Abstract
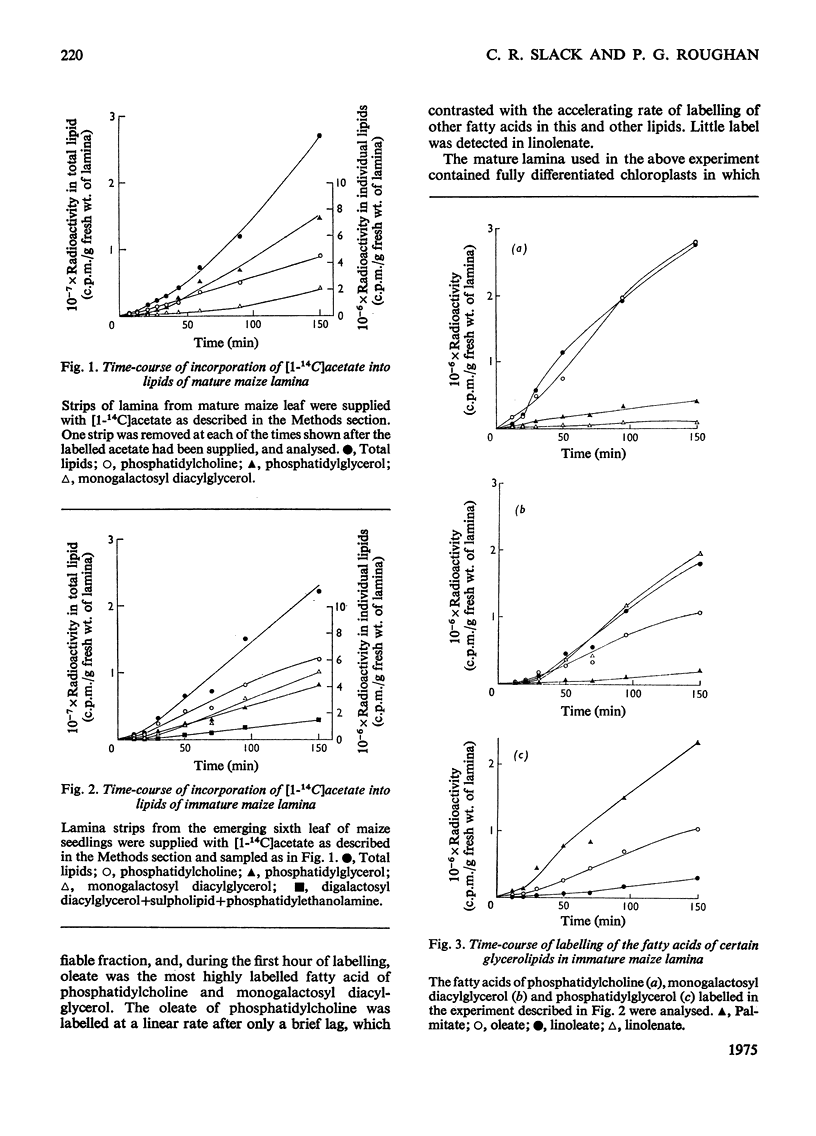
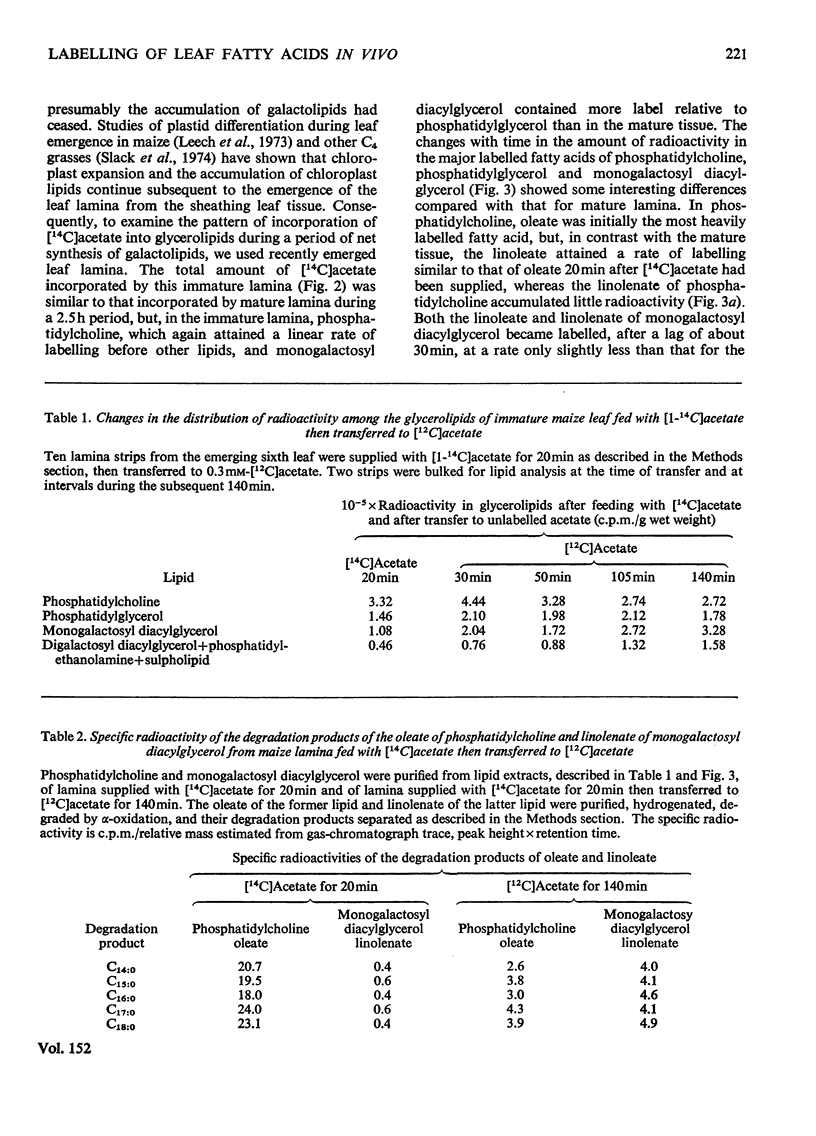
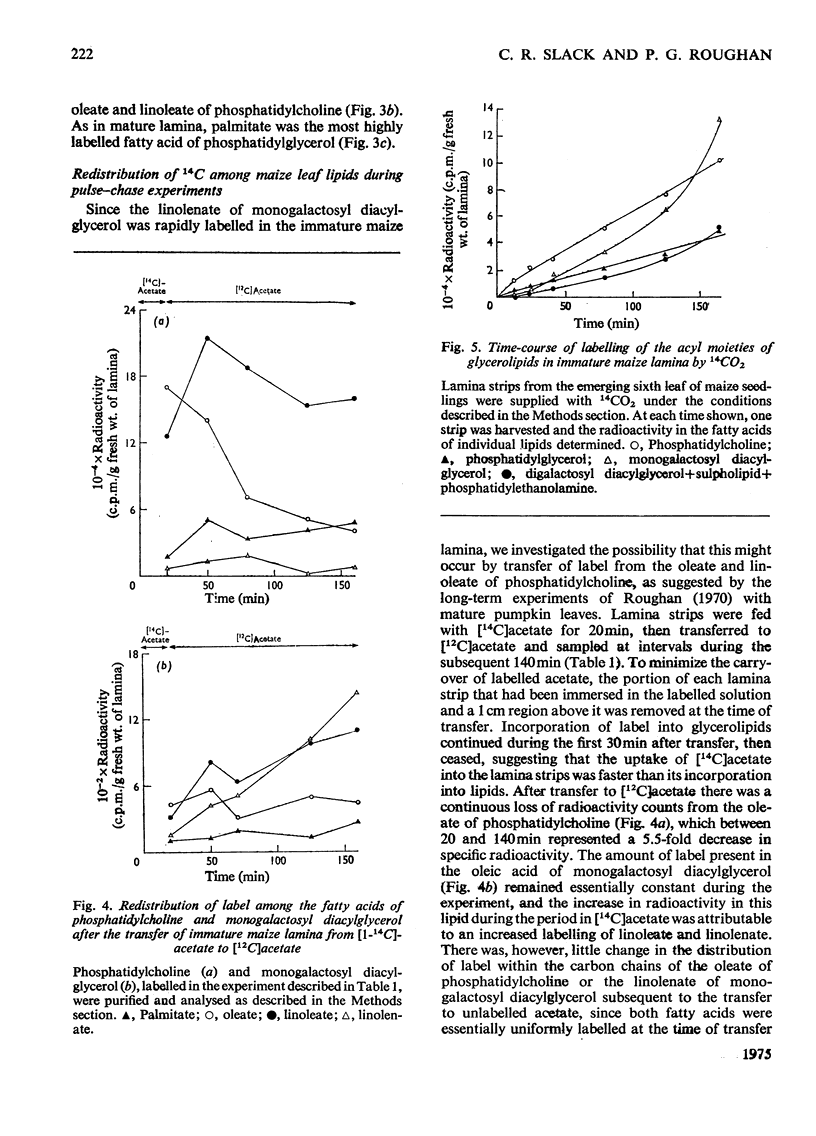
1. The patterns of incorporation of 14C into glycerolipid fatty acids of developing maize leaf lamina from supplied [1-14C]acetate and from 14CO2 during steady-state photosynthesis were similar. Oleate of phosphatidylcholine and palmitate of phosphatidylglycerol attained linear rates of labelling more rapidly than did other fatty acids, particularly the linoleate and linolenate of monogalactosyl diacylglycerol. 2. After the transfer of lamina from labelled to unlabelled acetate, there was a decrease in labelled oleate and linoleate of phosphatidylcholine and a concomitant increase in the amount of radioactivity in the linoleate and linolenate of monogalactosyl diacylglycerol. 3. The rapidly labelled phospholipids, phosphatidylcholine and phosphatidylglycerol, were shown by differential and sucrose-density-gradient centrifugation to be associated with different organelles, the former being mainly in a low-density membrane fraction, probably microsomal, and the latter mainly in chloroplasts. 4. During a 48h period after supplying spinach leaves with [14C]acetate, radioactivity was lost from the oleate of phosphatidylcholine present in fractions sedimented at 12000g and 105000g, and accumulated in the linolenate of monogalactosyl diacylglycerol of the chloroplast. 5. It is proposed that the phosphatidylcholine of some non-plastid membranes is intimately involved in the process of oleate desaturation and that this lipid serves as a donor of unsaturated C18 fatty acids to other lipids, principally monogalactosyl diacylglycerol, of the chloroplasts.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelqvist L. A. A simple and convenient procedure for the hydrogenation of lipids on the micro- and nanomole scale. J Lipid Res. 1972 Jan;13(1):146–148. [PubMed] [Google Scholar]
