Abstract
The rough pen shell Pinna rudis Linnaeus, 1758 (family Pinnidae) is a mollusc with an Atlantic–Mediterranean distribution, typically inhabiting coarse sandy substrates. Habitat degradation is considered the primary cause of population decline, leading to the designation ‘Vulnerable’ in certain regions. In this study, we conducted a genetic analysis of populations of P. rudis from Cabo Verde and compared them with populations from the Mediterranean and Macaronesia. We based our analysis on two mitochondrial DNA markers, cytochrome oxidase I (COI) and 16S rRNA, and one nuclear marker, 28S rRNA. The results showed a strong genetic structuring among Macaronesia populations, with each island tending to have unique or exclusive haplotypes, though some sharing occurred between islands. We found significant genetic divergence between the population from Cabo Verde and the other sampled population, suggesting that P. rudis is not monotypic, but may include several cryptic species. Bayesian and maximum-likelihood phylogenetic analysis, including all Pinnidae species, indicated that P. rudis from Gorée Island (Senegal) and Baía das Gatas (São Vicente Island) might be undergoing speciation. The high genetic structure found for P. rudis could be influenced by hydrodynamic barriers, local currents and hydrographic isolation, in association with the short larval duration (planktotrophic) reported for this species. Altogether, our findings highlight significant genetic divergence in P. rudis populations, possibly supporting speciation events in the Cabo Verde archipelago among widely distributed taxonomic groups.
Keywords: Pinna rudis; Cabo Verde; Genetic analysis; mtDNA, nDNA; Macaronesia
Introduction
The study of genetic diversity and evolutionary dynamics of species and populations in oceanic islands is fundamental for understanding their capacity for local adaptation, response to environmental changes and long-term survival (Carson, 1983). Sea-level fluctuations can cause major extinctions or promote population expansion (Cunha et al., 2011), and in some cases, these changes may result in the emergence of new taxonomic groups (Booy et al., 2000; Zhong et al., 2016). Identifying genetic structure within populations, often resulting from habitat fragmentation and reduced gene flow, is a critical aspect of implementing effective conservation measures to preserve the genetic diversity of distinct genetic units (Lemer et al., 2014).
Speciation in volcanic islands often occurs through one or a few inter-island founders (Carson, 1983), or through connectivity among islands. The relationship between an island’s geological history and its biodiversity has intensified the research interest in oceanic archipelagos, focusing on taxonomy, systematics, phylogeny, biogeographic patterns and species evolution. In addition, these studies often serve to outline more specific management and conservation strategies for natural populations (Marko, 2004; Wares & Cunningham, 2005). These aspects are of particular relevance in the Cabo Verde archipelago, where the geological history has played a significant role in shaping biodiversity.
In the Cabo Verde archipelago, phylogenetic studies have addressed the evolution of some marine species, primarily using mtDNA data. Among these, the gastropod Conus L, 1758, has been well-studied and serves as a notable example of marine diversity in this insular environment (Cunha et al., 2005, 2008, 2014; Cunha, Grande & Zardoya, 2009). However, despite bivalves being the second most common group of molluscs found in the islands, their diversity remains largely unstudied. Notably, mostly sessile species like Pinna rudis L, 1758, (Family Pinnidae) and Brachidontes puniceus Gmelin, 1791, (Mytilidae) are widely reported across the islands, yet genetic studies of these species have been scarce (Cunha et al., 2011). For sessile organisms, habitat degradation can threaten survival by causing population fragmentation, and potentially result in the emergence of new species (Basso et al., 2015; Whittaker & Fernández-Palacios, 2007).
P. rudis (Fig. 1) is a widely distributed bivalve, found mainly in the Mediterranean (Zotou et al., 2023), North Atlantic (archipelagos of Azores, Madeira, Canary Islands and Cabo Verde), extending as far west as the Caribbean (Miloslavich et al., 2010) and as far south as Saint Helena and the Gulf of Guinea (Barea-Azcón, Ballesteros-Duperón & Moreno, 2008; WoRMS, 2022). It inhabits small sandy spots on rocky bottoms, and rock crevices at depths ranging from 0 m up to 60 m (García-March & Kersting, 2006; Sempere et al., 2006). The species is characterized by a shell reaching 25–30 cm, five to ten radial ribs and large spines, and vary in color (from brown to pink-orange). Like its sister species P. nobilis L, 1758, it is believed that P. rudis is a sequential hermaphrodite with asynchronous gamete maturation to avoid self-fertilization. Although there is scarce information about its planktonic larval stage, it is reported to last 5 to 10 days in the water column (Sanna et al., 2013). Such duration likely limits larval dispersal capacity and consequently reduces gene flow between distant populations.
Figure 1. Specimen of Pinna rudis in its natural habitat, observed in Porto Grande Bay, São Vicente, Cabo Verde.
Information about P. rudis populations, including their biology, ecology and distribution is still limited (Gvozdenović et al., 2019). However, P. rudis plays an important ecological role as a filter-feeder and is considered a good bio-indicator of environmental pollution, being more abundant in less degraded areas or with less intense anthropic influence. It also contributes to local biodiversity by providing a hard substrate for colonization of other benthic species (flora and fauna) (Basso et al., 2015; Lopes, Monteiro & Santos, 2020). Despite its ecological importance, molecular studies on P. rudis are rare. While mitochondrial DNA data have been published for some congeneric taxa (just “congeners” can do as well), e.g., P. bicolor and P. nobilis (Katsares et al., 2008; Catanese, Coupé & Bunet, 2022), studies of P. rudis remain limited.
Classification of taxa in Pinnidae is challenging due to the variability in morphological characteristics and the lack of consistent morphological data for reliable identification (Bieler & Mikkelsen, 2006). Genetic data suggest that the family may include cryptic species (Lemer et al., 2014), as the plasticity of the shell renders it of limited taxonomic value. Currently, Pinna species are often identified based on morphological characteristics, which is particularly challenging at the juvenile stage. While Pinna species are present in Cabo Verde, no genetic studies have been conducted in the region. As genomic data become more accessible, integrating diverse data types and improving analytical models will be crucial for accurate taxonomic identification, particularly for cryptic species. This knowledge is ultimately crucial for advancing biodiversity conservation efforts. For example, a pilot study in the Mediterranean, using genetic information, enabled the identification of hybrids between P. rudis and P. nobilis, which may have contributed to saving P. nobilis from extinction by introducing new variants in the genetic pool (Vázquez-Luis et al., 2021).
Given the wide distribution of P. rudis and its short larval stage, genetic structuring within Macaronesian populations is expected. Previous studies on molluscs have shown that long distances between suitable substrates and oceanographic barriers, such as currents and deep areas, between the islands may have driven the adaptive radiation of some mollusc species in Cabo Verde, e.g., Conus (Cunha et al., 2005, 2008, 2014; Cunha, Grande & Zardoya, 2009; Duda & Lessios, 2009; Duda & Rolán, 2005; Puillandre et al., 2014; Tenorio, Afonso & Rolán, 2008; Tenorio & Afonso, 2004), Fissurella (Cunha et al., 2017), Euthria (Fraussen & Swinnen, 2016) and Nudibranchia gastropods (Calado, Ortea & Caballer, 2005; Ortea-Rato & Espinosa, 1998; Ortea, 1989; Pola et al., 2014; Rolán, 2005; Wirtz, 2009).
To better understand the evolutionary history of P. rudis, its current conservation status, and its potential resilience to ongoing habitat loss, it is essential to investigate its genetic diversity. It is against this background that the present study aims to improve our understanding of the genetic differentiation between populations of P. rudis in Cabo Verde, by combining genetic and morphological data. Additionally, the study seeks to reconstruct phylogenetic relationships between P. rudis populations from the northeast Atlantic, Mediterranean and Macaronesian regions. To this end, we sequenced portions of two mitochondrial markers, cytochrome C oxidase subunit I (COI) and 16S rRNA, as well as one nuclear marker, 28S ribosomal rRNA.
The findings of this study have important implications for the conservation and management of P. rudis, particularly in regions facing increasing human pressure. Understanding the genetic structure and potential cryptic diversity within these populations is essential to develop effective conservation strategies and to preserve the species and the unique genetic heritage they represent. Moreover, this research may contribute to our understanding of the influence of isolation and environmental heterogeneity on genetic diversity and the phylogenetic relationships in marine invertebrates.
Methodology
Study area
Cabo Verde is an archipelago located 600 km from the West African coast (Fig. 2), at 14°50′-17°20′N and 22°40′-25°30′W. It comprises 10 volcanic islands, ranging in age from approximately 3 to 158 Mya. The archipelago is divided into three basic groups: the northwestern group (Santo Antão, São Vicente, Santa Luzia and São Nicolau), the southern group (Santiago, Fogo and Brava), and the eastern group (Sal, Boavista and Maio). Boavista Island is situated about 570 km off Senegal, on the west African coast. Cabo Verde has a coastline of ca. 2,000 km, and a land area of 4,033 km2 (Medina et al., 2007; Freitas et al., 2019).
Figure 2. Location of the Cabo Verde Archipelago and the study islands in black (Santo Antão, São Vicente and Santa Luzia).
The archipelago is characterized by a narrow shelf and deep waters, reaching depths of up to 4,000 m between the islands (Mason, Coombs & Oliveira, 2005). Oceanographically, Cabo Verde is influenced by the North Equatorial counter-current and the Canary Current. The sea surface temperatures normally exceed 20 °C throughout the year. Together with the Canary Islands, Azores and Madeira, Cabo Verde forms part of the biogeographical region known as Macaronesia (Peña-Izquierdo et al., 2012). However, long-term data and more refined analysis suggest that Cabo Verde has unique biogeographic characteristics (Freitas et al., 2019). Studies on certain marine groups in Cabo Verde have even suggested the exclusion of this archipelago from Macaronesia, instead classifying it as part of a West African biogeographic sub-province (Freitas et al., 2019).
Sample collection and processing
Data collection was performed by SCUBA-diving at 0.5–4 m depth. Samples were collected at the following sites: Santo Antão-Porto Novo (17°0′4604″N/25°5′579″W), São Vicente–Laginha (16°53′5506″N/24°59′3634″W) and Baía das Gatas (16°54′755″N/24°54′2168″W); and Santa Luzia Islands–Portinho (16°45′945″N/24°45′3073″W), under permission from Cabo Verde government (permits DNA_N°08/2017). P. rudis specimens collected (n = 20) were photographed using a Panasonic Lumix DMC-TZ20 digital camera. The samples were transported to the ISECMAR-UTA Molecular Biology Laboratory and kept in the cold freezer (−28 °C) in separate bags. Initially, the goal was to sample approximately 10 specimens per site. However, due to very low concentrations of the species in some places and considering the conservation status of the species (which is included in Annex II of the Bern Convention as strictly protected species, and in the Barcelona Convention as threatened or endangered marine species) (Nebot-Colomer et al., 2016), it was not possible to collect that number for some localities. The specimens used for genetic analysis were deposited in the collection of the Universidade Técnica do Atlântico, São Vicente, Cabo Verde.
