Abstract
Sarcoidosis is a systemic granulomatous disease of unknown etiology, primarily affecting the lungs and the lymphatic system. Its diagnosis is challenging, and in many cases, it requires histopathological confirmation through the identification of non-caseating granulomas. The presented case illustrates its diagnostic complexity and highlights a rare, delayed complication associated with endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA). The patient developed a mediastinal abscess, a serious and uncommon post-procedural event, which likely resulted from microperforation of the bronchial wall during the aspiration. Symptoms developed several days after the procedure, with fever, chest pain, and signs of mediastinal infection. This case emphasizes the need for heightened clinical awareness and careful monitoring following EBUS-TBNA.
Keywords: endobronchial ultrasound (ebus), lymphadenopathy, mediastinal abscess, non-caseating granulomas, post-ebus complications, pulmonary inflammatory diseases, sarcoidosis
Introduction
Sarcoidosis is a chronic, systemic inflammatory disease characterized by the formation of non-caseating granulomas. It is more prevalent in melanodermic women under 50 years of age. Up to 60% of cases are asymptomatic and are diagnosed incidentally. Respiratory and constitutional symptoms are the most common among symptomatic patients, and extra-pulmonary involvement is frequent, affecting organs such as the eyes, liver, heart, nervous system, and kidneys. Usually, extra-hepatic gastrointestinal involvement is rare. Diagnosis typically requires the exclusion of other conditions, and in many cases, depends on histopathology to confirm the presence of granulomas [1,2].
Due to its variable and nonspecific presentation, sarcoidosis can be mistaken for other diseases, leading to a diagnostic delay. Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is a minimally invasive technique used for diagnosing lymph nodes and pulmonary tumors. When combined with real-time ultrasound, it improves accuracy, reduces risks, and is generally safer [3].
In this report, we describe a complex case that illustrates the need for a multidisciplinary approach, detailed diagnostic investigation, and the complications resulting from the EBUS-TBNA study.
Case presentation
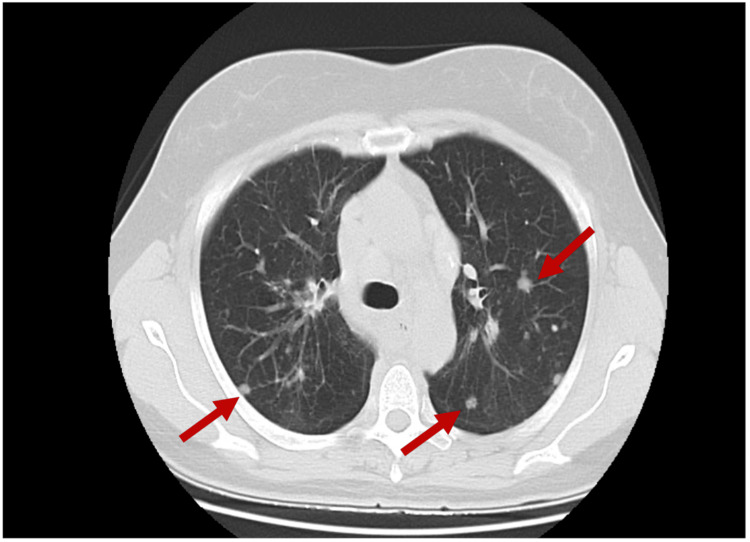
A 53-year-old female was referred to the Internal Medicine external consult with complaints of fatigue and dyspnea. Chest computed tomography (CT) revealed dispersed solid and subsolid pulmonary nodules in both lungs (Figure 1), associated with lymphadenopathy at the mediastinal and hilar levels. She had no significant medical history and wasn’t on any regular medication.
Figure 1. Chest CT (axial view) showing multiple solid and subsolid pulmonary nodules (red arrows).
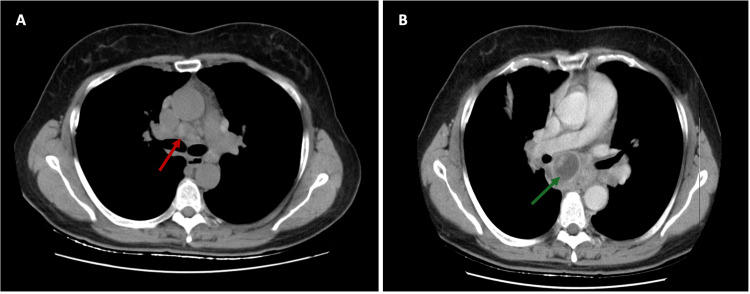
Complementary investigations including laboratory tests, serologies, angiotensin-converting enzyme (ACE), interferon-gamma release assay (IGRA), myeloperoxidase (MPO), proteinase 3 (PR3), and antinuclear antibodies (ANA) were carried out, all of which were negative. Transthoracic echocardiogram (TTE) was normal for her age, and a positron emission tomography (PET) scan was performed to exclude a neoplastic etiology. The PET scan showed lymph node activity and bilateral pulmonary parenchymal involvement, suggesting a possible inflammatory disease such as sarcoidosis or a lymphoproliferative disorder. Given the possibility of lymphoproliferative disease and with a subcarinal adenomegaly seen on CT (Figure 2A), an EBUS-TBNA was performed, and lymph nodes from station 7 (subcarinal) and 11L (left interlobar nodes) were sampled. Cytology and pathology reports did not reveal atypical cells or changes suggestive of granulomatous disease.