- Appleby R. S., Safford R., Nichols B. W. The involvement of lecithin and monogalactosyl diglyceride in linoleate synthesis by green and blue-green algae. Biochim Biophys Acta. 1971 Nov 5;248(2):205–211. doi: 10.1016/0005-2760(71)90008-7. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avron M., Gibbs M. Carbon dioxide fixation in the light and in the dark by isolated spinach chloroplasts. Plant Physiol. 1974 Feb;53(2):140–143. doi: 10.1104/pp.53.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N., Lynen F. Factors involved in fatty acyl CoA desaturation by fungal microsomes. The relative roles of acyl CoA and phospholipids as substrates. Eur J Biochem. 1971 Mar 11;19(2):200–210. doi: 10.1111/j.1432-1033.1971.tb01305.x. [DOI] [PubMed] [Google Scholar]
- Bartels C. T., James A. T., Nichols B. W. Metabolism of trans-3-hexadecenoic acid by Chlorella vulgaris and by lettuce leaf. Eur J Biochem. 1967 Dec;3(1):7–10. doi: 10.1111/j.1432-1033.1967.tb19492.x. [DOI] [PubMed] [Google Scholar]
- Douce R., Holtz R. B., Benson A. A. Isolation and properties of the envelope of spinach chloroplasts. J Biol Chem. 1973 Oct 25;248(20):7215–7222. [PubMed] [Google Scholar]
- Gurr M. I., Brawn P. The biosynthesis of polyunsaturated fatty acids by photosynthetic tissue. The composition of phosphatidyl choline species in Chlorella vulgaris during the formation of linoleic acid. Eur J Biochem. 1970 Nov;17(1):19–22. doi: 10.1111/j.1432-1033.1970.tb01126.x. [DOI] [PubMed] [Google Scholar]
- Gurr M. I., Robinson M. P., James A. T. The mechanism of formation of polyunsaturated fatty acids by photosynthetic tissue. The tight coupling of oleate desaturation with phospholipid synthesis in Chlorella vulgaris. Eur J Biochem. 1969 May 1;9(1):70–78. doi: 10.1111/j.1432-1033.1969.tb00577.x. [DOI] [PubMed] [Google Scholar]
- Harris R. V., James A. T. Linoleic and alpha-linolenic acid biosynthesis in plant leaves and green alga. Biochim Biophys Acta. 1965 Dec 2;106(3):456–464. doi: 10.1016/0005-2760(65)90062-7. [DOI] [PubMed] [Google Scholar]
- Harris R. V., James A. T. The fatty acid metabolism of Chlorella vulgaris. Biochim Biophys Acta. 1965 Dec 2;106(3):465–473. doi: 10.1016/0005-2760(65)90063-9. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. Photosynthesis by sugar-cane leaves. A new carboxylation reaction and the pathway of sugar formation. Biochem J. 1966 Oct;101(1):103–111. doi: 10.1042/bj1010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAMES A. T. The biosynthesis of long-chain saturated and unsaturated fatty acids in isolated plant leaves. Biochim Biophys Acta. 1963 Feb 19;70:9–19. doi: 10.1016/0006-3002(63)90714-5. [DOI] [PubMed] [Google Scholar]
