Abstract
Objective
This study aims to compare the effects of sevoflurane (SEV) and propofol (PRO) on postoperative cognitive dysfunction (POCD) in patients undergoing cardiac surgery (CS) under cardiopulmonary bypass (CPB), with a focus on evaluating the efficacy of these anesthetic agents in preventing POCD.
Methods
A total of 113 patients undergoing CS with CPB were grouped into two: PRO group (n = 58) and SEV group (n = 55). Baseline data, anesthesia effects (CPB duration, anesthesia time, respiratory recovery time, and anesthesia recovery time), Montreal Cognitive Assessment (MoCA) scores, POCD incidence, neurological function markers (NSE, S-100β, MMP9), and serum inflammatory markers (IL-6, IL-8, TNF-α) were analyzed. The study was conducted between March 2018 and May 2021.
Results
The PRO group showed significantly shorter anesthesia time (P < 0.05), respiratory recovery time (P < 0.05), and anesthesia recovery time (P < 0.05) compared to the SEV group. The postoperative MoCA score in the PRO group reduced markedly compared with the baseline, but still higher than that in the SEV group (P < 0.05). The incidence of POCD was significantly lower in the PRO group (5.17% vs. 27.27%, P = 0.001). The levels of NSE, S-100β, MMP9, IL-6, IL-8, and TNF-α were significantly elevated compared to baseline values, but still lower than those in the SEV group (P < 0.05 for all comparisons).
Conclusion
PRO is more effective than SEV in preventing POCD in patients undergoing CS with CPB. It provides superior anesthetic effects and offers better protection against neuronal damage and serum inflammation compared to SEV.
Clinical trial number
Not applicable.
Keywords: Cardiac surgery, Cardiopulmonary bypass, Sevoflurane, Propofol, Cognitive dysfunction, Postoperative cognitive complications, Neuropsychological tests
Introduction
Postoperative cognitive dysfunction (POCD) is a common adverse event in cardiovascular surgery, including cardiac surgery (CS), it refers to the cognitive decline that occurs in patients shortly after surgery (usually 24 to 72 h after surgery), manifested as a decrease in memory, attention, and executive function, which can lead to poor long-term recovery, impaired quality of life, and even an increased risk of death [1, 2]. This disease is common among middle-aged and elderly individuals, with age, depression, cognitive dysfunction, cardiovascular and cerebrovascular diseases, as well as intraoperative and postoperative complications being the main predisposing factors [3, 4]. According to epidemiological statistics, approximately 3 million patients undergo CS with cardiopulmonary bypass (CPB) in the United States each year, and up to 50% persist with POCD 30 months after discharge [5]. The formulation of anesthesia strategy plays a decisive role in the occurrence of POCD in patients undergoing CS under CPB. That is to say, appropriate anesthesia selection can help reduce the risk of delayed neurocognitive recovery while playing a certain role in brain function protection [6, 7]. This study attempts to reduce the risk of POCD in patients undergoing CS under CPB from the perspective of anesthesia management.
Sevoflurane (SEV) and propofol (PRO) are both commonly used to maintain anesthesia in patients undergoing CS with CPB, but no consensus has reached regarding which anesthetic strategy is more effective in preventing POCD [8, 9]. SEV is a halogenated inhalation anesthetic, which can also be applied to children, adult inpatients and outpatients undergoing surgery, with hypnotic, analgesic, and autonomic nerve block effects [10]. An animal experiment shows that the regulatory mechanisms of SEV on cognitive dysfunction and neuronal damage are influenced by Mind Bomb-2 (MIB-2), and down-regulating MIB-2 can significantly alleviate SEV expose-induced neuronal death and ferroptosis [11]. There is also evidence that SEV, assisted by electroacupuncture, is beneficial in reducing the risk of POCD in elderly patients [12]. PRO, as a selective regulator of inhibitory neurotransmitters, can modulate epigenetic pathways, affect genetic signal pathways to varying degrees, and influence patients’ immune function [13]. In addition, PRO can also be used as a sedative, with higher patient and physician satisfaction, faster patient recovery, and greater cost-effectiveness than other sedatives [14]. In a mouse experiment conducted by Nie Y et al. [15], PRO significantly attenuated the negative effects of isoflurane-induced neuroinflammation, apoptosis and cognitive function in offspring, suggesting the certain neuroprotective effect of PRO.
POCD pathogenesis is still poorly understood. The latest evidence suggests that the inflammatory response induced by surgery is a key figure in POCD development. Moreover, a recent study by Glumac et al. [16] showed that preoperative corticosteroid administration ameliorates inflammatory response prompted by surgery, and thereby, decreased the incidence and severity of POCD. Ischemia-reperfusion injury (IRI) and systemic inflammatory response syndrome (SIRS) are closely associated with POCD. IRI, which occurs when blood flow is restored to previously ischemic tissues, can trigger a cascade of inflammatory responses, leading to damage in both the brain and other organs. SIRS, a generalized inflammatory response triggered by surgical trauma, also exacerbates this cascade, affecting distant organs such as the kidneys, heart, and liver. Both IRI and SIRS contribute to the neuroinflammatory environment that underlies POCD by promoting the release of pro-inflammatory cytokines. These cytokines have been shown to increase neuronal apoptosis and dysfunction, further exacerbating cognitive decline post-surgery [17, 18]. Thus, reducing IRI and SIRS through appropriate perioperative management, including anti-inflammatory treatments, may be crucial in mitigating the risk of POCD.
This study hypothesizes that PRO is more effective than SEV in preventing POCD in patients undergoing cardiac surgery with CPB, through its superior ability to reduce inflammation and protect neuronal function, leading to better cognitive outcomes postoperatively.