Figure 2. Chest CT images (axial view) showing (A) Subcarinal adenomegaly with 17 mm in its short axis before EBUS-TBNA (red arrow) and (B) Posterior mediastinal abscess after EBUS-TBNA (green arrow).
EBUS-TBNA: endobronchial ultrasound-guided transbronchial needle aspiration
One month after the EBUS-TBNA, the patient presented with food impaction, retrosternal pain, fever, and elevated inflammatory markers, leading to an emergency room visit. A CT scan revealed a posterior mediastinal abscess (Figure 2B).
Due to the severity of the finding, the case was discussed with the Cardiothoracic Surgery team. The patient underwent drainage of a mediastinal abscess located in the subcarinal region. A necrotic lymph node conglomerate with purulent content was identified and debrided. Biopsies were obtained from station 7 lymph nodes. Additionally, a wedge resection of the right upper lobe (RUL) was performed to remove multiple nodular lesions. Histopathological examination confirmed the diagnosis of sarcoidosis, showing non-necrotizing granulomas in both the pulmonary parenchyma and lymph nodes.
The patient was hospitalized for 14 days and initially started on empirical piperacillin-tazobactam 4.5 g IV every eight hours. Later, Streptococcus constellatus was isolated from the purulent material obtained in the subcarinal abscess cavity. The antibiotic therapy was continued as the organism was found to be sensitive to piperacillin-tazobactam. The patient showed subsequent clinical and analytical improvement. Upon discharge, the patient was prescribed oral amoxicillin-clavulanate 875 mg + 125 mg every 12 hours, to complete a two-week course.
After discharge, due to persistent fatigue with medium exertion and changes in pulmonary function tests, the decision was made to initiate treatment with oral prednisolone (20-40 mg/day) with gradual tapering over six to nine months. The patient underwent screening for extrapulmonary sarcoidosis, including liver function tests, blood urea nitrogen, creatinine, glucose, electrolytes, serum calcium, ECG, and TTE, and all were normal. No cutaneous abnormalities were noted, and an ophthalmologic examination revealed no intraocular inflammation.
Discussion
The incidence of sarcoidosis ranges from 2.3 to 17.8 per 100,000 people annually, depending on the region and patient cohort, making it a rare disease [4]. Its presentation is variable, often mimicking other conditions. It is a multisystem disease characterized by fatigue, memory loss, shortness of breath, cough, pain, and dizziness [5]. Diagnostic tests are often suggestive but not definitive. While the literature highlights difficulties in diagnosing sarcoidosis, prognosis depends on factors such as organ involvement, treatment response, ethnicity, and age [6].
There is no specific test for diagnosing sarcoidosis, requiring criteria such as clinical evidence, imaging, non-caseating granulomas on biopsy, and the exclusion of other conditions. The diagnostic process is complex and time-consuming, leading to delays in diagnosis [7].
In this clinical case, the diagnosis of sarcoidosis was fortuitously reached following a rare complication. EBUS-TBNA is a minimally invasive technique used to diagnose mediastinal and hilar lymph nodes, pulmonary tumors near the airways, and masses at the lung apex close to the trachea [8]. Using real-time ultrasound improves sampling accuracy and reduces the risk of bleeding, being relatively safe and effective. EBUS-TBNA has a low complication rate; however, as reported in this case, complications can occur, including infectious complications such as mediastinitis, as well as pericarditis, hemorrhage, and pneumothorax. The incidence of complications varies depending on the medical environment and the operator [9].
While pneumothorax and pneumomediastinum are typically treated conservatively [10], mediastinitis often requires prolonged hospitalization, antibiotic therapy, and sometimes surgical intervention [11]. Fatalities are rare, occurring in less than 1% of major studies [12,13].
After discharge, the patient began treatment with corticosteroids due to persistent fatigue and changes in pulmonary function tests. Treatment for sarcoidosis is indicated when associated with hypercalcemia, potential cardiac or neurological involvement, or severe symptoms such as organ dysfunction [14]. If no clinical improvement is evident, increasing the dose of prednisolone or initiating immunosuppressive therapy with methotrexate should be considered for disease control and to prevent long-term complications [15].