- Jacobson B. S., Kannangara C. G., Stumpf P. K. The elongation of medium chain trienoic acids to -linolenic acid by a spinach chloroplast stroma system. Biochem Biophys Res Commun. 1973 Jun 19;52(4):1190–1198. doi: 10.1016/0006-291x(73)90626-8. [DOI] [PubMed] [Google Scholar]
- Kagawa T., Lord J. M., Beevers H. Lecithin synthesis during microbody biogenesis in watermelon cotyledons. Arch Biochem Biophys. 1975 Mar;167(1):45–53. doi: 10.1016/0003-9861(75)90439-7. [DOI] [PubMed] [Google Scholar]
- Kagawa T., Lord J. M., Beevers H. The origin and turnover of organelle membranes in castor bean endosperm. Plant Physiol. 1973 Jan;51(1):61–65. doi: 10.1104/pp.51.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannangara C. G., Jacobson B. S., Stumpf P. K. In vivo biosynthesis of -linolenic acid in plants. Biochem Biophys Res Commun. 1973 May 15;52(2):648–655. doi: 10.1016/0006-291x(73)90762-6. [DOI] [PubMed] [Google Scholar]
- Klenk E. The polyenoic fatty acid and the problem of essential fatty acids. Biochem Soc Symp. 1972;(35):41–48. [PubMed] [Google Scholar]
- Leech R. M., Rumsby M. G., Thomson W. W. Plastid differentiation, acyl lipid, and Fatty Acid changes in developing green maize leaves. Plant Physiol. 1973 Sep;52(3):240–245. doi: 10.1104/pp.52.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Beevers H. Intracellular distribution of enzymes of the cytidine diphosphate choline pathway in castor bean endosperm. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2429–2432. doi: 10.1073/pnas.69.9.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackender R. O., Leech R. M. The Galactolipid, Phospholipid, and Fatty Acid Composition of the Chloroplast Envelope Membranes of Vicia faba. L. Plant Physiol. 1974 Mar;53(3):496–502. doi: 10.1104/pp.53.3.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes A. C., Neyra C. A., Hageman R. H. Nitrite assimilation and amino nitrogen synthesis in isolated spinach chloroplasts. Plant Physiol. 1974 Mar;53(3):411–415. doi: 10.1104/pp.53.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris L. J. Separations of lipids by silver ion chromatography. J Lipid Res. 1966 Nov;7(6):717–732. [PubMed] [Google Scholar]