Morphological analyses
Morphological characters were selected based on the taxonomic descriptions in Poutiers (2016) and Abbot & Dance (2000). The biometric data obtained from 32 shell samples, resulted in a data matrix of five parameters: maximum shell length-L, maximum shell width-Lt, total shell weight W, L/W ratio, and Lt/W ratio (Supplemental Material, Table S1). We investigated the morphological variation within P. rudis across the different Cabo Verde sampling sites. Statistical differences in the mean sizes among the four groups were analysed using a one-way ANOVA, followed by a Tukey’s honestly significant difference (HSD) post hoc test. The level of significant difference was at p < 0.05. Principal components analysis (PCA) was conducted on log-transforming shell measurements to identify morphometric characters with the most variation among populations. Finally, a discriminant analysis (DA) was conducted to differentiate the meristic parameters of the four populations. All morphological analyses were performed using PAST3 software (Hammer, Harper & Ryan, 2001).
DNA isolation and sequencing
DNA extraction was performed from a portion of the posterior adductor muscle. Tissue was taken from the inside of the muscle to avoid polysaccharides that may reduce the efficiency of DNA extraction (Panova et al., 2016). Tissue samples were stored in a 2-ml Eppendorf tube with 96% alcohol at −21 °C until DNA extraction. Genomic DNA was extracted following Sambrook & Russel (2001) using a sodium chloride protocol. Muscle tissue (100–250 mg) was excised and homogenized in extraction buffer (0.05 M Tris, 0.1 M EDTA, pH 7) with 70 µl of SDS and 14 µl proteinase K (10 ng per µl). The homogenates were incubated at 55 °C for 4 h, and DNA was precipitated with isopropanol. DNA pellets were washed with 70% ethanol, dried and suspended in 100 µl of water. Extraction products were visualized using 1% agarose gel electrophoresis stained with Sybr Green. Samples were then stored at 4 °C. DNA concentration was estimated using a spectrophotometer. Samples with low DNA concentrations (n = 12) were reprocessed using the EZNA Tissue DNA Kit (Omega Bio-Tek) according to the manufacturer’s instructions.
Fragments of the two mitochondrial—16S (490 bp) and COI (644 bp)—and one nuclear marker—a fragment of 28S rRNA (ca. 2Kb)—were amplified using a polymerase chain reaction (PCR) with primers showing a certain level of polymorphism within the used specimens, according to Lemer et al. (2014, 2016) and Vázquez-Luis et al. (2021). (See Table 1 for complete primer information). In some cases the primers used to amplify the COI fragment were unsuccessful, probably due to the presence of polysaccharides (Bessetti, 2007). Therefore, a second primer pair jgHCO-2198 and jgLCO-1490 (Geller et al., 2013) was used for those cases. PCR was performed in a 25-µL solution containing: 1X PCR buffer, 25 mM of dNTP, 0.5 mM of each primer, 2.5 mM of MgCl2, 2 μl of template DNA, 01 μg/μl BSA and 0.3 U of Platinum Taq DNA Polymerase (Invitrogen, Carlsbad, CA, USA). Reactions were carried out using the following cycling parameters: initial denaturing at 94 °C for 5 min, 45 cycles of 94 °C for 45 s, 48–50 °C (16S rRNA), 48–55 °C (COI), or 52 °C (28S rRNA), for 1 min, and 72 °C for 90 s and a final extension at 72 °C for 10 min double-stranded.
Table 1. Primers sequences used in this study and the annealing temperature at which successful amplifications were performed.
| Gene | Primer name | Sequence (5′ – 3′) | Annealing temperature (°C) | Source |
|---|---|---|---|---|
| 16S | 16Sar | CGCCTGTTTATCAAAAACAT | 48–50 | Palumbi (1996) |
| 16Sbr | CCGGTCTGAACTCAGATCACGT | 48–50 | Palumbi (1996) | |
| COI | HCO-2198 | TAAACTTCAGGGTGACCAAAAAATCA | 48–49 | Folmer et al. (1994) |
| LCO-1490 | GGTCAACAAATCATAAAGATATTGGGG | 48–49 | Folmer et al. (1994) | |
| jgHCO2198 | TAIACYTCIGGRTGICCRAARAAYCA | 50–55 | Geller et al. (2013) | |
| jgLCO1490 | TITCIACIAAYCAYAARGAYATTGG | 50–55 | Geller et al. (2013) | |
| 28S | 28Sa | GAC CCGTCTTGAAACACGGA | 52 | Whiting et al. (1997) |
| 28Srdb | CCACAGCGCCAGTTCTGCTTAC | 52 | Whiting et al. (1997) | |
| 28Srd1a | CCCSCGTAAYTTAGG CATAT | 52 | Edgecombe & Giribet (2006) | |
| 28Srd4b | CCTTGGTCC GTG TTTCAAGAC | 52 | Edgecombe & Giribet (2006) | |
| 28Srd4.8a | ACCTATTCTCAAACTTTA ATGG | 52 | Schwendinger & Giribet (2005) | |
| 28Srd7b1 | GACTTCCCTTAC TACAT | 52 | Schwendinger & Giribet (2005) |
PCR products were visualized using 1% agarose gel electrophoresis stained with Sybr Green. Approximately 10 ng of the purified product was used as a template for sequencing, performed in an automated sequencer (ABI PRISM 3700; Applied Biosystems, Foster City, CA, USA) using the BigDye® Terminator v31 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA), following the manufacturer’s instructions.
Sequence data
A manual check of misreads in COI, 16S rRNA and 28S rRNA chromatograms was performed with Bioedit v7053 for Windows (Hall, 1999). Taxonomic confirmation was done through a BLAST search (National Center for Biotechnology Information, 2023). The translation of COI was inferred using the invertebrate mtDNA genetic code in Unipro UGENE version 129 (Okonechnikov et al., 2012). Multiple sequence alignments were performed with the Clustal W algorithm present in BioEdit, with a gap opening penalty of 10,000 and a gap extension penalty of 0,05. The alignments obtained were optimized manually when needed. Regions of poor alignment within the ribosomal RNA genes, typically unpaired loops and bulges of varying sizes bounded by stem regions, were excluded from the phylogenetic analyses using GBlocks Server v091b (Castresana, 2000). The haplotypes (mtDNA) were determined separately and from concatenated (1,073 bp) sequences from the two regions of the mitochondrial gene encoding the COI and 16S rRNA fragments.
Phylogenetic analysis
For the phylogenetic analyses, additional COI, 16S rRNA, and 28S rRNA sequences were downloaded from GenBank for the nine species of the Pinnidae family (P. rudis, P. carnea Gmelin, 1791, P. atropurpurea G B Sowerby I, 1825, P. bicolor Gmelin, 1791, P. dolabrata Lamarck, 1819, P. muricata L, 1758, P. nobilis, P. trigonalis Pease, 1861 and P. epica Jousseaume, 1894) and the outgroup Streptopinna saccata (L, 1758). The genus Streptopinna was chosen as an outgroup based on its proximity to the genus Pinna according to Lemer et al. (2014). The sequences used in gene tree reconstruction are detailed in Table 2.
Table 2. Sequences of mitochondrial COI, 16S and nuclear 28S genes of Pinnidae species used in the genetic analysis.
The 20 new sequences from Cabo Verde were the only ones obtained in this study and the GenBank accession numbers are presented here for each marker. The total number of specimens per species is given as well as the location where it was found and the GenBank accession numbers for each. Streptopinna saccata species was included in the species tree reconstruction as outgroup.