Materials and methods
Study design
This is a prospective, comparative study conducted at [Hospital Name], located in [City, Country]. The study included patients undergoing cardiac surgery with CPB between March 2018 and May 2021. We compared the effects of two anesthetic agents, SEV and PRO, on POCD in these patients. The study was approved by the hospital’s ethics committee, and informed consent was obtained from all participants.
Randomization method
The participants in this study were randomly assigned to either the PRO group or the SEV group using a computer-generated random number sequence. The randomization was performed by an independent researcher who was not involved in the patient recruitment or data analysis. To ensure balance in key baseline characteristics, stratified randomization was used. Patients were randomly assigned in a 1:1 ratio to the two groups. The allocation was concealed from the investigators through the use of sealed opaque envelopes, ensuring that both the researchers and the participants were blinded to the group assignments until the moment of intervention.
Sample size
Sample size calculation for this study was based on a similar study conducted by Schoen, J et al. [19]. Given the primary endpoint of POCD, we estimated that a difference of 5% in the POCD incidence between the two groups (propofol vs. sevoflurane) would be clinically significant. To achieve a power of 80% and a significance level of 5% (alpha = 0.05), the required sample size was calculated to be 50 patients per group. Assuming a 10% drop-out rate, 55 patients were enrolled in each group, leading to a total sample size of 110 participants.
Patient information
Eligibility criteria: Aged 18 to 80 years; No severe carotid artery stenosis, neuropsychiatric diseases, chronic respiratory diseases or severe endocrine diseases before surgery; left ventricular ejection fraction ≥ 35%; complete medical records. Exclusion criteria: Preoperative use of cardioprotective drugs; chronic obstructive pulmonary disease (COPD) or diabetes or inflammatory disorders; surgical contraindications; other concurrent operations; past coronary heart disease or cardiomyopathy. The research was conducted under the approval of the hospital’s ethics committee. In this study, 113 patients who underwent CS under CPB, including coronary artery bypass grafting (CABG), valve replacement surgeries, and other complex cardiac surgeries requiring CPB were strictly selected according to the above eligibility and exclusion criteria. The research was approved by the hospital’s ethics committee (Ethical Review No. [number]).PRO group (n = 58) received PRO and SEV group (n = 55) received SEV. The two patient cohorts exhibited clinical comparability without any notable differences in baseline data (P > 0.05).
Methods
Preoperative analgesia was provided by intramuscular injection of 0.3 mg of scopolamine and 10 mg of morphine 1 h before anesthesia induction. Radial artery catheterization was performed after entering the operating room, and electrocardiogram, heart rate, blood oxygen and other vital signs were monitored. After that, rapid induction was carried out by intravenous injection of midazolam (0.1 mg/kg), sufentanil (0.8 µg/kg), vecuronium bromide (0.1 mg/kg), and etomidate (0.3 mg/kg) in turn. Subsequently, endotracheal intubation was performed and a ventilator was connected. Intraoperative analgesia was maintained with continuous infusion of sufentanil (0.1–0.2 µg/kg/hr) to ensure adequate pain relief throughout the procedure. The perfusion flow of the CPB machine was set to 50–80 mL/(kg·min) [20], and the cold crystalloid cardioplegia (Thomas’ solution) supplemented with local hypothermia was used to protect the myocardium. Postoperative analgesia was provided by continuous infusion of sufentanil (0.05–0.1 µg/kg/hr) for the first 24 h after surgery, adjusted based on the patient’s pain level and recovery. In cases of inadequate pain relief, additional doses of morphine (1–2 mg every 4 h) were used as needed for breakthrough pain.
The PRO group received target controlled infusion of PRO with plasma target concentration of 2 to 4 µg/mL. In the SEV group, 1–2% SEV was continuously inhaled, the mean arterial pressure (MAP) was maintained at 50–80 mmHg, and the BIS was 40–55. To ensure sufficient anesthesia depth during the surgical process. If the BIS value deviates from this range, adjust the infusion rate of propofol or the concentration of sevoflurane to maintain BIS within the target range. The anesthesiologist carefully controls these adjustments to avoid insufficient and excessive anesthesia, reducing the impact on POCD outcomes. Blood gas levels were continuously monitored throughout the procedure to ensure the maintenance of optimal pH, oxygen, and carbon dioxide levels. The target pH during CPB was maintained at 7.35–7.45, and the arterial oxygen saturation (SaO₂) was kept above 95% to prevent ischemic damage. The oxygenator used in the CPB circuit was carefully monitored to ensure adequate gas exchange, and adjustments to the flow of oxygen and carbon dioxide were made accordingly. Continuous ultrafiltration (CUF) was employed to manage excess fluid and improve hemodynamic stability during CPB. This technique was used to optimize the patient’s fluid balance and minimize the risk of edema or volume overload. Additionally, modified ultrafiltration (MUF) was utilized in some cases to remove excess fluid and improve hemodynamics by concentrating the blood and removing inflammatory mediators. The use of zero-balanced ultrafiltration (ZBUF) and zero-balanced continuous ultrafiltration (ZCUF) was also considered for patients with more complex fluid management needs. These techniques help to maintain optimal volume status and reduce the inflammatory response during CPB. For myocardial protection, cold crystalloid cardioplegia (Thomas’ solution) was used in combination with local hypothermia. The cardioplegia was delivered through a retrograde and antegrade infusion technique to ensure adequate myocardial protection during the procedure. The temperature of the cardioplegia solution was maintained at 4–8 °C to minimize ischemic damage to the myocardium. CPB was stopped when the circulation was stabilized. Vital signs were continuously monitored 24 h after surgery. Monitor postoperative bleeding in all patients. Perform chest tube drainage 24 h before surgery to monitor and manage any excess fluid accumulation. Record the amount of bleeding and intervene if significant bleeding is observed. Once the patient reaches the standards of spontaneous breathing, adequate oxygenation, and stable hemodynamics, extubation is performed. In the case of delayed extubation, reasons were recorded, such as difficulty breathing or low level of consciousness. All patients were extubated within 6 h after surgery, and the average extubation time was recorded for reference.
