Abstract
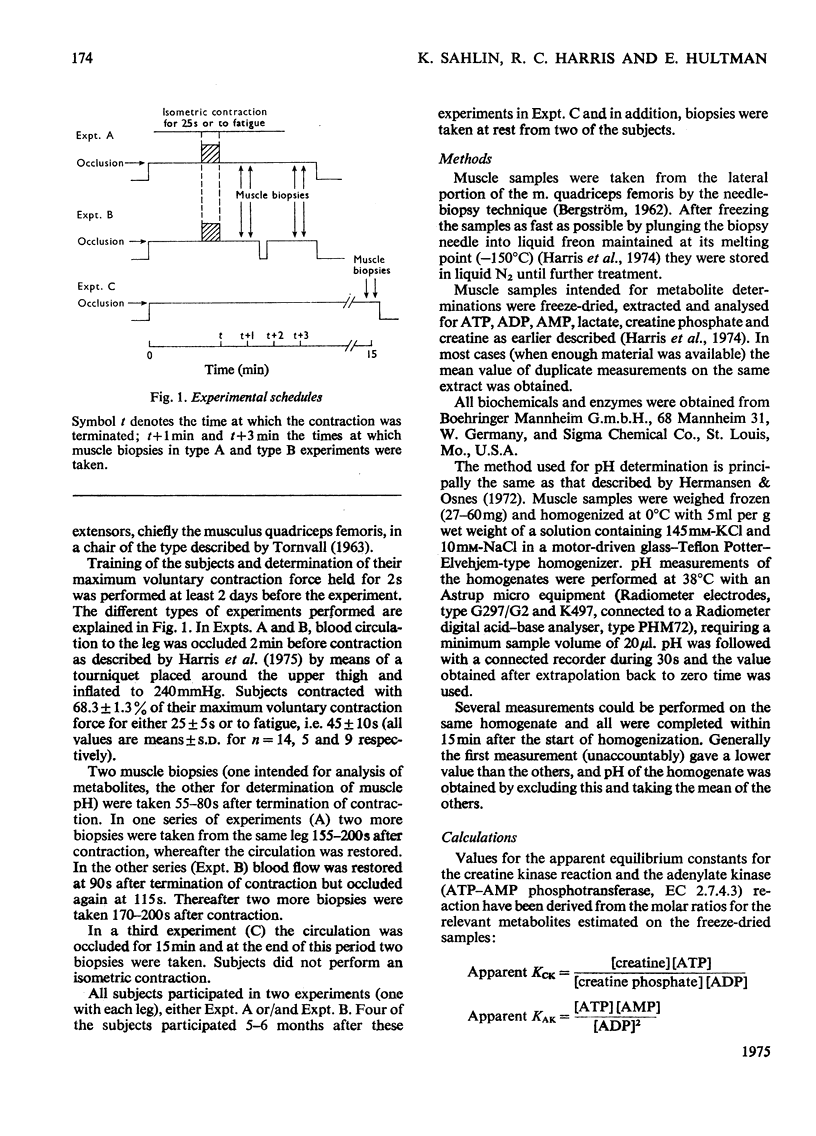
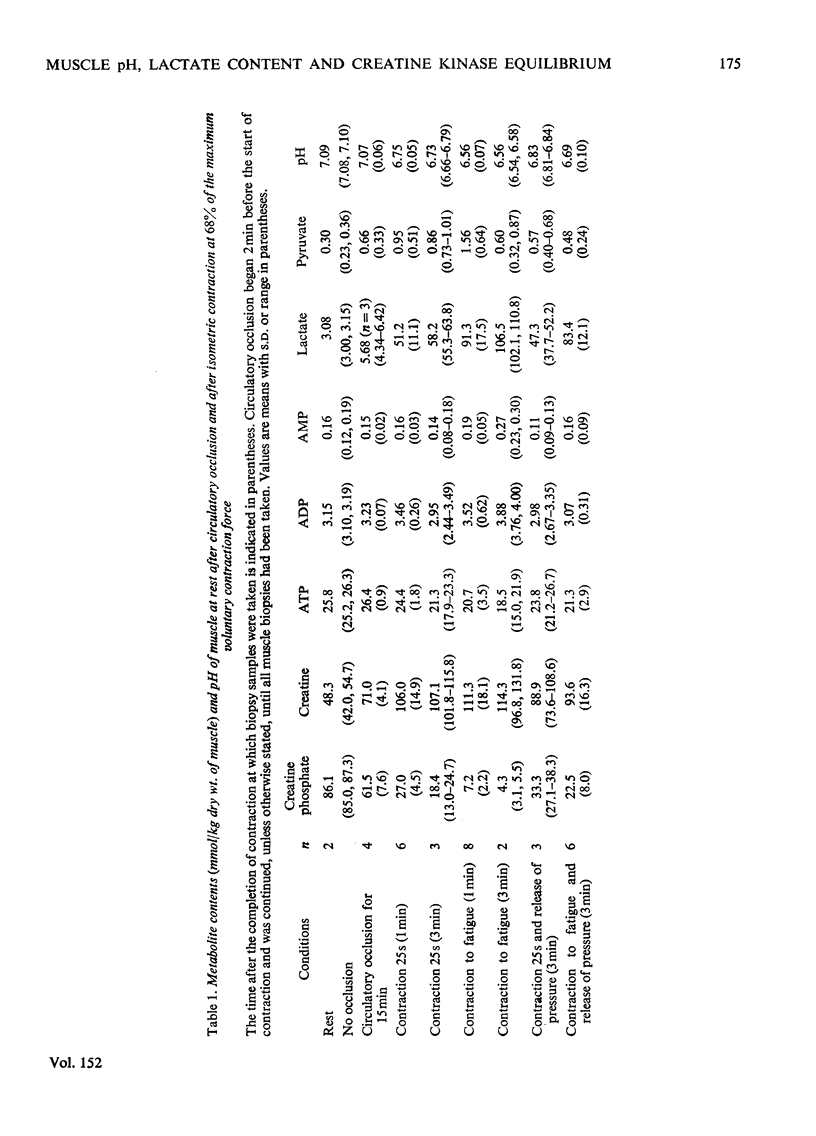
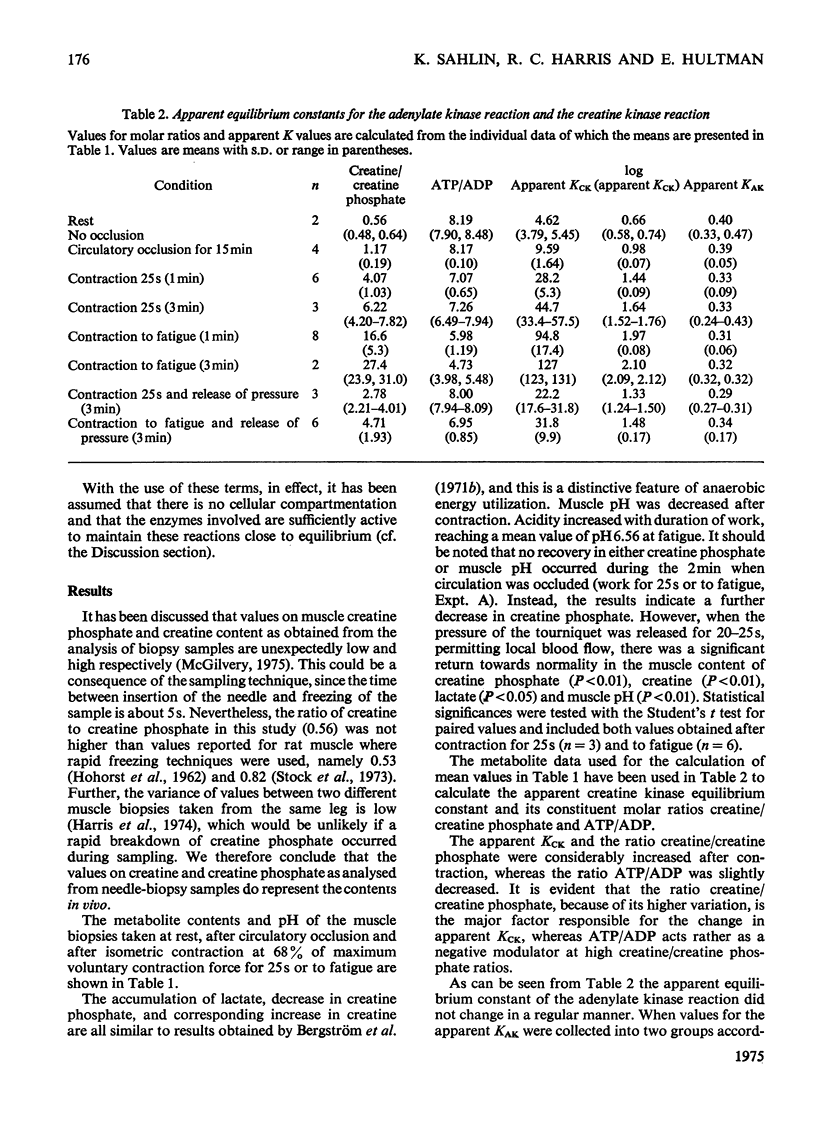
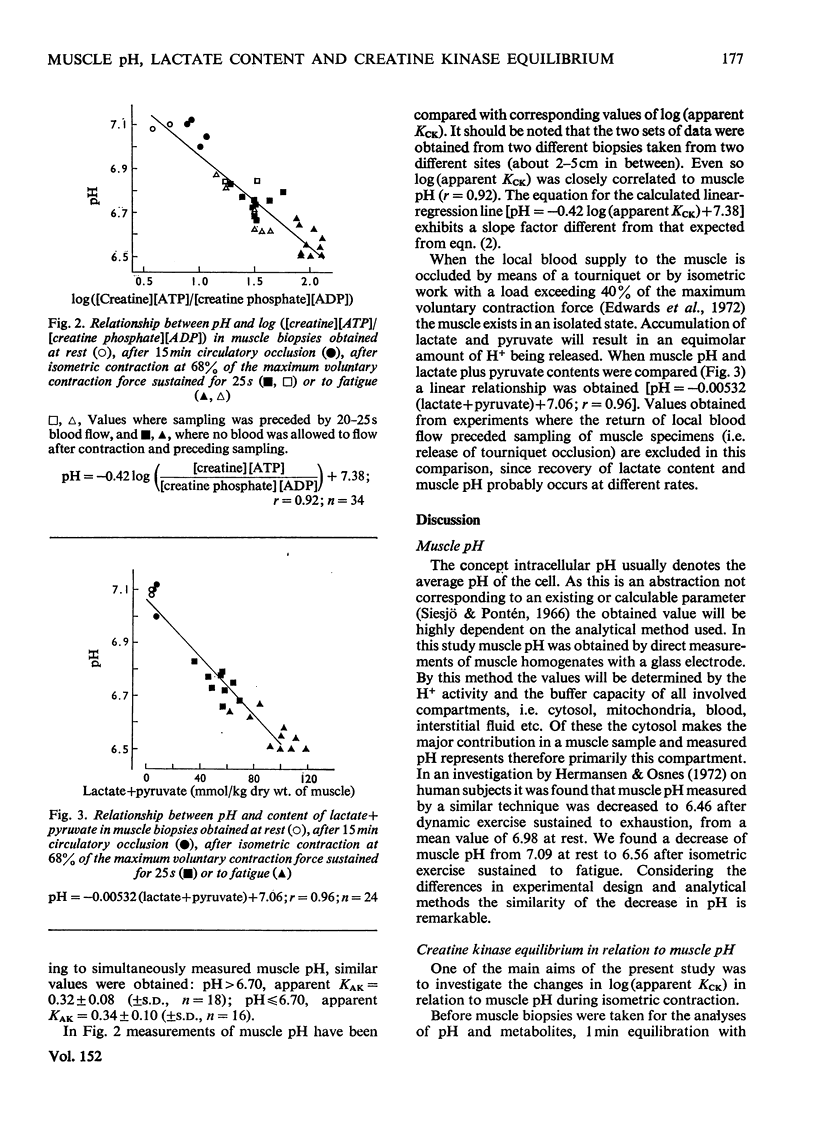
Muscle biopsies taken from the musculus quadriceps femoris of man were analysed for pH, ATP, ADP, AMP, creatine phosphate, creatine, lactate and pyruvate. Biopsies were taken at rest, after circulatory occlusion and after isometric contraction. Muscle pH decreased from 7.09 at rest to 6.56 after isometric exercise to fatigue. Decrease in muscle pH was linearly related to accumulation of lactate plus pyruvate. An increase of 22μmol of lactate plus pyruvate per g of muscle resulted in a fall of 0.5pH unit. The apparent equilibrium constant of the creatine kinase reaction (apparent KCK) increased after isometric contraction and a linear relationship between log(apparent KCK) and muscle pH was obtained. The low content of creatine phosphate in muscle after contraction as analysed from needle-biopsy samples is believed to be a consequence of an altered equilibrium state of the creatine kinase reaction. This in turn is attributed mainly to a change in intracellular pH.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlborg B., Bergström J., Ekelund L. G., Guarnieri G., Harris R. C., Hultman E., Nordesjö L. O. Muscle metabolism during isometric exercise performed at constant force. J Appl Physiol. 1972 Aug;33(2):224–228. doi: 10.1152/jappl.1972.33.2.224. [DOI] [PubMed] [Google Scholar]