| Taxon | Location 1 | Location 2 | Region | N | ID | Specimens | 16S rRNA | COI | 28S rRNA |
|---|---|---|---|---|---|---|---|---|---|
| Pinna rudis Linnaeus, 1774 | Cabo Verde–Santo Antão | Porto Novo | Atlantic Ocean | 6 | VA1 | PRUD (VA1, 1–6) | OQ055700–OQ055705 |
OQ026776
OQ026781 |
OQ026797
OQ026802 |
| Cabo Verde–São Vicente | Laginha | 6 | VV1 | PRUD (VV1, 1–6) | OQ05576–OQ055711 | OQ026782–OQ026787 | OQ026803–OQ026808 | ||
| Baía das Gatas | 5 | VV2 | PRUD (VV2, 1–5) | OQ055712–OQ055716 | OQ026788–OQ026792 | OQ026809–OQ026813 | |||
| Cabo Verde–Santa Luzia | Portinho | 3 | VL1 | PRUD (VL1, 1–3) | OQ055717–OQ055719 | OQ026793–OQ026795 | OQ026814–OQ026816 | ||
| Spain | Murcia | Mediterranean Sea | 1 1 |
Pr | Pr13 | – | KJ366482 | KJ366289 | |
| Pr | Pr15 | KJ365726 | KJ366483 | KJ366291 | |||||
| Columbrete island | Mediterranean Sea | 1 | Pr | Pr44 | KJ365731 | – | KJ366298 | ||
| Canarias island | El Hierro | Atlantic Ocean | 1 | Pr | Pr24 | KJ365727 | KJ366484 | KJ366292 | |
| 1 | Pr | Pr25 | KJ365728 | KJ366485 | KJ366293 | ||||
| 1 | Pr | Pr28 | KJ365729 | KJ366486 | KJ366294 | ||||
| 1 | Pr | Pr29 | – | KJ366487 | KJ366295 | ||||
| Tenerife | Atlantic Ocean | 1 | Pr | Pr40 | KJ365730 | – | KJ366296 | ||
| 1 | Pr | Pr42 | – | KJ366488 | KJ366297 | ||||
| 1 | Pr | Pr57 | KJ365733 | KJ366489 | KJ366301 | ||||
| 1 | Pr | Pr58 | KJ365734 | KJ366490 | KJ366302 | ||||
| 1 | Pr | Pr59 | – | – | KJ366303 | ||||
| Fuerteventura | Atlantic Ocean | 1 | Pr | Pr56 | KJ365732 | – | KJ366300 | ||
| Azores | São Miguel | Atlantic Ocean | 1 | AZ1 | MZ USP 114025 | KJ365520 | KJ366319 | KJ366026 | |
| 1 | AZ2 | MZ USP 114038-1 | KJ365521 | KJ366320 | KJ366027 | ||||
| 1 | AZ3 | MZ USP 114038-2 | KJ365522 | KJ366321 | KJ366028 | ||||
| Senegal | Gorée island | Atlantic Ocean | 1 | SN1 | MNHN IM-2013-7009 | KJ365642 | – | KJ366172 | |
| 1 | SN2 | MNHN IM-2013-7010 | – | – | KJ366181 | ||||
| Pinna carnea Gmelin, 1791 | Panama | Bocas del Toro | Atlantic Ocean | 1 | PA1 | MCZ MAL-381150 | KJ365533 | KJ366336 | KJ366044 |
| Florida | Long Key Channe | Atlantic Ocean | 1 | FL1 | UF 437518 | KJ365608 | KJ366396 | KJ366126 | |
| French Antilles | Guadeloupe | Atlantic Ocean | 1 | FA1 | MNHN IM-2013-7108 | KJ365634 | KJ366408 | KJ366157 | |
| Pinna atropurpurea Sowerby I, 1825 | Japan | Okinawa | Pacific Ocean | 1 | JA1 | UF 351968C | KJ365595 | – | KJ366112 |
| Philippines | Mactan island | Pacific Ocean | 1 | PH1 | FLMNH_MO_349489 | KJ365632 | – | KJ366154 | |
| 1 | PH2 | MCZ MAL-381081 | KJ365564 | KU987199 | KJ366080 | ||||
| Pinna bicolor Gmelin, 1791 | Oman | Bar Al Hikman Peninsula | Red Sea | 1 | OM1 | UF_367994 | KJ365599 | – | KJ366116 |
| Mozambique | Inhaca island | Indian Ocean | 1 | MO1 | MNHN IM-2013-7077 | KJ365682 | – | KJ366225 | |
| Indian Ocean | 1 | MO2 | MNHN IM-2013-7078 | KJ365691 | – | KJ366234 | |||
| China | southern China coast | Pacific Ocean | 1 | PB | PB1 | JN182725 | |||
| Pinna dolabrata Lamarck, 1819 | Australia | Woody island | Pacific Ocean | 1 | AU1 | MNHN IM-2013-7088 | KJ365700 | – | KJ366246 |
| 1 | AU2 | MNHN IM-2013-7087 | KJ366236 | ||||||
| Heron island | Pacific Ocean | 1 | AU3 | UF 437384 | KJ365590 | – | KJ366107 | ||
| Pinna muricata Linnaeus, 1758 | Madagascar | Nosy be | Indian Ocean | 1 | MA1 | UF 423478_a | KJ365579 | KJ366375 | KJ366095 |
| Djibouti | Red Sea | 1 | DJ1 | UF 455816 | KJ365585 | KJ366381 | KJ366102 | ||
| Guam | Pago bay | Pacific Ocean | 1 | GU1 | MO_ 298856 | KJ365627 | KJ366402 | KJ366148 | |
| Pinna nobilis Linnaeus, 1758 | Spain | Columbretes | Mediterranean Sea | 1 | Pn | Pn8 | KJ365725 | – | KJ366286 |
| Palma de Mallorca | Mediterranean Sea | 1 | Pn | Pn68 | KJ365721 | – | KJ366281 | ||
| 1 | ML4 | mallorca4 | – | KY321774 | – | ||||
| Múrcia | Mediterranean Sea | 1 | ML16 | murcia16 | – | KY321811 | – | ||
| Pinna trigonalis W. H. Pease, 1861 | French Polynesia | Marquesas islands | Pacific Ocean | 1 | FP1 | MNHN IM-2013-7035 | KJ365661 | KJ366434 | KJ366198 |
| 1 | FP2 | MNHN IM-2013-7038 | KJ365687 | KJ366457 | KJ366230 | ||||
| 1 | FP3 | MNHN IM-2013-7045 | KJ365679 | KJ366451 | KJ366221 | ||||
|
Pinna (Abyssopinna) epica Jousseaume, 1894 |
Papua New Guinea | Pacific Ocean | 1 | PG1 | MNHN IM-2013-7084 | KJ365667 | KJ366440 | KJ366205 | |
| New Caledonia | South of Grande Terre | 1 | nC1 | MNHN IM-2013-7116 | KJ365639 | KJ366414 | KJ366165 | ||
| New Caledonia | North of Grande Terre | 1 | nC2 | MNHN IM-2013-7023 | KJ365694 | KJ366462 | KJ366239 | ||
| Streptopinna saccata (Linnaeus, 1758) | Hawaii | French Frigate Shoals | Pacific Ocean | 1 | Ssaca1 | UF 413844 | KJ365601 | KJ366391 | KJ366119 |
| French Polynesia | Tahiti | 1 | Ssaca2 | MCZ MAL-381010 | KJ365742 | KJ366495 | KJ366314 |
A total of three databases were created for each marker, including the sequences from Genbank. To compare the relationships of P. rudis haplotypes between the Atlantic and Mediterranean, maximum parsimony networks (MP) analyses of mtDNA and nDNA were performed independently, using the statistical parsimony procedure in TCS121 (Clement, Posada & Crandall, 2000). The non-rooted haplotype networks based on the 95% parsimony were constructed separately for mitochondrial and nuclear markers. The results were visualized with tcsBU (Santos et al., 2015), treating gaps as a 5th state for the nDNA.
A second phylogenetic analysis was conducted using maximum likelihood estimation (ML), Bayesian (BI) and BEAST inference to reconstruct the phylogenetic tree of Pinna species. The best-fit nucleotide substitution model was identified using PartitionFinder v111 (Lanfear et al., 2012), according to Akaike’s (1973) Information Criterion. Codon partitioning was applied to COI to minimize the effects of codon position saturation (Salemi, 2009) and to account for different rates of evolution of each codon (Pond, Poon & Frost, 2009). The best common models of nucleotide substitution selected and implemented in MrBayes, RAXML and BEAST were: COI partitions: TRN + G (1st partition), GTR + X (2nd and 3rd partitions); 16S rRNA and 28S rRNA partitions: GTR + I + G (for all partitions in BEAST).
For BI analyses in MrBayes v326 software (Ronquist et al., 2012), five million generations were run with sampling every 1,000 generations, discarding the first 25% of generations as burn-in. Parameter convergence was checked using Tracer v17 (Rambaut & Drummond, 2013; Rambaut et al., 2018), ensuring ESS values > 100. A final Bayesian majority-rule consensus tree was obtained for each data set. ML analyses were performed with RAxML v8116 (Stamatakis, 2006) using a rapid hill-climbing algorithm and 10,000 bootstrap pseudoreplicates. BEAST version 2.2.1 (Bouckaert et al., 2014) was used to estimate the species tree, with a 5,000 burn-in (corresponding to 10% of the total samples in each run). Results were checked in TRACER v15 (Rambaut & Drummond, 2013) to determine adequate burn-in. The convergence and ESS were also assessed with Tracer after discarding the burn-in samples. The final species tree was plotted with Tree Annotator v245 (http://tree.bio.ed.ac.uk/software/beast/). Consensus trees were visualized in FigTree version 143 (Rambaut, 2012). Final modifications, such as the insertion of posterior values and branch colouring, were performed with Inkscape version 1.0 (https://www.inkscape.org/).
Results
Morphological analyses
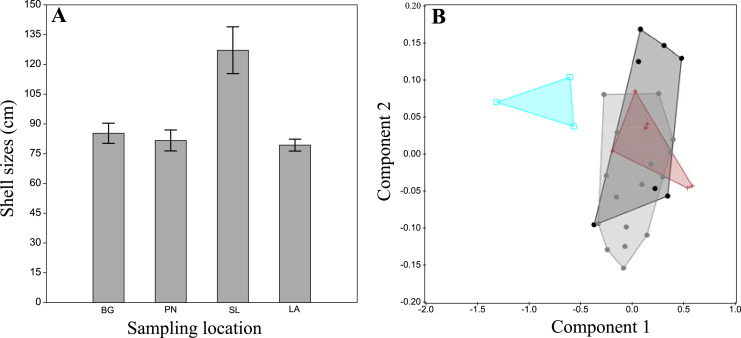
The examination of morphological variation within P. rudis across different Cabo Verde sampling sites revealed no distinct patterns in shell morphology, colour and shape of the adductor muscle impressions. Size of the 32 specimens analysed ranged from 124.5 cm in Porto Novo to 309 cm in Portinho (Fig. 3A). Mean sizes (±SEM) were highest in Portinho (mean = 254.16 ± 27.42 cm), followed by Baía das Gatas (mean = 177.4 ± 10.50 cm), Laginha (mean = 163.09 ± 4.57 cm), and lowest in Porto Novo (mean = 158.66 ± 11.47 cm). A one-way ANOVA indicates statistically significant differences in mean size (F = 11.67, p < 0.005) between the Santa Luzia and others tree groups. The differences in means were not significant (p > 0.05) between the São Vicente and Santo Antão groups.
Figure 3. Morphological analysis of P. rudis specimens in the laboratory.
(A) A plot of the results of average sizes (with error bar) and (B) the Principal Components Analysis result of the Pinna rudis specimen’s measurements from Cabo Verde. Legends: black circles-Baía das Gatas (BG), grey circles-Laginha (LA), blue squares-Portinho (PT) and red crosses-Porto Novo (PN).
PCA analysis and Discriminant Analysis (DA) did not reveal any separation between the two clades of P. rudis from Cabo Verde sampling sites (Fig. 3B). However, a distinction was noted for samples from Santa Luzia, likely related to the overall sample size. Principal components 1 and 2 accounted for 91.03% of the variation among the samples.
Sequenced data
A total of 65 mitochondrial DNA sequences of P. rudis were analysed in this study. These comprised 40 sequences generated in this study (20 COI and 20 16S rRNA sequences), and additional sequences obtained from online databases (12 COI and 13 16S rRNA sequences). For the 28S rRNA gene, 38 sequences were analysed, including 20 new sequence samples and 18 from previous studies. The final concantenated alignments for COI, 16S rRNA and 28S rRNA genes totalized 3,444 bp. No stop codons were found at least in one reading frame of the COI alignment. BLAST results confirmed the identification of P. rudis specimens. The sequences obtained in this study have been deposited in GenBank (OQ055700–OQ055719, OQ026776–OQ026795, OQ026797–OQ026816. See Table 2 for sequence details).