Analysis indexes
The primary outcome of this study was the incidence of POCD, which was observed and recorded at 24 and 72 h post-surgery. Cognitive function was evaluated pre- and post-operatively by the Montreal Cognitive Assessment Scale (MoCA), with a total score of 30 (≥ 26 normal). POCD was defined as a decrease of at least 2 points in MoCA score within 24 and 72 h after surgery, and significant changes in cognitive function compared to preoperative levels. If the MoCA score of the patient is below 26 points at these two time points and there are symptoms of cognitive decline, it is judged as POCD. CPB duration, anesthesia time, respiratory recovery time, and anesthesia recovery time were counted. Blood samples were collected from the superior vena cava immediately after anesthesia induction (T0), at the end of surgery (T1), 12 h after surgery (T2), and 24 h after surgery (T3), to determine plasma concentrations of neuron-specific enolase (NSE), S-100β, and matrix metalloproteinase 9 (MMP9). Enzyme-linked immunosorbent assays (ELISAs) were performed to detect the levels of inflammatory factors such as interleukin (IL)-6, IL-8 and tumor necrosis factor (TNF)-α in serum obtained by centrifugation at the above four time points.
Statistical processing of data
Data were processed by SPSS19.0, and the significance threshold was set as P < 0.05. We used the (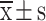 ) and n(%) to statistically describe continuous and categorical variables, respectively; the comparison of continuous variables employed the independent samples t test (between groups) and the paired t test (within groups before and after treatment), while categorical variables were tested by the χ2 test.
) and n(%) to statistically describe continuous and categorical variables, respectively; the comparison of continuous variables employed the independent samples t test (between groups) and the paired t test (within groups before and after treatment), while categorical variables were tested by the χ2 test.
Results
Comparative analysis of baseline data
Initially, a total of 130 patients participated in the study. However, during the screening process, 7 patients were excluded due to not meeting the inclusion criteria. Exclusion criteria are as follows: (1) Preoperative use of cardiac protective drugs (n = 2); (2) COPD or diabetes or inflammatory disorders (n = 8); (3) Other concurrent operations (n = 4); (4) Past coronary heart disease or cardiomyopathy (n = 3). As a result, 113 patients were included in the final analysis. These patients were randomly assigned to the PRO group (n = 58) and the SEV group (n = 55). Although these groups were randomly assigned, the slight differences in sample size between the two groups (58 in the PRO group and 55 in the SEV group) were due to minor biases in the recruitment process, such as patient preferences or other logistical factors that did not affect the integrity of the study. Importantly, no significant differences was observed between the two groups at baseline (P > 0.05). Shown in Fig. 1; Table 1.
Fig. 1.
Inclusion and exclusion flowchart
Table 1.
Comparative analysis of baseline data
| Indicators | PRO group (n = 58) | SEV group (n = 55) | χ2/t | P |
|---|---|---|---|---|
| Sex | 0.493 | 0.482 | ||
| Male | 27 (46.55) | 22 (40.00) | ||
| Female | 31 (53.45) | 33 (60.00) | ||
| Age (years) | 54.43 ± 11.44 | 56.40 ± 11.13 | 0.927 | 0.356 |
| Weight (kg) | 60.16 ± 6.52 | 61.05 ± 7.26 | 0.686 | 0.494 |
| EUROScore classification (grade) | 0.688 | 0.441 | ||
| Low Risk | 25 (43.10%) | 28 (50.91%) | ||
| Intermediate Risk | 28 (48.28%) | 25 (45.45%) | ||
| High Risk | 5 (8.62%) | 2 (3.64%) | ||
| Educational level | 2.312 | 0.315 | ||
| Junior high school | 42 (72.41) | 33 (60.00) | ||
| Senior high school | 3 (5.17) | 6 (10.91) | ||
| University or above | 13 (22.41) | 16 (29.09) | ||
| Type of surgery | ||||
| CABG | 30 (51.72%) | 28 (50.91%) | 0.021 | 0.985 |
| Valve replacement surgery | 20 (34.48%) | 19 (34.55%) | 0.004 | 0.952 |
| Other complex cardiac surgeries (e.g., congenital, heart failure) | 8 (13.79%) | 8 (14.55%) | 0.000 | 1.000 |
| Pre-existing conditions | 2 (3.45%) | 3 (5.45%) | 0.298 | 0.588 |
| Thyroid disorders | ||||
| Renal disease | 1 (1.72%) | 1 (1.82%) | 0.001 | 0.971 |
Comparative analysis of anesthesia effects
The anesthesia effects between the two groups were compared in terms of CPB duration, anesthesia time, respiratory recovery time, and anesthesia recovery time. Although the CPB duration was similar in both the PRO group and the SEV group (P > 0.05), significant differences were observed in other parameters. Specifically, the PRO group demonstrated markedly shorter anesthesia time (P = 0.043), respiratory recovery time (P = 0.037), and anesthesia recovery time (P = 0.002) compared to the SEV group. Shown in Fig. 2.
Fig. 2.