- BATE-SMITH E. C., BENDALL J. R. Factors determining the time course of rigor mortis. J Physiol. 1949 Dec 15;110(1-2):47–65. doi: 10.1113/jphysiol.1949.sp004420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström J., Guarnieri G., Hultman E. Carbohydrate metabolism and electrolyte changes in human muscle tissue during heavy work. J Appl Physiol. 1971 Jan;30(1):122–125. doi: 10.1152/jappl.1971.30.1.122. [DOI] [PubMed] [Google Scholar]
- CARLSON F. D., SIGER A. The creatine phosphoryltransfer reaction in iodoacetate-poisoned muscle. J Gen Physiol. 1959 Nov;43:301–313. doi: 10.1085/jgp.43.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOHORST H. J., REIM M., BARTELS H. Studies on the creatine kinase equilibrium in muscle and the significance of ATP and ADP levels. Biochem Biophys Res Commun. 1962 Apr 3;7:142–146. doi: 10.1016/0006-291x(62)90163-8. [DOI] [PubMed] [Google Scholar]
- Harris R. C., Hultman E., Kaijser L., Nordesjö L. O. The effect of circulatory occlusion on isometric exercise capacity and energy metabolism of the quadriceps muscle in man. Scand J Clin Lab Invest. 1975 Jan;35(1):87–95. [PubMed] [Google Scholar]
- Harris R. C., Hultman E., Nordesjö L. O. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest. 1974 Apr;33(2):109–120. [PubMed] [Google Scholar]