Haplotype network reconstruction
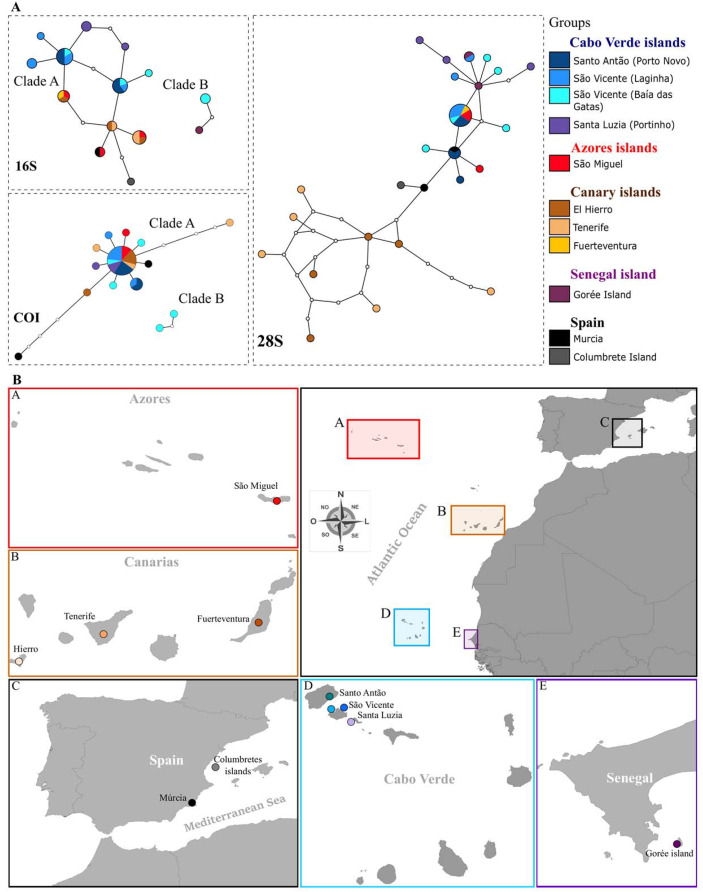
The genetic structure of P. rudis populations across Macaronesia was inferred from 103 mitochondrial and nuclear DNA sequences from the archipelagos of the Azores, Madeira, Canaries, Cabo Verde and the Mediterranean (Table 2). Statistical parsimony networks for COI, 16S rRNA and 28S rRNA haplotypes are shown in Figs. 4A and 4B. Moderate diversity values were observed for both mtDNA and nDNA, with the highest diversity found in the 28S rRNA (see Table 3 for more detail).
Figure 4. Haplotype networks and Map of sampling localities.
(A) Haplotype networks (95% parsimony connection limit) for all sequenced P. rudis COI, 16S and 28S data. Lines connecting white dots represent mutation steps between haplotypes, while white dots represent theoretical intermediate haplotypes. (B) Map of sampling localities included in the present work: A-Azores islands, B-Canary Islands, C-Spain, D–Cabo Verde islands, E-Gorée Island.
Table 3. Genetic diversity indices for Pinna rudis in each sampling site.
| Sampling places | mtDNA (16S, 470 pb) | mtDNA (COI, 594 pb) | nDNA (28S, 2,079 pb) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | h | Hd ± SD | π ± SD | k | N | S | Hd ± SD | π ± SD | k | N | h | Hd ± SD | π ± SD | k | |
| Cabo Verde islands | 20 | 8 | 0.853 ± 0.052 | 0.010 ± 0.004 | 5.07 | 20 | 8 | 0.695 ± 0.108 | 0.018 ± 0.010 | 10.82 | 20 | 11 | 0.763 ± 0.103 | 0.002 ± 0.001 | 4.41 |
| Spain | 2 | 2 | 1.000 ± 0.500 | 0.006 ± 0.003 | 3.00 | 2 | 2 | 1.000 ± 0.500 | 0.010 ± 0.005 | 6.00 | 3 | 3 | 1.000 ± 0.272 | 0.001 ± 0.001 | 1.33 |
| Canarias islands | 7 | 3 | 0.762 ± 0.115 | 0.003 ± 0.001 | 1.52 | 7 | 4 | 0.714 ± 0.181 | 0.003 ± 0.001 | 1.71 | 10 | 10 | 1.000 ± 0.045 | 0.004 ± 0.001 | 5.44 |
| Azores | 3 | 3 | 1.000 ± 0.272 | 0.006 ± 0.002 | 2.67 | 3 | 2 | 0.667 ± 0.314 | 0.001 ± 0.001 | 0.67 | 3 | 2 | 0.667 ± 0.314 | 0.001 ± 0.000 | 1.33 |
| Senegal | 1 | 1 | – | - | – | 0 | – | – | – | – | 2 | 2 | 1.000 ± 0.500 | 0.002 ± 0.001 | 1.00 |
| Total/average | 33 | 11 | 0.866 ± 0.035 | 0.010 ± 0.003 | 4.89 | 32 | 14 | 0.720 ± 0.087 | 0.012 ± 0.007 | 7.69 | 38 | 15 | 0.693 ± 0.092 | 0.005 ± 0.001 | 2.54 |
Note:
The number of individuals used for mitochondrial and nuclear (N), number of haplotypes (h), haplotype diversity (Hd), nucleotide diversity (π), standard deviation (SD) (shown in italic), and mean of pairwise differences (k) are shown for all locality.
For COI, the species’ haplotype diversity (h) was 0.720 (+ 0.087), corresponding to 14 haplotypes, and nucleotide diversity (π) was 0.012 (+ 0.007). The COI network revealed two poorly structured networks: one star-shaped and largely shared, the other more localized, including samples from Baía das Gatas and São Vicente Islands (Fig. 4A). For 16 rRNA, haplotypic diversity was also high (h = 0.866 ± 0.035) and nucleotide diversity was low (π 0.010 ± 0.003). In this marker, all Cabo Verde haplotypes were unique to the archipelago, highlighting the separation of these populations from the rest. Notably, Santa Luzia’s haplotypes were not shared with any other locality, further suggesting local differentiation. Two distinct clades emerged in both mitochondrial markers, with Cabo Verde samples from Baía das Gatas and São Vicente (Fig. 4A) constituting a separate group, clustering with samples from Gorée, Senegal. Few mutational steps separated the haplotypes from other localities, with Mediterranean populations (Murcia and Columbrete Island) showing the highest divergence. The North Atlantic islands (Azores and Canary Islands) shared most haplotypes (see Figs. 4A and 4B).
For the 28S rRNA marker, the average genetic diversity values showed a slightly different pattern to that observed with the mtDNA markers (h = 0.693 ± 0.092; π 0.005 ± 0.001). The genetic divergence shows 26 distinct haplotypes and many private haplotypes identified. In total, 18 sites were variable with eight being parsimony informative. Samples from the Canary Islands were mostly separated from the others, although with few mutational steps between them. Interestingly, Azorean samples were genetically closer to those from Cabo Verde, despite the Canary Islands’ geographic proximity to Cabo Verde (see Figs. 4A and 4B and Table 3). Cabo Verde showed a high degree of endemic haplotypes, with only two haplotypes shared with the Azores and Murcia populations. The samples from Santa Luzia and Baía das Gatas, which showed variation in the mitochondrial networks, also highlighted differentiation in the 28S rRNA marker, though with fewer mutational steps.
Phylogenetic relationships within Pinna species
Phylogenetic analyses produced trees with similar general topologies for mitochondrial data, differing only in the position of some haplotypes within internal groups, and the statistical support of clades. The 28S rRNA sequences showed a similar trend, although with low bootstrap support due to low genetic variation (i.e., replacement of a single nucleotide). Maximum likelihood bootstrap support (BP) and posterior Bayesian probability values (PP) were congruent with each other, with PP values generally higher, as expected (Suzuki, Glazko & Nei, 2002) (Supplemental Files, Figs. S1 and S2).
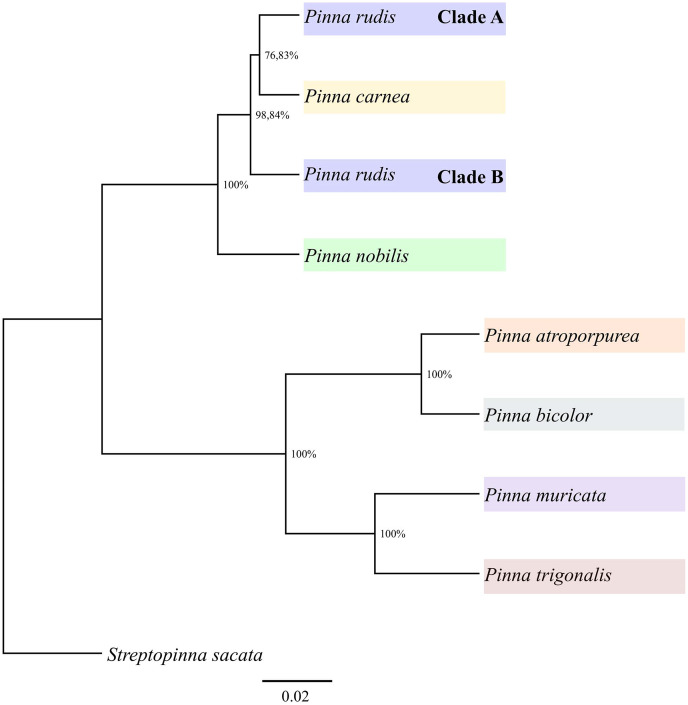
All Pinna species examined were monophyletic across all analyses, with high support, except for the P. rudis (Supplemental Files, Figs. S1 and S2) and P. nobilis in the mitochondrial data. The paraphyly of P. nobilis may result from incomplete data for the two mitochondrial markers. Both ML and PP analyses based on mitochondrial sequences showed two clades within P. rudis populations from Cabo Verde (Clades A and B), with bootstrap support >70%, consistent with the haplotype network reconstruction. Clade A comprised many Cabo Verde specimens grouped along with those from other Macaronesian islands and the Mediterranean, while Clade B grouped Cabo Verde samples with those from Gorée, Senegal. The average distance between the two clades was 25%, with intra-clade distances of 0.3% and 0.2%, for Clade A and Clade B, respectively. Clade B was formed by three sequences that represent two haplotypes of specimens that were collected in Baía das Gatas and on the island of Gorée, Senegal. Clade A is sister to P. carnea with bootstrap support >70% and Clade B to those in the mitochondrial and nuclear gene trees (Supplemental Files, Figs. S1 and S2).
The BEAST analyses corroborated these findings, revealing two clades within P. rudis separated by Pinna carnea (Fig. 5), although the relationships within clades differ from the previous analysis. Specimens of P. rudis from Senegal and Cabo Verde (Clade B) were genetically distinct from those in the Mediterranean, Azores and Canary Islands (Clade A). Clade B shows a sister group to the remaining P. rudis (Clade A), P. nobilis and P. carnea.
Figure 5. Phylogenetic relationships of Pinna genera based on a Bayesian inference analysis of mitochondrial COI, 16S rRNA and nuclear 28S rRNA markers produced by BEAST.
The Streptopinna sacata sequence was used as an outgroup.
Discussion
This study provides a comprehensive analysis of genetic variation in P. rudis by combining data from both this and previous studies across the Mediterranean Sea, Macaronesia and coastal Senegal. This approach enhances our understanding of large-scale genetic patterns and allows us to revisit previous phylogenetic analyses of the Pinna genus conducted by Lemer et al. (2014). The haplotype network reconstructions, incorporating sequences from various regions revealed a distinct level of population structuring, that generally aligns with the geographic distribution of these populations.