Comparative analysis of anesthesia effects. (A) Comparison of CPB duration between PRO and SEV groups. (B) Comparison of anesthesia time between PRO and SEV groups. (C) Comparison of respiratory recovery time between PRO and SEV groups. (D) Comparison of anesthesia recovery time between PRO and SEV groups. Notes: *P < 0.05 and **P < 0.01 vs. SEV group
Comparative analysis of MoCA scores
Both the PRO group and the SEV group had similar preoperative MoCA scores (P > 0.05), indicating comparable baseline cognitive function prior to surgery. Postoperatively, both groups experienced a significant decline in MoCA scores (P = 0.021), reflecting a general postoperative cognitive decline. However, the PRO group showed significantly higher MoCA scores than the SEV group at both postoperative time points, with a statistically significant difference observed (P = 0.004). Shown in Fig. 3.
Fig. 3.
Comparative analysis of MoCA scores. Notes: *P < 0.05 and **P < 0.01 vs. before intervention; aP < 0.05 vs. SEV group
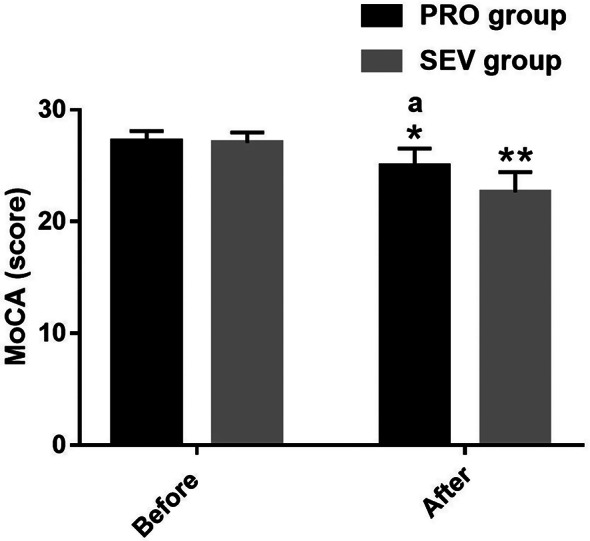
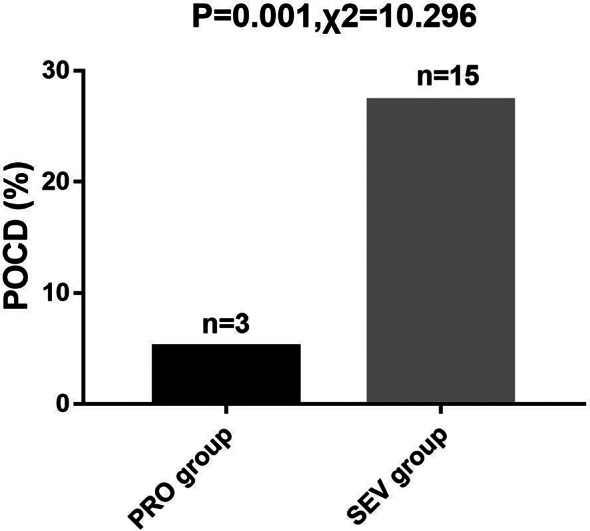
Comparative analysis of POCD
The incidence of postoperative cognitive dysfunction (POCD) was observed and recorded in both the PRO group and the SEV group. The PRO group exhibited a notably lower incidence of POCD compared to the SEV group, with 5.17% of patients in the PRO group experiencing POCD, compared to 27.27% in the SEV group (P = 0.001). Shown in Fig. 4.
Fig. 4.
Comparative analysis of POCD
Comparative analysis of neurological function indexes
We compared NSE, S-100β, and MMP9, all neurological function indexes, at various time points. No statistical inter-group differences were identified at T0 (P > 0.05); while they all rose markedly in both groups at T1 (PRO group: P = 0.036、0.032、0.005; SEV group: P = 0.005、0.004、0.009) and T2 (PRO group: P = 0.044、0.042、0.035; SEV group: P = 0.004、0.004、0.003), with lower NSE, S-100β, MMP9 in PRO group (T1: P = 0.046、0.045、0.031; T2: P = 0.019、0.025、0.037); at T3, the these indexes of both groups reduced significantly, with no obvious inter-group difference (P > 0.05). Shown in Fig. 5.
Fig. 5.
Comparative analysis of neurological function indexes. (A) Comparison of NSE between PRO and SEV groups at various time points. (B) Comparison of S-100β between PRO and SEV groups at various time points. (C) Comparison of MMP9 between PRO and SEV groups at various time points. Notes: *P < 0.05 and **P < 0.01 vs. T0; aP < 0.05 vs. SEV group
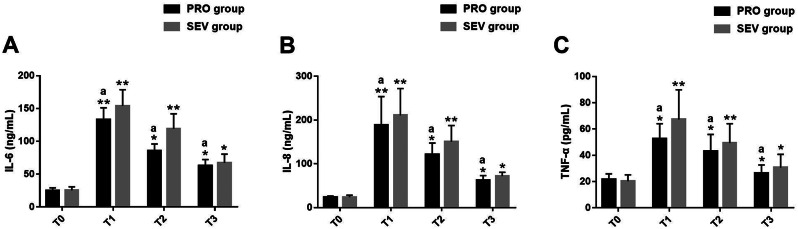
Comparative analysis of serum inflammatory indexes
Serum inflammatory indexes such as IL-6, IL-8 and TNF-α were measured at different time points. The two groups differed little in these inflammatory indexes at T0 (P > 0.05); all of them elevated significantly at T1 in both groups (PRO group: P = 0.006、0.002、0.033; SEV group: P = 0.009、0.005、0.003), but showed a gradual decrease at T2 (PRO group: P = 0.026、0.039、0.041; SEV group: P = 0.005、0.005、0.003) and T3 (PRO group: P = 0.046、0.044、0.031; SEV group: P = 0.028、0.029、0.033); lower levels of IL-6, IL-8 and TNF-α were determined in PRO group versus SEV group at T1-T3 (P < 0.05) (T1: P = 0.022、0.036、0.031; T2: P = 0.037、0.018、0.028; T3: P = 0.016、0.022、0.044).