Conclusions
This case highlights the diagnostic challenges of sarcoidosis, with a more rapid diagnosis achieved following a rare and potentially fatal complication from EBUS-TBNA. Although this is a minimally invasive procedure associated with a low complication rate, severe complications such as mediastinitis have been reported. Given the growing use of EBUS-TBNA, it is crucial to establish an educational system to ensure its safe and effective performance and maintain vigilance for potential complications.
Disclosures
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Joana Castro Vieira, Mafalda Maria Santos, João Vieira Afonso, Mariana Simão de Magalhães, Ana Cristina Teotónio
Acquisition, analysis, or interpretation of data: Joana Castro Vieira
Drafting of the manuscript: Joana Castro Vieira
Critical review of the manuscript for important intellectual content: Joana Castro Vieira, Mafalda Maria Santos, João Vieira Afonso, Mariana Simão de Magalhães, Ana Cristina Teotónio
Supervision: Joana Castro Vieira, Mafalda Maria Santos, João Vieira Afonso, Mariana Simão de Magalhães, Ana Cristina Teotónio
References
- 1.Neurosarcoidosis: pathophysiology, diagnosis, and treatment. Bradshaw MJ, Pawate S, Koth LL, Cho TA, Gelfand JM. Neurol Neuroimmunol Neuroinflamm. 2021;8 doi: 10.1212/NXI.0000000000001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarcoidosis: causes, diagnosis, clinical features, and treatments. Jain R, Yadav D, Puranik N, Guleria R, Jin JO. J Clin Med. 2020;9:1081. doi: 10.3390/jcm9041081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The utility of endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal or hilar lymph node evaluation in extrathoracic malignancy: benign or malignant? Parmaksız ET, Caglayan B, Salepci B, Comert SS, Kiral N, Fidan A, Sarac G. Ann Thorac Med. 2012;7:210–214. doi: 10.4103/1817-1737.102171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epidemiology of sarcoidosis: current findings and future directions. Arkema EV, Cozier YC. Ther Adv Chronic Dis. 2018;9:227–240. doi: 10.1177/2040622318790197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The elusive sarcoidosis, an eight-year journey to the diagnosis of sarcoidosis: a case report. Vacaru A, Nguyen JP, Youn SJ, Lien D. Cureus. 2023;15:0. doi: 10.7759/cureus.39400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical manifestations, diagnosis, and treatment of sarcoidosis. Ungprasert P, Ryu JH, Matteson EL. Mayo Clin Proc Innov Qual Outcomes. 2019;3:358–375. doi: 10.1016/j.mayocpiqo.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diagnosis and management of sarcoidosis. Soto-Gomez N, Peters JI, Nambiar AM. https://pubmed.ncbi.nlm.nih.gov/27175719/ Am Fam Physician. 2016;93:840–848. [PubMed] [Google Scholar]
- 8.Steward M, Dickson C. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2023. Sonography endobronchial assessment, protocols, and interpretation. [Google Scholar]
- 9.Complications associated with endobronchial ultrasound-guided transbronchial needle aspiration: a nationwide survey by the Japan Society for Respiratory Endoscopy. Asano F, Aoe M, Ohsaki Y, et al. https://pmc.ncbi.nlm.nih.gov/articles/PMC3655828/ Respir Res. 2013;14:50. doi: 10.1186/1465-9921-14-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pulmonary sarcoidosis presenting with acute respiratory failure: a report of a case diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration on ventilation after intubation. Taniguchi J, Nakashima K, Ito H, et al. Intern Med. 2020;59:2291–2295. doi: 10.2169/internalmedicine.4624-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mediastinitis in the intensive care unit patient: a narrative review. Pastene B, Cassir N, Tankel J, Einav S, Fournier PE, Thomas P, Leone M. Clin Microbiol Infect. 2020;26:26–34. doi: 10.1016/j.cmi.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Linear endobronchial ultrasound in the era of personalized lung cancer diagnostics-a technical review. Oezkan F, Eisenmann S, Darwiche K, Gassa A, Carbone DP, Merritt RE, Kneuertz PJ. J Clin Med. 2021;10:5646. doi: 10.3390/jcm10235646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Recent advances in convex probe endobronchial ultrasound: a narrative review. Wu J, Wu C, Zhou C, Zheng W, Li P. Ann Transl Med. 2021;9:419. doi: 10.21037/atm-21-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King TF, Culver DA. UpToDate. Waltham, MA: UpToDate; [ Dec; 2024 ]. 2024. Treatment of pulmonary sarcoidosis: Initial approach. [Google Scholar]
- 15.ERS clinical practice guidelines on treatment of sarcoidosis. Baughman RP, Valeyre D, Korsten P, et al. Eur Respir J. 2021;58:2004079. doi: 10.1183/13993003.04079-2020. [DOI] [PubMed] [Google Scholar]