The population of P. rudis from Cabo Verde showed great haplotypic and nucleotide diversity across all markers used. Specifically, Baía das Gatas revealed a great genetic diversity, indicating the presence of highly divergent haplotypes in sympatry. This phenomenon suggests the potential for sympatric speciation, where multiple evolutionary units coexist within the same geographical area and can interbreed. Such events will be eventually lead to the emergence of distinct species (Hart et al., 2018).
The mitochondrial markers separated two differentiated evolutionary units within Cabo Verde, demonstrating a clear separation in the haplotype network. The Baía das Gatas samples comprised unique haplotypes distinct from those in other regions. This high level of diversity is likely advantageous for population resilience, potentially detecting evolutionary processes (Zhan et al., 2021; Uricoechea Patiño et al., 2023).
The two locations in São Vicente, Baía das Gatas and Laginha, demonstrated a certain degree of haplotype sharing (two in 16S rRNA and one in 28S rRNA), likely due to their proximity. This connectivity might be influenced by the small-scale current regime around the island (Medina et al., 2007). However, the absence of the most divergent haplotype of Baía das Gatas in the population of Laginha Beach may be due to the relatively low number of samples analysed in both populations but could also suggest that ecological differences between these sites affects the distribution of evolutionary units. Baia das Gatas, characterized by its shallow lagoon behind a breakwater wall (Schönfeld & Lübbers, 2020) and limited hydrodynamism, may have historically influenced genetic connectivity. Fossil data indicating past submersion of Baia das Gatas supports the idea that historical sea level changes could have influenced current genetic diversity and connectivity (Boekschoten & Best, 1988).
Across Macaronesia and the Mediterranean, the haplotype networks showed some structuration within P. rudis, although mutation steps were insufficient to separate between the Micronesian archipelagos (Freitas et al., 2019), except for the unique haplotypes from Baía das Gatas (see Figs. 4A and 4B). The 16S rRNA marker again highlighted population structure, with Cabo Verde samples showing distinct clades. The most divergent samples from Baía das Gatas formed a separate clade (Clade B) with Gorée Island, while other samples grouped into Clade A. This result corroborates previous findings by Lemer et al. (2014), who also observed significant separation of Gorée samples.
Long geographical distances and short larval durations in this species may contribute to the observed genetic structure (Nicolas et al., 1989; Wright et al., 2015). Additionally, sex-biased dispersion, where one sex disperses more, could further influence genetic structuring. According to Harrison, York & Young (2014), when sex-biased dispersion occurs in fragmented populations, genotypes of the more dispersive sex can be expected to be more randomly distributed among populations than genotypes of the other sex. Evidence of sex-biased dispersal has been documented in other marine molluscs, such as Mytilus galloprovincialis (Quesada, Skibinski & Skibinski, 1996) and Cerastoderma glaucum (Tarnowska et al., 2010), where male genotypes are more randomly distributed. The observed higher structuring in mtDNA compared to nDNA in Cabo Verde populations of P. rudis may suggest a similar phenomenon, with mitochondrial markers showing more pronounced differentiation (Dávalos & Russell, 2014; Yu et al., 2022).
Marine invertebrates with sessile adult stages often show patterns influenced by their larval dispersion capabilities (Butler, Vicente & de Gaulejac, 1993; Lee & Boulding, 2009). Some larvae spend relatively long periods in plankton and passive dispersion through ocean currents can allow sufficient gene flow to ensure relative genetic homogeneity within the geographic range of the species (Lee & Boulding, 2009). The high number of unique haplotypes in Cabo Verde suggests weak biogeographic affinity with the other archipelagos. Freitas et al. (2019) presented evidence for distinct biogeographic units of six marine groups with very different dispersal capacities within Macaronesia, with Cabo Verde forming a separate unit due to limited genetic exchange with other units (larvae, propagules, rafting adults, colonization events). This might be explained by the fact the Cabo Verde archipelago is situated within the Sahelian upwelling ecoregion in a single biogeographic unit, the West African Transition Province. Although the tendency for planktotrophic species is to show less or no genetic divergence at the regional level, a few cases of population structure have been reported for species with planktonic larvae. Sanna et al. (2013) showed the existence of population under-structuring in P. nobilis in the Mediterranean Sea, due to the presence of hydrodynamic barriers, local currents and hydrographic isolation. Indeed, P. rudis demonstrates a notable population structure, possibly due to reduced genetic flow and limited larval dispersion.
Another factor to consider is the relatively low level of genetic erosion in P. rudis (Bijlsma & Loeschcke, 2012), due to the species limited human exploitation because of the low muscle mass and the low population density. Exploitation can also lead to decreased genetic differentiation between populations. When substantial portions of a population are harvested, the remaining individuals may not represent the full genetic scale of the original population. This can result in homogenized genetic characteristics, with fewer haplotypes and reduced differentiation, contrary to the findings in P. rudis (Gandra et al., 2020). Indeed, in the sister species P. nobilis, higher mitochondrial variability was observed in Corsica-Sardinia, Elba Island, Sicily and the Lagoon of Venice, compared to populations in the Aegean and Tunisian coast. This suggests that human disturbance or bio-ecological changes may contribute to the loss of genetic variability (Sanna et al., 2024). The current study’s data are inconclusive as to supporting or rejecting these hypotheses.
Bayesian and maximum likelihood analysis of the same dataset showed a pattern remarkably similar to the haplotype network, providing support for the existence of two highly divergent clades in P. rudis, thus corroborating the findings of Lemer et al. (2014). Specifically, the sequences of P. rudis off the coast of Senegal and Baía das Gatas were distinctly and earlier separated from those of P. carnea and the remaining P. rudis populations from the Mediterranean, Atlantic Ocean (Canaries and Azores) and Cabo Verde. The average genetic distance between the two clades was considerable (6.4%) based on all two mitochondrial markers concatenated. For comparison, Wu et al. (2023) found that Lepidodesma aligera and Lepidodesma languilati were separated by an average genetic distance of only 4% based on COI marker.
According to Hart & Sunday (2007), the 95% parsimony connection limit, based on conventional DNA barcoding can be a useful tool for species discovery, potentially more effective than nuclear alleles. This limit appears to have a higher true-positive rate for discovering new cryptic species when applied to mtDNA. In contrast, frequent recombination between nuclear alleles may slow the rate at which ancestral polymorphisms shared among recently diverged species are lost from one (or both) through lineage sorting, thus reducing the rate at which haplotype differences between sister species approach the parsimony connection limit. Our data shows that P. rudis from Senegal and Baía das Gatas (São Vicente Island) may be a candidate species. This finding indicates previously unrecognized diversity within P. rudis, which may lead to a re-evaluation of the evolutionary relationships within the species. This has important implications for the taxonomy and conservation of these evolutionary units, which hold unique genetic heritage crucial to biodiversity preservation (Sanna et al., 2024). Further research should involve additional morphological and genetic data from the Baía das Gatas and Gorée populations to more accurately delineate species morphotypes.
Our study did not reveal significant morphological differences between the two clades of P. rudis. Similar findings were reported in other species, such as P. saccata, where morphological stasis was observed despite genetic differences (Lemer et al., 2014). Genetic information does not always align with morphological data. For example, analysis of the morphological and molecular differences between Ruditapes philippinarum (Adams & Reeve, 1850) and Ruditapes decussatus (Linnaeus, 1758) in the northeastern Adriatic Sea showed significant morphological similarities, with the species differentiation confirmed only by differences in the 16S rRNA gene analysis (Nerlović, Korlević & Mravinac, 2016). Conversely, morphological analysis of two Corbicula fluminea (Müller, 1774) populations in the Minho River estuary and the Lima River estuary (NW Portugal) showed significant shell shape differences, while genetic analysis revealed identical mtCOI sequence, indicating that both populations belong to C. fluminea (Sousa et al., 2007).
Environmental conditions can impose stabilizing selection in morphology, reducing or eliminating the morphological changes that might accompany speciation, since there are often few ways for an organism to adapt to harsh environments (Nevo, 2001). Consequently, speciation does not always involve morphological change (the morphological stasis). The true number of biological species is probably higher than the current count of nominal species, most of which are often outlined based solely on morphological characteristics (Bickford et al., 2007).
Conclusion
This study highlights significant genetic divergence within P. rudis, revealing the potential cryptic speciation, particularly in the populations of the coast of Senegal and Baía das Gatas. Despite the limited sample size, the results reveal significant genetic divergence, suggesting the possible existence of a previously unrecognized species. The minimal morphological variation alongside this genetic differentiation highlights the limitations of relying only on traditional taxonomy and emphasizes the value of molecular data in species identification and delimitation. Additionally, the study demonstrates the role of human disturbance in shaping genetic diversity, showing higher mitochondrial variability in regions less impacted by human activities, as the population from Santa Luzia with larger and, consequently, older samples. On the other hand, the sample group from Baía das Gatas is likely to be under more human disturbance. Hence there is a need for more extensive sampling, in number and space, and deeper investigation into the evolutionary relationships within the genus Pinna. Understanding these dynamics is critical for developing effective conservation strategies to protect their unique genetic units, which are essential for maintaining biodiversity in these marine ecosystems.
Supplemental Information
Phylogenetic relationships of Pinna species inferred from the combined Maximum Likelihood and Baesian analysis of two mitochondrial markers (COI and 16S rRNA). Tree topology was obtained from Maximum Likelihood analysis. Samples from Cabo Verde are in blue. The two clades of Pinna rudis form Cabo Verde (A and B) are indicated by labelled bars. Scale bar represents 0.4 substitutions per site. The bootstrap values for ML and BA appear one after the other at each node, respectively. The Streptopinna sacata sequences were used as outgroups.
Phylogenetic relationships of Pinna species inferred from the combined Maximum Likelihood and Bayesian analysis of 28S rRNA. Tree topology was obtained from Maximum Likelihood analysis. Samples from Cabo Verde are in blue. Scale bar represents 0.004 substitutions per site. The bootstrap values for ML and BA appear one after the other at each node, respectively. The Streptopinna sacata sequences were used as outgroups.
Raw Data - Biometric data obtained from 5 parameters in a total of 32 shell samples. Legend: L- Maximum shell length, LT- Maximum shell width, W - Total shell weight; Fraction of total length by shell weight - L / W, Fraction of total width by shell weight - Lt / W.
Haplotype frequency distributions of COI P. rudis samples
Haplotype frequency distributions of 16S RNA P. rudis samples
Haplotype frequency distributions of 28S RNA P. rudis samples
Acknowledgments
This study was part of Evandro P. Lopes’ BIODIV PhD Thesis. We thank Rui Freitas and Corrine Almeida of ISECMAR for help in developing the methodology; Commander Nuno Almeida for material supply for the assembly of the experiment; biology students at ISECMAR for their support in the data collection; the Direcção Nacional do Ambiente (DNA), as well as anonymous reviewers for their comments on the manuscript. This article is output from the Portuguese-Cabo Verde TwinLab, established between CIBIO/InBIO and Uni-CV.