Discussion
The main finding of this study is that among patients undergoing cardiac surgery and using CPB, those anesthetized with SEV performed better in terms of POCD incidence, cognitive recovery, and postoperative recovery time compared to propofol. Specifically, patients in the SEV group showed fewer cognitive impairment symptoms in a shorter period of time after surgery, and cognitive test results at 3 months after surgery showed that the cognitive function recovery rate of this group of patients was faster than that of the PRO group.
The risk of POCD in patients undergoing CS may also be affected by intraoperative trauma in addition to anesthesia [21, 22]. Moreover, the incidence of POCD was significantly higher in patients undergoing CS under CPB compared to other patients [23]. Therefore, the formulation of a reasonable anesthesia strategy will help to protect the cerebral blood oxygen supply and the central nervous system of patients, which is of great help to prevent POCD [24].
At present, the comparison of clinical advantages between SEV and PRO in surgical patients is still controversial. For example, Schoen J et al. [25] reported that SEV is more effective than PRO in promoting short-term postoperative cognitive recovery in patients undergoing CS. According to Zhang Y et al. [26], SEV is not as effective as PRO in preventing delayed neurocognitive recovery in elderly patients undergoing major cancer surgery. In our study, the anesthetic effects were evaluated in terms of CPB duration, anesthesia time, respiratory recovery time, and anesthesia recovery time. The PRO group was significantly shorter than the SEV group in all of the above indexes except the comparable CPB duration, suggesting that PRO has a better anesthetic effect for patients undergoing CS under CPB. Shortening anesthesia time and recovery times could positively impact patient outcomes by reducing the duration of exposure to anesthetic agents, potentially decreasing the risk of POCD. Faster recovery from anesthesia and respiratory function may also limit the time patients spend in a vulnerable post-anesthesia state, contributing to improved cognitive function and reducing the incidence of POCD in the PRO group [27].
MoCA evaluation of cognitive function found that MoCA in PRO group decreased significantly after intervention, but was still significantly higher compared with SEV group, suggesting that PRO has certain protective effect on cognitive function in patients undergoing CS with CPB. According to statistics, the incidence of POCD in patients undergoing CPB-assisted CS receiving PRO intervention was significantly lower (5.17% vs. 27.27%), suggesting that PRO can help prevent the occurrence of POCD and reduce the risk of POCD by 22.10%. Li S et al. [28] reported that the prevention of PRO against POCD may be related to the activation of AMP-dependent protein kinase (AMPK)/mammalian target of rapamycin (mTOR) pathway-mediated autophagy by Sirtuin 3 (SIRT3). It should be pointed out that this study mainly focuses on POCD, rather than postoperative delayed cognitive recovery, and the incidence of POCD is closely related to early postoperative anesthesia selection. Postoperative cognitive recovery is usually a long-term process that may gradually occur within a few months after surgery, and the outcome measures of this study focus on changes in cognitive function in the early postoperative period.
On the other hand, NSE, as a glycolytic enzyme, is expressed in neuronal cytoplasm, platelets, red blood cells and other environments, which can reflect neuronal damage and be used as an auxiliary predictor of POCD [29]. Given the unusually high levels of S-100β in POCD patients, it may help to indicate the occurrence of POCD in patients undergoing CS to some extent [30]. MMP9 is an endoprotease closely linked to the inflammatory reaction of the nervous system, which can reflect blood-brain barrier damage and brain tissue inflammation [31, 32]. After testing these neurological function indexes, it was found that NSE, S-100β, and MMP9 in PRO group were significantly increased at T1 and T2 compared with T0 but still lower than those in SEV group; while at T3, these indexes of both groups were equivalent to those at T0, with no statistical inter-group difference. It is suggested that PRO intervention in patients undergoing CS under CPB is beneficial to alleviate neurological damage and thus plays a certain role in protecting brain function. In the study of Tang S et al. [33], PRO intervention in patients undergoing CS under CPB obviously improved postoperative cognitive function, with markedly better inhibitory effects on NSE, S-100β and MMP9 than SEV, which is consistent with our findings. The quantification of serum inflammatory indicators showed that IL-6, IL-8, and TNF-α in PRO group were significantly higher at T1 versus T0; all these indexes dropped step by step at T2 and T3, with lower IL-6, IL-8 and TNF-α levels in PRO group compared with SEV group at all time points (except T0). It indicates that PRO intervention has obvious and more potent inhibitory effects than SEV on serum inflammation of patients undergoing CS under CPB. In terms of mechanism, Research has shown that an increase in inflammation may lead to apoptosis of hippocampal cells, resulting in POCD [34], and PRO may help to relatively reduce activated microglia in the hippocampus, significantly increase the expression of miR-223-3p, reduce inflammation, and thus reduce POCD [35]. Meanwhile, numerous studies show that, reduced incidence of cognitive decline associated with PRO use [36]. This indicates that SEV and higher cytokine levels may lead to a higher incidence of POCD. These findings suggest that the ability of PRO to regulate inflammatory responses may play a key role in reducing the incidence of POCD.
