jambwa K, et al. Characterisation and antibiotic resistance of Yersinia enterocolitica from various meat categories, South Africa. Onderstepoort J Vet Res. 2022;89(1):e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Plaza-Rodríguez C, Alt K, Grobbel M, … Wildlife as sentinels of antimicrobial resistance in Germany? frontiersin.org; 2021. [DOI] [PMC free article] [PubMed]
- 117.Laborda P, Sanz-García F, Ochoa-Sánchez LE, Gil-Gil T, Hernando-Amado S, Martínez JL. Wildlife and Antibiotic Resistance. Front Cell Infect Microbiol. 2022;12:873989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cristóbal-Azkarate J, Dunn JC, Day JM, Amábile-Cuevas CF. Resistance to antibiotics of clinical relevance in the fecal microbiota of Mexican wildlife. PLoS ONE. 2014;9(9):e107719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Arnold KE, Williams NJ, Bennett M. ‘Disperse abroad in the land’: the role of wildlife in the dissemination of antimicrobial resistance. Biol Lett. 2016;12(8). [DOI] [PMC free article] [PubMed]
- 120.Miller RV, Gammon K, Day MJ. Antibiotic resistance among bacteria isolated from seawater and penguin fecal samples collected near Palmer Station, Antarctica. Can J Microbiol. 2009;55(1):37–45. [DOI] [PubMed] [Google Scholar]
- 121.WHO. Critically important antimicrobials for human medicine, 6th revision. Geneva, Switzerland: World Health Organization; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.