Funding Statement
Funding for this study review was provided by Research Grant for a Master in the area of marine biology reference ICETA 2016-31, by the European Regional Development Fund (ERDF) through a complete program and by National Funds through FCT - Foundation for Science and Technology (project PTDC/MAR-BIO/0902/2014-POCI-01-0145-FEDER-016550). The APC was supported by the European Union’s Horizon 2020 Research and Innovation Programme under the Grant Agreement Number 857251. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Evandro P. Lopes conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Sarah Santos performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Raquel Xavier performed the experiments, analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Joana L. Santos performed the experiments, authored or reviewed drafts of the article, and approved the final draft.
M. Pilar Cabezas performed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Fernando Sequeira conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
António M. Santos conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Data Availability
References
- Abbot & Dance (2000).Abbot RT, Dance SP. Compendium of seashells A full-colour guide to more than 4,200 of the world’s marine shells. Corona: Odyssey Publishing; 2000. [Google Scholar]
- Akaike (1973).Akaike H. 2nd International Symposium on Information Theory. Budapest, Hungary: Akademiai Kiado; 1973. Information theory as an extension of the maximum likelihood principle; pp. 267–281. [Google Scholar]
- Barea-Azcón, Ballesteros-Duperón & Moreno (2008).Barea-Azcón JM, Ballesteros-Duperón E, Moreno D. Libro Rojo de los Invertebrados de Andalucía 4 Tomos. Seville: Consejería de Medio Ambiente, Junta de Andalucía; 2008. [Google Scholar]
- Basso et al. (2015).Basso L, Vázquez-Luis M, García-March JR, Deudero S, Alvarez E, Vicente N, Duarte CM, Hendriks IE. The pen shell, Pinna nobilis: a review of population status and recommended research priorities in the Mediterranean Sea. Advances in Marine Biology. 2015;71(1–2):109–160. doi: 10.1016/bs.amb.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Bessetti (2007).Bessetti BJ. An introduction to PCR inhibitors. Promega Notes. 2007;40:11. [Google Scholar]
- Bickford et al. (2007).Bickford D, Lohman DJ, Sodhi NS, Ng PKL, Meier R, Winker K, Ingram KK, Das I. Cryptic species as a window on diversity and conservation. Trends in Ecology & Evolution. 2007;22(3):148–155. doi: 10.1016/j.tree.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Bieler & Mikkelsen (2006).Bieler R, Mikkelsen PM. Bivalvia-a look at the branches. Zoological Journal of the Linnean Society. 2006;148(3):223–235. doi: 10.1111/j.1096-3642.2006.00255.x. [DOI] [Google Scholar]
- Bijlsma & Loeschcke (2012).Bijlsma R, Loeschcke V. Genetic erosion impedes adaptive responses to stressful environments. Evolutionary Applications. 2012;5(2):117–129. doi: 10.1111/j.1752-4571.2011.00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boekschoten & Best (1988).Boekschoten GJ, Best MB. Fossil and recent shallow water corals from the Atlantic Islands off Western Africa-CANCAP-contribution no 56. Zoologische Mededelingen. 1988;62(8):99–112. [Google Scholar]
- Booy et al. (2000).Booy G, Hendriks RJJ, Smulders MJM, Van Groenendael JM, Vosman B. Genetic diversity and the survival of populations. Plant Biology. 2000;2(4):379–395. doi: 10.1055/s-2000-5958. [DOI] [Google Scholar]
- Bouckaert et al. (2014).Bouckaert R, Heled J, Kühnert D, Vaughan T, Wu C-H, Xie D, Suchard MA, Rambaut A, Drummond AJ. BEAST 2: a software platform for bayesian evolutionary analysis. PLOS Computer Biology. 2014;10(4):e1003537. doi: 10.1371/journal.pcbi.1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler, Vicente & de Gaulejac (1993).Butler A, Vicente N, de Gaulejac B. Ecology of the pterioid bivalves Pinna bicolor Gmelin and Pinna nobilis L. Marine Life. 1993;3(1–2):37–45. [Google Scholar]
- Calado, Ortea & Caballer (2005).Calado G, Ortea J, Caballer M. A new species of the genus Flabellina Voigt 1834 (Mollusca: Nudibranchia) from the Cape Verde Islands. Journal of Conchology. 2005;38(6):663–672. doi: 10.5962/p.408184. [DOI] [Google Scholar]
- Carson (1983).Carson HL. Genetical processes of evolution on high oceanic islands. GeoJournal. 1983;7:543–547. doi: 10.1007/BF00218528. [DOI] [Google Scholar]
- Castresana (2000).Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular Biology and Evolution. 2000;17(4):540–552. doi: 10.1093/oxfordjournals.molbev.a026334. [DOI] [PubMed] [Google Scholar]
- Catanese, Coupé & Bunet (2022).Catanese G, Coupé S, Bunet R. Mitogenome sequence comparison in the endangered congeneric Pinna nobilis and Pinna rudis bivalves. Molecular Biology Reports. 2022;49(5):3627–3635. doi: 10.1007/s11033-022-07202-w. [DOI] [PubMed] [Google Scholar]
- Clement, Posada & Crandall (2000).Clement M, Posada D, Crandall KA. TCS: a computer program to estimate gene genealogies. Molecular Ecology. 2000;9(10):1657–1659. doi: 10.1046/j.1365-294x.2000.01020.x. [DOI] [PubMed] [Google Scholar]
- Cunha et al. (2017).Cunha RL, Assis JM, Madeira C, Seabra R, Lima FP, Lopes EP, Williams ST, Castilho R. Drivers of Cape Verde archipelagic endemism in keyhole limpets. Scientific Reports. 2017;7(1):41817. doi: 10.1038/srep41817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha et al. (2005).Cunha R, Castilho R, Rüber L, Zardoya R. Patterns of cladogenesis in the venomous marine gastropod genus conus from the cape Verde Islands. Systematic Biology. 2005;54(4):634–650. doi: 10.1080/106351591007471. [DOI] [PubMed] [Google Scholar]
- Cunha, Grande & Zardoya (2009).Cunha RL, Grande C, Zardoya R. Neogastropod phylogenetic relationships based on entire mitochondrial genomes. BMC Evolutionary Biology. 2009;9(1):210. doi: 10.1186/1471-2148-9-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha et al. (2014).Cunha RL, Lima FP, Tenorio MJ, Ramos AA, Castilho R, Williams ST. Evolution at a different pace: distinctive phylogenetic patterns of cone snails from two ancient oceanic archipelagos. Systematic Biology. 2014;63(6):971–987. doi: 10.1093/sysbio/syu059. [DOI] [PubMed] [Google Scholar]
- Cunha et al. (2011).Cunha RL, Lopes EP, Reis DM, Castilho R. Genetic structure of Brachidontes puniceus populations in Cape Verde archipelago shows signature of expansion during the last glacial maximum. Journal of Molluscan Studies. 2011;77(2):175–181. doi: 10.1093/mollus/eyr001. [DOI] [Google Scholar]
- Cunha et al. (2008).Cunha RL, Tenorio MJ, Afonso C, Castilho R, Zardoya R. Replaying the tape: recurring biogeographical patterns in Cape Verde Conus after 12 million years. Molecular Ecology. 2008;17(3):885–901. doi: 10.1111/j.1365-294X.2007.03618.x. [DOI] [PubMed] [Google Scholar]
- Dávalos & Russell (2014).Dávalos LM, Russell AL. Sex-biased dispersal produces high error rates in mitochondrial distance-based and tree-based species delimitation. Journal of Mammalogy. 2014;95(4):781–791. doi: 10.1644/14-MAMM-A-107. [DOI] [Google Scholar]
- Duda & Lessios (2009).Duda TF, Lessios HA. Connectivity of populations within and between major biogeographic regions of the tropical Pacific in Conus ebraeus, a widespread marine gastropod. Coral Reefs. 2009;28(3):651–659. doi: 10.1007/s00338-009-0485-9. [DOI] [Google Scholar]
- Duda & Rolán (2005).Duda TF, Rolán E. Explosive radiation of Cape Verde Conus, a marine species flock. Molecular Ecology. 2005;14(1):267–272. doi: 10.1111/j.1365-294X.2004.02397.x. [DOI] [PubMed] [Google Scholar]
- Edgecombe & Giribet (2006).Edgecombe GD, Giribet G. A century later—a total evidence re-evaluation of the phylogeny of scutigeromorph centipedes (Myriapoda: Chilopoda) Invertebrate Systematics. 2006;20(5):503–525. doi: 10.1071/IS05044. [DOI] [Google Scholar]
- Folmer et al. (1994).Folmer O, Black MB, Hoeh WR, Lutz RA, Vrijenhoek RC. DNA primers for amplification of mitochondrial cytochrome C oxidase subunit I from metazoan invertebrates. Molecular Marine Biology Biotechnology. 1994;3(5):294–299. [PubMed] [Google Scholar]
- Fraussen & Swinnen (2016).Fraussen K, Swinnen F. A review of the genus Euthria Gray, 1839 (Gastropoda: Buccinidae) from the Cape Verde Archipelago. Xenophora Taxonomy. 2016;11:9–31. [Google Scholar]
- Freitas et al. (2019).Freitas R, Romeiras M, Silva L, Cordeiro R, Madeira P, González J, Wirtz P, Falcón JM, Brito A, Floeter SR, Afonso P, Porteiro F, Viera-Rodríguez MA, Neto AI, Haroun R, Farminhão JNM, Rebelo AC, Baptista L, Melo CS, Martínez A, Núñez J, Berning B, Johnson ME, Ávila SP. Restructuring of the ‘Macaronesia’ biogeographic unit: a marine multi-taxon biogeographical approach. Scientific Reports. 2019;9:15792. doi: 10.1038/s41598-019-51786-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandra et al. (2020).Gandra M, Assis J, Martins MR, Abecasis D. Reduced global genetic differentiation of exploited marine fish species. Molecular Biology and Evolution. 2020;38(4):1402–1412. doi: 10.1093/molbev/msaa299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-March & Kersting (2006).García-March JR, Kersting DK. Preliminary data on the distribution and density of Pinna nobilis and Pinna rudis in the Columbretes Islands Marine Reserve (Western Mediterranean, Spain). International Congress on Bivalvia Universitat Autònoma de Barcelona.2006. [Google Scholar]
- Geller et al. (2013).Geller J, Meyer C, Parker M, Hawk H. Redesign of PCR primers for mitochondrial cytochrome C oxidase subunit I for marine invertebrates and application in all-taxa biotic surveys. Molecular Ecology Resources. 2013;13(5):851–861. doi: 10.1111/1755-0998.12138. [DOI] [PubMed] [Google Scholar]