- Nichols B. W. Fatty acid metabolism in the chloroplast lipids of green and blue-green algae. Lipids. 1968 Jul;3(4):354–360. doi: 10.1007/BF02530939. [DOI] [PubMed] [Google Scholar]
- Nichols B. W., Harris P., James A. T. The biosynthesis of trans-delta-3-hexadecenoic acid by chlorella vulgaris. Biochem Biophys Res Commun. 1965 Dec 9;21(5):473–479. doi: 10.1016/0006-291x(65)90407-9. [DOI] [PubMed] [Google Scholar]
- Nichols B. W., James A. T., Breuer J. Interrelationships between fatty acid biosynthesis and acyl-lipid synthesis in Chlorella vulgaris. Biochem J. 1967 Aug;104(2):486–496. doi: 10.1042/bj1040486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J. S., Benson A. A. Isolation and fatty acid composition of the plant sulfolipid and galactolipids. J Lipid Res. 1964 Jul;5(3):432–434. [PubMed] [Google Scholar]
- Roughan P. G., Boardman N. K. Lipid Composition of Pea and Bean Leaves during Chloroplast Development. Plant Physiol. 1972 Jul;50(1):31–34. doi: 10.1104/pp.50.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G. Turnover of the glycerolipids of pumpkin leaves. The importence of phosphatidylcholine. Biochem J. 1970 Mar;117(1):1–8. doi: 10.1042/bj1170001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouser G., Kritchevsky G., Simon G., Nelson G. J. Quantitative analysis of brain and spinach leaf lipids employing silicic acid column chromatography and acetone for elution of glycolipids. Lipids. 1967 Jan;2(1):37–40. doi: 10.1007/BF02531998. [DOI] [PubMed] [Google Scholar]
- STUMPF P. K., JAMES A. T. The biosynthesis of long-chain fatty acids by lettuce chloroplast preparations. Biochim Biophys Acta. 1963 Feb 19;70:20–32. doi: 10.1016/0006-3002(63)90715-7. [DOI] [PubMed] [Google Scholar]
- Talamo B., Chang N., Bloch K. Desaturation of oleyl phospholipid to linoleyl phospholipid in Torulopsis utilis. J Biol Chem. 1973 Apr 25;248(8):2738–2742. [PubMed] [Google Scholar]