- Hermansen L., Osnes J. B. Blood and muscle pH after maximal exercise in man. J Appl Physiol. 1972 Mar;32(3):304–308. doi: 10.1152/jappl.1972.32.3.304. [DOI] [PubMed] [Google Scholar]
- KUBY S. A., NODA L., LARDY H. A. Adenosinetriphosphate-creatine transphosphorylase. III. Kinetic studies. J Biol Chem. 1954 Sep;210(1):65–82. [PubMed] [Google Scholar]
- MacMillan V., Siesjö B. K. Intracellular pH of the brain in arterial hypoxemia, evaluated with the CO 2 method and from the creatine phosphokinase equilibrium. Scand J Clin Lab Invest. 1972 Oct;30(2):117–125. doi: 10.3109/00365517209081100. [DOI] [PubMed] [Google Scholar]
- NIHEI T., NODA L., MORALES M. F. Kinetic properties and equilibrium constant of the adenosine triphosphate-creatine transphosphorylase-catalyzed reaction. J Biol Chem. 1961 Dec;236:3203–3209. [PubMed] [Google Scholar]
- NODA L., KUBY S. A., LARDY H. A. Adenosinetriphosphate-creatine transphosphorylase. IV. Equilibrium studies. J Biol Chem. 1954 Sep;210(1):83–95. [PubMed] [Google Scholar]
- Newbold R. P., Scopes R. K. Post-mortem glycolysis in ox skeletal muscle. Effect of temperature on the concentrations of glycolytic intermediates and cofactors. Biochem J. 1967 Oct;105(1):127–136. doi: 10.1042/bj1050127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose I. A. The state of magnesium in cells as estimated from the adenylate kinase equilibrium. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1079–1086. doi: 10.1073/pnas.61.