- Gvozdenović et al. (2019).Gvozdenović S, Mačić V, Pešić V, Nikolić M, Peraš I, Mandić M. Review on Pinna rudis (Linnaeus, 1758) (bivalvia: Pinnidae) presence in the mediterranean. Agriculture and Forestry. 2019;65:115–126. doi: 10.17707/AgricultForest.65.4.10. [DOI] [Google Scholar]
- Hall (1999).Hall T. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Hammer, Harper & Ryan (2001).Hammer Ø, Harper DAT, Ryan PD. PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica. 2001;4:1–9. [Google Scholar]
- Harrison, York & Young (2014).Harrison XA, York JE, Young AJ. Population genetic structure and direct observations reveal sex-reversed patterns of dispersal in a cooperative bird. Molecular Ecology. 2014;23(23):5740–5755. doi: 10.1111/mec.12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart et al. (2018).Hart KM, Iverson AR, Fujisaki I, Lamont MM, Bucklin D, Shaver DJ. Sympatry or syntopy? Investigating drivers of distribution and co-occurrence for two imperiled sea turtle species in Gulf of Mexico neritic waters. Ecology and Evolution. 2018;8(24):12656–12669. doi: 10.1002/ece3.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart & Sunday (2007).Hart MW, Sunday J. Things fall apart: biological species form unconnected parsimony networks. Biology Letters. 2007;3(5):509–512. doi: 10.1098/rsbl.2007.0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsares et al. (2008).Katsares V, Tsiora A, Galinou-Mitsoudi S, Imsiridou A. Genetic structure of the endangered species Pinna nobilis (Mollusca: Bivalvia) inferred from mtDNA sequences. Biologia. 2008;63(3):412–417. doi: 10.2478/s11756-008-0061-8. [DOI] [Google Scholar]
- Lanfear et al. (2012).Lanfear R, Calcott B, Ho SYW, Guindon S. PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution. 2012;29(6):1675–1701. doi: 10.1093/molbev/mss020. [DOI] [PubMed] [Google Scholar]
- Lee & Boulding (2009).Lee HJ, Boulding EG. Spatial and temporal population genetic structure of four northeastern Pacific littorinid gastropods: the effect of mode of larval development on variation at one mitochondrial and two nuclear DNA markers. Molecular Ecology. 2009;18(10):2166–2184. doi: 10.1111/j.1365-294X.2009.04169.x. [DOI] [PubMed] [Google Scholar]
- Lemer et al. (2014).Lemer S, Bug B, Bemi A, Giribet G. First molecular phylogeny of the circumtropical bivalve family Pinnidae (Mollusca, Bivalvia): evidence for high levels of cryptic species diversity. Molecular Phylogenetics and Evolution. 2014;75:11–23. doi: 10.1016/j.ympev.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Lemer et al. (2016).Lemer S, Combosch D, Sotto F, Giribet G, Dumale D, Soliman V. The family Pinnidae (Bivalvia) in the Philippine archipelago: observations on its distribution and phylogeography. Nautilus. 2016;130(4):137–145. [Google Scholar]
- Lopes, Monteiro & Santos (2020).Lopes EP, Monteiro N, Santos AM. Epibiotic assemblages on the pen shell Pinna rudis (Bivalvia, Pinnidae) at Matiota Beach, São Vicente Island, Cabo Verde. African Journal of Marine Science. 2020;42(1):13–21. doi: 10.2989/1814232X.2019.1700826. [DOI] [Google Scholar]
- Marko (2004).Marko PB. ‘What’s larvae got to do with it?’ Disparate patterns of post-glacial population structure in two benthic marine gastropods with identical dispersal potential. Molecular Ecology. 2004;13(3):597–611. doi: 10.1046/j.1365-294X.2004.02096.x. [DOI] [PubMed] [Google Scholar]
- Mason, Coombs & Oliveira (2005).Mason E, Coombs SH, Oliveira PB. An overview of the literature concerning the oceanography of the eastern North Atlantic region. Relatórios Científcos e Técnicos IPIMAR Serie Digital. 2005;33:1–57. [Google Scholar]
- Medina et al. (2007).Medina A, Brêthes JC, Sévigny JM, Zakardjian B. How geographic distance and depth drive ecological variability and isolation of demersal fish communities in an archipelago system (Cape Verde, Eastern Atlantic Ocean) Marine Ecology. 2007;28(3):404–417. doi: 10.1111/j.1439-0485.2007.00163.x. [DOI] [Google Scholar]
- Miloslavich et al. (2010).Miloslavich P, Díaz JM, Klein E, Alvarado JJ, Díaz C, Gobin J, Escobar-Briones E, Cruz-Motta JJ, Weil E, Cortés J, Bastidas AC, Robertson R, Zapata F, Martín A, Castillo J, Kazandjian A, Ortiz M. Marine biodiversity in the Caribbean: regional estimates and distribution patterns. PLOS ONE. 2010;5(8):e11916. doi: 10.1371/journal.pone.0011916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Biotechnology Information (2023).National Center for Biotechnology Information Standard nucleotide BLAST. 2023. https://blast.ncbi.nlm.nih.gov/Blast.cgi. [5 November 2023]. https://blast.ncbi.nlm.nih.gov/Blast.cgi
- Nebot-Colomer et al. (2016).Nebot-Colomer E, Vázquez-Luis M, García-March JR, Deudero S. Population structure and growth of the threatened pen shell, Pinna rudis (Linnaeus, 1758) in a Western Mediterranean Marine Protected Area. Mediterranean Marine Science. 2016;17(3):785–793. doi: 10.12681/mms.1597. [DOI] [Google Scholar]
- Nerlović, Korlević & Mravinac (2016).Nerlović V, Korlević M, Mravinac B. Morphological and molecular differences between the invasive bivalve Ruditapes philippinarum (Adams & Reeve, 1850) and the native species Ruditapes decussatus (Linnaeus, 1758) from the Northeastern Adriatic Sea. Journal of Shellfish Research. 2016;35(1):31–39. doi: 10.2983/035.035.0105. [DOI] [Google Scholar]
- Nevo (2001).Nevo E. Evolution of genome-phenome diversity under environmental stress. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(11):6233–6240. doi: 10.1073/pnas.101109298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas et al. (1989).Nicolas J, Fernandez-Palacios J, Ferrer FJ, Nieto E. Inter-island floristic similarities in the Macaronesian region. Plant Ecology. 1989;84(2):117–125. [Google Scholar]
- Okonechnikov et al. (2012).Okonechnikov K, Golosova O, Fursov M, Varlamov A, Vaskin Y, Efremov I, German Grehov OG, Kandrov D, Rasputin K, Syabro M, Tleukenov T. Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics. 2012;28(8):1166–1167. doi: 10.1093/bioinformatics/bts091. [DOI] [PubMed] [Google Scholar]
- Ortea (1989).Ortea JA. Descripción de algunos moluscos opistobranquios nuevos recolectados en el Archipiélago de Cabo Verde. Publicaçoes Ocasionais da Sociedade Portuguesa de Malacologia. 1989;13:17–43. [Google Scholar]
- Ortea-Rato & Espinosa (1998).Ortea-Rato J, Espinosa J. Estudio de nueve especies del género Flabellina Voight, 1834 (Mollusca: Nudibranchia) colectadas en Angola, Cabo Verde, Costa Rica, Cuba y Portugal, con la descripción de tres especies nuevas. [Nine species of the genus Flabellina Voight, 1834 (Mollusca: Nudibranchia) found in Angola, Cape Verd, Costa Rica, Cuba and Portugal are studied with the description of three new species] Avicennia. 1998;8/9:135–148. [Google Scholar]
- Palumbi (1996).Palumbi S. Nucleic acids II: polymerase chain reaction. In: Hillis D, Moritz C, Mable B, editors. Molecular Systematics. Second Edition. Sunderland, Massachusetts: Sinauer Associates; 1996. pp. 205–247. [Google Scholar]
- Panova et al. (2016).Panova M, Aronsson H, Cameron RA, Dahl P, Godhe A, Lind U, Ortega-Martinez O, Pereyra R, Tesson SVM, Wrange AL, Blomberg A, Johannesson K. DNA extraction protocols for whole-genome sequencing in marine organisms. In: Leray M, Knowlton N, editors. Marine Genomics: Methods in Molecular Biology. New York: Humana Press; 2016. pp. 13–44. [DOI] [PubMed] [Google Scholar]
- Peña-Izquierdo et al. (2012).Peña-Izquierdo J, Pelegrí JL, Pastor MV, Castellanos P, Emelianov M, Gasser M, Salvador J, Vázquez-Domínguez E. The continental slope current system between Cape Verde and the Canary Islands. Scientia Marina. 2012;76(S1):65–78. doi: 10.3989/scimar.03607.18C. [DOI] [Google Scholar]
- Pola et al. (2014).Pola M, Carmona L, Calado G, Cervera JL. A new nudibranch, Flabellina albomaculata sp. nov (Flabellinidae), from the Cape Verde Archipelago with comparisons among all eastern Atlantic violet Flabellina spp. Marine Biology Research. 2014;11(2):218–222. doi: 10.1080/17451000.2014.923102. [DOI] [Google Scholar]
- Pond, Poon & Frost (2009).Pond SLK, Poon AFY, Frost SDW. Estimating selection pressures on alignments of coding sequences. In: Lemey P, Salemi M, Vandamme A, editors. The Phylogenetic Handbook: A Practical Approach to Phylogenetic Analysis and Hypothesis. Cambridge: Cambridge University Press; 2009. pp. 419–445. [Google Scholar]
- Poutiers (2016).Poutiers JM. Bivalves (Acephala, Lamellibranchia, Pelecypoda) In: Carpenter KE, Angelis N, editors. The Living Marine Resources of the Eastern Central Atlantic Volume 2 Bivalves, Gastropods, Hagfishes, Sharks, Batoid Fishes And Chimaeras. Rome: FAO; 2016. pp. 665–896. FAO Species Identification Guide for Fishery Purposes. [Google Scholar]
- Puillandre et al. (2014).Puillandre N, Bouchet P, Duda TF, Kauferstein S, Kohn AJ, Olivera BM, Watkins M, Meyer C. Molecular phylogeny and evolution of the cone snails (Gastropoda, Conoidea) Molecular Phylogenetics and Evolution. 2014;78:290–303. doi: 10.1016/j.ympev.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada, Skibinski & Skibinski (1996).Quesada H, Skibinski DAG, Skibinski DOF. Sex-biased heteroplasmy and mitochondrial DNA inheritance in the mussel Mytilus galloprovincialis Lmk. Current Genetics. 1996;29(5):423–426. doi: 10.1007/BF02221509. [DOI] [PubMed] [Google Scholar]
- Rambaut (2012).Rambaut A. FigTree Version 14 3. Edinburgh: University of Edinburgh; 2012. [Google Scholar]
- Rambaut & Drummond (2013).Rambaut A, Drummond AJ. Tracer v1 5. 2013. http://beast.bio.ed.ac.uk/Trace. [10 November 2022]. http://beast.bio.ed.ac.uk/Trace