In this study, the interventions of SEV and PRO showed significant differences in postoperative cognitive outcomes, which may be partially explained by the neuroprotective effects observed through clinical biochemical markers measured in this study. The biomarkers of postoperative neuronal damage, including NSE, S-100 β, and MMP9, were elevated in both groups, indicating a certain degree of neuronal damage. However, compared with the SEV group, the PRO group showed significantly reduced levels of these biomarkers at 12 and 24 h postoperatively, indicating that propofol may provide greater protection against neuronal damage. The reduction of neuroinflammatory markers is associated with a lower incidence of POCD observed in the PRO group, supporting the hypothesis that neuronal damage and reduced inflammation contribute to better cognitive recovery. In addition, postoperative inflammatory markers such as IL-6, IL-8, and TNF - α were significantly increased in both groups, but the decrease was more significant in the PRO group. This suggests that the anti-inflammatory effect of propofol may further help reduce the risk of POCD, as excessive inflammation is associated with cognitive dysfunction. These findings suggest that PRO may have both neuroprotective and anti-inflammatory effects, which can help improve cognitive outcomes after CPB cardiac surgery. The combination of reducing neuronal damage and better controlling postoperative inflammation may play a key role in the observed decrease in POCD incidence. Further research investigating these biomarkers and their relationship with cognitive recovery can provide valuable insights into the potential mechanisms of action of different anesthetics.
This study has some limitations. Firstly, as a single center study with a small sample size, the external validity of the results may be limited. Secondly, the study mainly evaluated short-term cognitive function after surgery, lacking long-term follow-up data and failing to fully assess the long-term effects of POCD. In addition, the dosage of anesthetic drugs and other intraoperative interventions were not fully controlled, which may affect the research results. Furthermore, POCD and MoCA scores were not measured separately at 24 h and 72 h post-surgery, which could have provided more precise data on the immediate and early cognitive decline. The short follow-up period, limited to the immediate postoperative phase, is another limitation of the study, as it does not capture the long-term effects of POCD. In addition, cerebral oxygen saturation was not measured during surgery, which may provide more insights into the relationship between cerebral oxygenation and postoperative cognitive function. Future research should adopt a multi center, large sample design to further explore the long-term effects of anesthetic drugs on POCD, considering differences between different patient populations, and combining with cerebral oxygenation monitoring to evaluate their potential impact on cognitive outcomes.
In summary, PRO is significantly preventive against POCD in patients undergoing CS under CPB, with obvious anesthesia advantages in anesthesia time, respiratory recovery time and anesthesia recovery time, which can protect patients’ cognitive function, reduce nerve damage and inhibit serum inflammation. It has clinical advantages over SEV and can provide a better anesthesia choice for patients undergoing CS under CPB.
Acknowledgements
Not applicable.
Author contributions
NZ Substantial contributions to the conception or design of the work and drafting the work or revising it critically for important intellectual content; RQ and BL acquisition, analysis abd interpretation of data for the work; All autuors final approval of the version to be published; DZ agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This study was supported by the University-level scientific research project of Ningxia Medical University XM2018179.
Data availability
he simulation experiment data used to support the findings of this study are available from the corresponding author upon request.
Declarations
Ethical approval
This study was approved by the Ethics Committee of the General Hospital of Ningxia Medical University (Approval No.2018 − 271). Informed consent was obtained from all the participants. All methods were carried out in accordance with Declaration of Helsinki.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Butz M, Gerriets T, Sammer G, El-Shazly J, Tschernatsch M, Huttner HB, Braun T, Boening A, Mengden T, Choi YH, Schoenburg M, Juenemann M. Effects of postoperative cognitive training on neurocognitive decline after heart surgery: a randomized clinical trial. Eur J Cardiothorac Surg. 2022;62(5):ezac251. 10.1093/ejcts/ezac251. PMID: 35415742. [DOI] [PubMed]
- 2.Ntalouka MP, Arnaoutoglou E, Tzimas P. Postoperative cognitive disorders: an update. Hippokratia. 2018;22(4):147–54. PMID: 31695301; PMCID: PMC6825421. [PMC free article] [PubMed]