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERAYDARIAN K., MOMMAERTS W. F., WALLNER A. The amount and compartmentalization of adenosine diphosphate in muscle. Biochim Biophys Acta. 1962 Dec 17;65:443–460. doi: 10.1016/0006-3002(62)90447-x. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K., Folbergrová J., MacMillan V. The effect of hypercapnia upon intracellular pH in the brain, evaluated by the bicarbonate-carbonic acid method and from the creatine phosphokinase equilibrium. J Neurochem. 1972 Nov;19(11):2483–2495. doi: 10.1111/j.1471-4159.1972.tb01308.x. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K., Pontén U. Intracellular pH--true parameter or misnomer? Ann N Y Acad Sci. 1966 Apr 1;133(1):78–86. doi: 10.1111/j.1749-6632.1966.tb50711.x. [DOI] [PubMed] [Google Scholar]
- Smith E. C. The buffering of muscle in rigor; protein, phosphate and carnosine. J Physiol. 1938 Apr 14;92(3):336–343. doi: 10.1113/jphysiol.1938.sp003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock W., Bohn H. J., Isselhard W. Die Restitution des Energiestoffwechsels der Skeletmuskulatur der Ratte nach langdauernder Ischämie. Res Exp Med (Berl) 1973;159(4):306–320. doi: 10.1007/BF01851605. [DOI] [PubMed] [Google Scholar]
- Veloso D., Guynn R. W., Oskarsson M., Veech R. L. The concentrations of free and bound magnesium in rat tissues. Relative constancy of free Mg 2+ concentrations. J Biol Chem. 1973 Jul 10;248(13):4811–4819. [PubMed] [Google Scholar]