- Rambaut et al. (2018).Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA. Posterior summarization in Bayesian phylogenetics using tracer 17. Systematic Biology. 2018;67(5):901–904. doi: 10.1093/sysbio/syy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolán (2005).Rolán E. Malacological Fauna from the Cape Verde Archipelago: Part 1. Harxheim: ConchBooks; 2005. [Google Scholar]
- Ronquist et al. (2012).Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. MrBayes 32: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology. 2012;61(3):539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salemi (2009).Salemi M. Genetic distances and nucleotide substitution models. In: Lemey P, Salemi M, Vandamme A, editors. The Phylogenetic Handbook: A Practical Approach to Phylogenetic Analysis and Hypothesis. Cambridge: Cambridge University Press; 2009. pp. 126–140. [Google Scholar]
- Sambrook & Russel (2001).Sambrook J, Russel DW. Molecular cloning, 3-volume set: a laboratory manual. New York: Cold Spring Harboc Laboratory Press; 2001. [Google Scholar]
- Sanna et al. (2024).Sanna D, Azzena I, Locci C, Ankon P, Kružić P, Manfrin C, Pallavicini A, Ciriaco S, Segarich M, Batistini E, Scarpa F, Casu M. Reconstructing the evolutionary history of pinna nobilis: new genetic signals from the past of a species on the brink of extinction. Animals. 2024;14:114. doi: 10.3390/ani14010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna et al. (2013).Sanna D, Cossu P, Dedola GL, Scarpa F, Maltagliati F, Castelli A, Franzoi P, Lai T, Cristo B, Curini-Galletti M, Francalacci P, Casu M. Mitochondrial DNA reveals genetic structuring of Pinna nobilis across the Mediterranean Sea. PLOS ONE. 2013;8(6):e67372. doi: 10.1371/journal.pone.0067372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos et al. (2015).Santos AM, Cabezas MP, Tavares AI, Xavier R, Branco M. TcsBU: a tool to extend TCS network layout and visualization. Bioinformatics. 2015;32(4):627–628. doi: 10.1093/bioinformatics/btv636. [DOI] [PubMed] [Google Scholar]
- Schwendinger & Giribet (2005).Schwendinger PJ, Giribet G. The systematics of the south-east Asian genus Fangensis Rambla (Opiliones: Cyphophthalmi: Stylocellidae) Invertebrate Systematics. 2005;19(4):297–323. doi: 10.1071/IS05023. [DOI] [Google Scholar]
- Schönfeld & Lübbers (2020).Schönfeld J, Lübbers J. Checklist, assemblage composition, and biogeographic assessment of Recent benthic foraminifera (Protista, Rhizaria) from São Vincente, Cape Verde. Zootaxa. 2020;4731(2):151–192. doi: 10.11646/zootaxa.4731.2.1. [DOI] [PubMed] [Google Scholar]
- Sempere et al. (2006).Sempere JB, Jerez PS, Luis MV, Martínez CO. Evaluación de las Especies Marinas de Interés Ecológico del LIC de las Islas Columbretes. Servicio de Conservación de la Biodiversidad–Conselleria de Território y Vivenda. Alicante: Instituto Multidisciplinar para el Estudio del Medio; 2006. [Google Scholar]
- Sousa et al. (2007).Sousa R, Freire R, Rufino M, Méndez J, Gaspar M, Antunes C, Guilhermino L. Genetic and shell morphological variability of the invasive bivalve Corbicula fluminea (Müller, 1774) in two Portuguese estuaries. Estuarine, Coastal and Shelf Science. 2007;74(1–2):166–174. doi: 10.1016/j.ecss.2007.04.011. [DOI] [Google Scholar]
- Stamatakis (2006).Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22(21):2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Suzuki, Glazko & Nei (2002).Suzuki Y, Glazko GV, Nei M. Overcredibility of molecular phylogenies obtained by Bayesian phylogenetics. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(25):16138–16143. doi: 10.1073/pnas.212646199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnowska et al. (2010).Tarnowska K, Chenuil A, Nikula R, Féral JP, Wołowicz M. Complex genetic population structure of the bivalve Cerastoderma glaucum in a highly fragmented lagoon habitat. Marine Ecology Progress Series. 2010;406:173–184. doi: 10.3354/meps08549. [DOI] [Google Scholar]
- Tenorio & Afonso (2004).Tenorio MJ, Afonso CML. Description of four new species of Conus from the Cape Verde Islands (Gastropoda, Conidae) Visaia. 2004;1(2):24–37. [Google Scholar]
- Tenorio, Afonso & Rolán (2008).Tenorio MJ, Afonso CML, Rolán E. New endemic species of Conus (Gastropoda, Conidae) from the Islands of São Nicolau, Santo Antão and Sal in the Cape Verde Archipelago. Vita Malacologica. 2008;6:1–10. [Google Scholar]
- Uricoechea Patiño et al. (2023).Uricoechea Patiño D, Collins A, García OJR, Santos Vecino G, Cuenca JVR, Bernal JE, Benavides Benítez E, Vergara Muñoz S, Briceño Balcázar I. High mitochondrial haplotype diversity found in three pre-hispanic groups from colombia. Genes. 2023;14(10):1853. doi: 10.3390/genes14101853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Luis et al. (2021).Vázquez-Luis M, Nebot-Colomer E, Deudero S, Planes S, Boissin E. Natural hybridization between pen shell species: Pinna rudis and the critically endangered Pinna nobilis may explain parasite resistance in P. nobilis. Molecular Biology Reports. 2021;48(1):997–1004. doi: 10.1007/s11033-020-06063-5. [DOI] [PubMed] [Google Scholar]
- Wares & Cunningham (2005).Wares JP, Cunningham CW. Diversification before the most recent glaciation in Balanus glandula. Biological Bulletin. 2005;208(1):60–68. doi: 10.2307/3593101. [DOI] [PubMed] [Google Scholar]
- Whiting et al. (1997).Whiting MF, Carpenter JC, Wheeler QD, Wheeler WC. The Strepsiptera problem: phylogeny of the holometabolous insect orders inferred from 18S and 28S ribosomal DNA sequences and morphology. Systematic Biology. 1997;46(1):1–68. doi: 10.1093/sysbio/46.1.1. [DOI] [PubMed] [Google Scholar]
- Whittaker & Fernández-Palacios (2007).Whittaker RJ, Fernández-Palacios JM. Island biogeography: ecology, evolution, and conservation. Oxford: Oxford University press; 2007. [Google Scholar]
- Wirtz (2009).Wirtz P. Thirteen new records of marine invertebrates and two of fishes from Cape Verde Islands. Life and Marine Sciences. 2009;26:51–56. [Google Scholar]
- WoRMS (2022).WoRMS World register of marine species. 2022. https://www.marinespecies.org/ [15 November 2022]. https://www.marinespecies.org/
- Wright et al. (2015).Wright D, Bishop JM, Matthee CA, von der Heyden S. Genetic isolation by distance reveals restricted dispersal across a range of life histories: implications for biodiversity conservation planning across highly variable marine environments. Diversity and Distributions. 2015;21(6):698–710. doi: 10.1111/ddi.12302. [DOI] [Google Scholar]
- Wu et al. (2023).Wu R, Liu L, Zhang L, Jin D, Wu X, Liu X, Xie Z, Li Z. Molecular phylogeny and comparative morphology reveal the species validity and systematic position of Lepidodesma (Bivalvia: Unionidae) Organisms Diversity and Evolution. 2023;24:51–66. doi: 10.1007/s13127-023-00627-6. [DOI] [Google Scholar]
- Yu et al. (2022).Yu M, Liu Q, Wu YY, Guo P, Yang K. Genetic diversity and sex-biased dispersal in the brown spotted pitviper (Protobothrops mucrosquamatus): evidence from microsatellite markers. Ecology and Evolution. 2022;12(3):1–13. doi: 10.1002/ece3.8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan et al. (2021).Zhan HX, Hao ZP, Tang R, Zhu LN, Zhou JJ, Hou SM. High genetic diversity and strong genetic structure of Strongyllodes variegatus populations in oilseed rape production areas of China. BMC Ecology and Evolution. 2021;21:18. doi: 10.1186/s12862-021-01752-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong et al. (2016).Zhong X, Feng D, Yu H, Kong L, Li Q. Genetic variation and breeding signature in mass selection lines of the pacific oyster (Crassostrea gigas) assessed by SNP markers. PLOS ONE. 2016;11(3):e0150868. doi: 10.1371/journal.pone.0150868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotou et al. (2023).Zotou M, Papadakis O, Catanese G, Stranga Y, Ragkousis M, Kampouris TE, Aga-Spyridopoulou RN, Papadimitriou E, Koutsoubas D, Katsanevakis S. New kid in town: Pinna rudis spreads in the eastern Mediterranean. Mediterranean Marine Science. 2023;24(3):709–721. doi: 10.12681/MMS.35343. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic relationships of Pinna species inferred from the combined Maximum Likelihood and Baesian analysis of two mitochondrial markers (COI and 16S rRNA). Tree topology was obtained from Maximum Likelihood analysis. Samples from Cabo Verde are in blue. The two clades of Pinna rudis form Cabo Verde (A and B) are indicated by labelled bars. Scale bar represents 0.4 substitutions per site. The bootstrap values for ML and BA appear one after the other at each node, respectively. The Streptopinna sacata sequences were used as outgroups.
Phylogenetic relationships of Pinna species inferred from the combined Maximum Likelihood and Bayesian analysis of 28S rRNA. Tree topology was obtained from Maximum Likelihood analysis. Samples from Cabo Verde are in blue. Scale bar represents 0.004 substitutions per site. The bootstrap values for ML and BA appear one after the other at each node, respectively. The Streptopinna sacata sequences were used as outgroups.
Raw Data - Biometric data obtained from 5 parameters in a total of 32 shell samples. Legend: L- Maximum shell length, LT- Maximum shell width, W - Total shell weight; Fraction of total length by shell weight - L / W, Fraction of total width by shell weight - Lt / W.
Haplotype frequency distributions of COI P. rudis samples
Haplotype frequency distributions of 16S RNA P. rudis samples
Haplotype frequency distributions of 28S RNA P. rudis samples
Data Availability Statement
The following information was supplied regarding data availability:
The sequences are available at GenBank: OQ055700–OQ055719 (16S rRNA); OQ026776–OQ026795 (COI); OQ026797–OQ026816 (28S rRNA).
The other raw data are available in the Supplemental Files.