- 3.Rundshagen I. Postoperative cognitive dysfunction. Dtsch Arztebl Int. 2014;111(8):119–25. 10.3238/arztebl.2014.0119. PMID: 24622758; PMCID: PMC3959222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhushan S, Li Y, Huang X, Cheng H, Gao K, Xiao Z. Progress of research in postoperative cognitive dysfunction in cardiac surgery patients: A review article. Int J Surg. 2021;95:106163. 10.1016/j.ijsu.2021.106163. Epub 2021 Nov 4. PMID: 34743049. [DOI] [PubMed]
- 5.Shaefi S, Marcantonio ER, Mueller A, Banner-Goodspeed V, Robson SC, Spear K, Otterbein LE, O’Gara BP, Talmor DS, Subramaniam B. Intraoperative oxygen concentration and neurocognition after cardiac surgery: study protocol for a randomized controlled trial. Trials. 2017;18(1):600. 10.1186/s13063-017-2337-1. PMID: 29254495; PMCID: PMC5735533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasputytė G, Bukauskienė R, Širvinskas E, Lenkutis T, Vimantaitė R, Andrejaitienė J. Effects of Combined and General Anesthesia on Cognitive Functions for Patients Undergoing Cardiac Surgery Under CPB. Heart Surg Forum. 2021;24(4):E593-E597. 10.1532/hsf.3885. PMID: 34473033. [DOI] [PubMed]
- 7.Dong W, Li X, Wang X, Cheng X, Kong L, Guo Z, Jing H. Influence of Dexmedetomidine on Cognitive Function and Inflammatory Factors in Rats and Analysis of Its Molecular Mechanism after Cardiac Surgery under Cardiopulmonary Bypass. Cell Mol Biol (Noisy-le-grand). 2022;68(2):119–125. 10.14715/cmb/2022.68.2.17. PMID: 35869718. [DOI] [PubMed]
- 8.Yonekura H, Sumiyoshi M, Matsunari Y, Sakai M, Kamei M. Volatile Agents versus Propofol in Cardiac Surgery: Comment. Anesthesiology. 2021;134(1):131–132. 10.1097/ALN.0000000000003591. PMID: 33035291. [DOI] [PubMed]
- 9.Bonanni A, Signori A, Alicino C, Mannucci I, Grasso MA, Martinelli L, Deferrari G. Volatile Anesthetics versus Propofol for Cardiac Surgery with Cardiopulmonary Bypass: Meta-analysis of Randomized Trials. Anesthesiology. 2020;132(6):1429–1446. 10.1097/ALN.0000000000003236. PMID: 32205551. [DOI] [PubMed]
- 10.Wang Z, Wang Z, Wang A, Li J, Wang J, Yuan J, Wei X, Xing F, Zhang W, Xing N. The neuroprotective mechanism of sevoflurane in rats with traumatic brain injury via FGF2. J Neuroinflammation. 2022;19(1):51. 10.1186/s12974-021-02348-z. PMID: 35177106; PMCID: PMC8855620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao L, Gong H, Huang H, Tuerhong G, Xia H. Participation of mind Bomb-2 in Sevoflurane Anesthesia induces cognitive impairment in aged mice via modulating ferroptosis. ACS Chem Neurosci. 2021;12(13):2399–408. 10.1021/acschemneuro.1c00131. Epub 2021 Jun 13. PMID: 34121396. [DOI] [PubMed] [Google Scholar]
- 12.Gao XQ, Zhang ZY, Ma WH. [Effects of electroacupuncture assistant general anesthesia on postoperative cognitive dysfunction of aged patients]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012;32(5):591-3. Chinese. PMID: 22679714. [PubMed]
- 13.Gao X, Mi Y, Guo N, Luan J, Xu H, Hu Z, Wang N, Zhang D, Gou X, Xu L. The mechanism of propofol in cancer development: an updated review. Asia Pac J Clin Oncol. 2020;16(2):e3–11. 10.1111/ajco.13301. Epub 2020 Jan 22. PMID: 31970936. [DOI] [PubMed] [Google Scholar]
- 14.Stogiannou D, Protopapas A, Protopapas A, Tziomalos K. Is propofol the optimal sedative in gastrointestinal endoscopy? Acta Gastroenterol Belg. 2018;81(4):520–4. PMID: 30645922. [PubMed]
- 15.Nie Y, Li S, Yan T, Ma Y, Ni C, Wang H, Zheng H. Propofol Attenuates Isoflurane-Induced Neurotoxicity and Cognitive Impairment in Fetal and Offspring Mice. Anesth Analg. 2020;131(5):1616–1625. 10.1213/ANE.0000000000004955. PMID: 33079886. [DOI] [PubMed]
- 16.Glumac S, Kardum G, Sodic L, Supe-Domic D, Karanovic N. Effects of dexamethasone on early cognitive decline after cardiac surgery: A randomised controlled trial. Eur J Anaesthesiol. 2017;34(11):776–784. 10.1097/EJA.0000000000000647. PMID: 28985195. [DOI] [PubMed]
- 17.Zhuang YM, Xu JY, Zheng K, Zhang H. Research progress of postoperative cognitive dysfunction in cardiac surgery under cardiopulmonary bypass. Ibrain. 2023;10(3):290–304. 10.1002/ibra.12123. Published 2023 Aug 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kristek G, Radoš I, Kristek D et al. Influence of postoperative analgesia on systemic inflammatory response and postoperative cognitive dysfunction after femoral fractures surgery: a randomized controlled trial [published correction appears in Reg Anesth Pain Med. 2019;44(10):e2. doi: 10.1136/rapm-2018-000023corr1]. Reg Anesth Pain Med. 2019;44(1):59–68. 10.1136/rapm-2018-000023 [DOI] [PubMed]
- 19.Schoen J, Husemann L, Tiemeyer C et al. Cognitive function after sevoflurane- vs propofol-based anaesthesia for on-pump cardiac surgery: a randomized controlled trial. Br J Anaesth. 2011;106(6):840–850. 10.1093/bja/aer091. PMID: 21518736. [DOI] [PubMed]
- 20.Alexander Wahba M, Milojevic C, Boer, Filip MJJ, De Somer T, van den Gudbjartsson TJ, Jones V, Lomivorotov F, Merkle M, Ranucci G, Kunst L, Puis, February, EACTS/EACTA/EBCP Committee Reviewers., 2019 EACTS/EACTA/EBCP guidelines on cardiopulmonary bypass in adult cardiac surgery, European Journal of Cardio-Thoracic Surgery, Volume 57, Issue 2, 2020, Pages 210–251, doi.org: 10.1093/ejcts/ezz267 [DOI] [PubMed]
- 21.Lu B, Yuan H, Mo L, Sun D, Liu R, Zhou H, Zhai X, Wang R, Chen J, Meng B. Effects of different types of non-cardiac surgical trauma on hippocampus-dependent memory and neuroinflammation. Front Behav Neurosci. 2022;16:950093. 10.3389/fnbeh.2022.950093. PMID: 36035019; PMCID: PMC9399929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiberg S, Holmgaard F, Zetterberg H, Nilsson JC, Kjaergaard J, Wanscher M, Langkilde AR, Hassager C, Rasmussen LS, Blennow K, Vedel AG. Biomarkers of Cerebral Injury for Prediction of postoperative cognitive dysfunction in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2022;36(1):125–32. 10.1053/j.jvca.2021.05.016. Epub 2021 May 16. PMID: 34130895. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell SJ, Merry AF. Perspective on cerebral Microemboli in Cardiac surgery: significant problem or much ado about nothing? J Extra Corpor Technol. 2015;47(1):10–5. PMID: 26390674; PMCID: PMC4566815. [PMC free article] [PubMed] [Google Scholar]
- 24.Klamt JG, Vicente WVA, Garcia LV, Carmona F, Abrão J, Menardi AC, Manso PH. Neuroprotective anesthesia regimen and Intensive Management for Pediatric Cardiac surgery with cardiopulmonary bypass: a review and initial experience. Braz J Cardiovasc Surg. 2017 Nov-Dec;32(6):523–9. 10.21470/1678-9741-2016-0064. PMID: 29267616; PMCID: PMC5731303. [DOI] [PMC free article] [PubMed]
- 25.Schoen J, Husemann L, Tiemeyer C, Lueloh A, Sedemund-Adib B, Berger KU, Hueppe M, Heringlake M. Cognitive function after sevoflurane- vs propofol-based anaesthesia for on-pump cardiac surgery: a randomized controlled trial. Br J Anaesth. 2011;106(6):840–50. 10.1093/bja/aer091. Epub 2011 Apr 25. PMID: 21518736. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Shan GJ, Zhang YX, Cao SJ, Zhu SN, Li HJ, Ma D, Wang DX. First Study of Perioperative Organ Protection (SPOP1) investigators. Propofol compared with sevoflurane general anaesthesia is associated with decreased delayed neurocognitive recovery in older adults. Br J Anaesth. 2018;121(3):595–604. Epub 2018 Jul 27. PMID: 30115258. [DOI] [PubMed] [Google Scholar]
- 27.Arefayne NR, Berhe YW, van Zundert AA. Incidence and factors related to prolonged Postoperative Cognitive decline (POCD) in Elderly patients following surgery and anaesthesia: a systematic review. J Multidiscip Healthc. 2023;16:3405–13. 10.2147/JMDH.S431168. Published 2023 Nov 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li S, Zhou Y, Hu H, Wang X, Xu J, Bai C, Yuan J, Zhang D. SIRT3 Enhances the Protective Role of Propofol in Postoperative Cognitive Dysfunction via Activating Autophagy Mediated by AMPK/mTOR Pathway. Front Biosci (Landmark Ed). 2022;27(11):303. 10.31083/j.fbl2711303. PMID: 36472103. [DOI] [PubMed]
- 29.Zbóril S, Schmidt AP, Oses JP, Wiener CD, Portela LV, Souza DO, Auler JOC, Junior, Carmona MJC, Fugita MS, Flor PB, Cortopassi SRG. S100B protein and neuron-specific enolase as predictors of postoperative cognitive dysfunction in aged dogs: a case-control study. Vet Anaesth Analg. 2020;47(6):740–7. Epub 2020 Jul 7. PMID: 32800537. [DOI] [PubMed] [Google Scholar]
- 30.He X, Wen LJ, Cui C, Li DR, Teng JF. The significance of S100β protein on postoperative cognitive dysfunction in patients who underwent single valve replacement surgery under general anesthesia. Eur Rev Med Pharmacol Sci. 2017;21(9):2192–8. PMID: 28537663. [PubMed] [Google Scholar]
- 31.Choudhari OK, Rani A, Kampani G, Kaur C, Sengupta A. Matrix Metalloproteinase-9 gene polymorphism and its methylation in Stroke patients. Malays J Med Sci. 2021;28(6):32–41. 10.21315/mjms2021.28.6.4. Epub 2021 Dec 22. PMID: 35002488; PMCID: PMC8715877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li YJ, Wang ZH, Zhang B, Zhe X, Wang MJ, Shi ST, Bai J, Lin T, Guo CJ, Zhang SJ, Kong XL, Zuo X, Zhao H. Disruption of the blood-brain barrier after generalized tonic-clonic seizures correlates with cerebrospinal fluid MMP-9 levels. J Neuroinflammation. 2013;10:80. 10.1186/1742-2094-10-80. PMID: 23829879; PMCID: PMC3706217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang S, Huang W, Zhang K, Chen W, Xie T. Comparison of effects of propofol versus sevoflurane for patients undergoing cardiopulmonary bypass cardiac surgery. Pak J Med Sci. 2019 Jul-Aug;35(4):1072–5. 10.12669/pjms.35.4.1279. PMID: 31372145; PMCID: PMC6659056. [DOI] [PMC free article] [PubMed]
- 34.Liu C, Wu J, Li M et al. Smad7 in the hippocampus contributes to memory impairment in aged mice after anesthesia and surgery. J Neuroinflammation. 2023;20(1):175. Published 2023 Jul 28. 10.1186/s12974-023-02849-z [DOI] [PMC free article] [PubMed]
- 35.Lian F, Cao C, Deng F, Liu C, Zhou Z. Propofol alleviates postoperative cognitive dysfunction by inhibiting inflammation via up-regulating mir-223-3p in aged rats. Cytokine. 2022;150:155783. 10.1016/j.cyto.2021.155783. [DOI] [PubMed] [Google Scholar]
- 36.O’Bryan LJ, Atkins KJ, Lipszyc A, Scott DA, Silbert BS, Evered LA. Inflammatory biomarker levels after propofol or sevoflurane anesthesia: a Meta-analysis. Anesth Analg. 2022;134(1):69–81. 10.1213/ANE.0000000000005671. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
he simulation experiment data used to support the findings of this study are available from the corresponding author upon request.