Abstract
Context
Several data from the literature have focused on the relationship between congenital sensorineural hearing loss, as well as acquired hearing loss, and their impact on cognition and the risk of dementia. However, few studies have been conducted on this subject in countries where access to hearing rehabilitation measures is limited. Thus, the objective of the present study was to investigate the relationship between sensorineural hearing loss and cognitive disorders in a correlational approach.
Methods
This is a cross-sectional and analytical study conducted in the ENT department of the Center for the Disabled (visual, auditory, and mental) “Village Bondeko”, from June to September 2023, involving 150 adults (≥20 years) with acquired sensorineural hearing loss; without a history of neuropsychic disorders. Sensorineural hearing loss was confirmed by tonal threshold audiometry and characterized according to WHO criteria; cognitive disorders were defined according to the MoCA scale.
Results
In total, 150 adults, including 78 men and 72 women, were collected in the present study. The average age was 54.11 ± 20 years, with extremes ranging from 20 to 87 years. In univariate analysis, there was a significant association (p<0.0001) between the degree of hearing loss and cognitive disorders. The analysis of variance (ANOVA) comparing the means demonstrated a very significant correlation (p ˂ 0.0001) between the decline in cognitive functions and the severity of hearing impairment. The low level of education, marital status, and cardiovascular risk factors were associated with cognitive disorders; however, no association was demonstrated between advancing age, gender, socioeconomic status, and cognitive disorders in the study population.
Conclusion
The present study has demonstrated the existence of an association between sensorineural hearing loss and cognitive disorders, involving a multidisciplinary and early management of sensorineural hearing loss.
Keywords: sensorineural hearing loss, cognition, Kinshasa, Village Bondeko
Introduction
Sensorineural hearing loss (SNHL) currently constitutes the third leading cause of disability in the world, affecting 1.57 million people,1 80% of whom live in developing countries.2 Compared to high-income countries, where prevalences around 4 to 6.4% are observed in adults, the SSA reports prevalence rates of hearing loss three times higher.3
In addition to the negative economic impact of SNHL, SNHL is responsible for communication difficulties with the surrounding environment. In children, it is responsible for delayed language acquisition and decreased academic performance.4 In adults, the consequences are rather social isolation, depression, and cognitive decline with a risk of dementia.5 Cognitive functions are essential elements for maintaining autonomy and quality of life; they refer to the mental processes involved in attention, language, reasoning, memory, problem-solving ability, and visuospatial capacity, which can be accompanied by behavioral disorders at certain stages of the disease.6
The natural history of cognitive impairments, especially severe ones, is still poorly understood, but it is increasingly suspected that a silent period of around 15 to 20 years often precedes the clinical diagnosis of Alzheimer’s-type cognitive disorders. This reality reinforces the importance of thoroughly understanding the factors that can influence cognitive disorders such as low education level, marital status, hypertension, diabetes, alcohol and tobacco use, aging, and socioeconomic status; thus enabling the implementation of targeted interventions, especially among middle-aged and elderly adults.7
According to the Lancet Commission report in 2020, sensorineural hearing loss has been identified as the main target in strategies for preventing cognitive disorders such as dementia. Thus, eliminating hearing loss in midlife could reduce the risk of developing dementia by 9%.8
Several systematic reviews and meta-analyses have highlighted an association between both congenital and acquired sensorineural hearing loss and a decline in cognitive performance.9–11
Recent studies on the association (correlation) between cognitive disorders and sensorineural hearing loss stipulate that the risk of developing cognitive disorders is increased by 24% in the case of deafness (average threshold at 39 dB) and that there is a linear association between the risk of occurrence of cognitive disorders, their severity, and the severity of hearing impairment.12
However, the majority of previous studies demonstrating the association between hearing loss and cognitive decline are based on data from Western countries.13 Indeed, in Sub-Saharan Africa, the knowledge of this association remains very poorly documented; however, the high prevalence of disabling hearing loss, as well as the harmful societal impacts of sensorineural deafness in developing countries, should constitute a public health priority,14 particularly in the D.R. Congo, where access to hearing rehabilitation measures is limited by the lack of effective healthcare coverage.
The advent of the COVID-19 pandemic in 2019 from China15 and after 2020 in the rest of the world,16 including the DRC,17 general deafness,18 and cognitive disorders/dementia are compelling evidence (Evidence Based Medicine) of an anxiety-depressive and pro-dementia state.19
The hypothesis is proposed that the prolonged exposure to military conflicts, inter-ethnic crises, auditory trauma, and the widespread poverty of Congolese people contribute to an epidemic-like prevalence of sensorineural hearing loss and cognitive disorders in Kinshasa.
Thus, the objective of the present study was to investigate the association between sensorineural hearing loss and cognitive disorders using a correlational approach; among patients followed at the ENT department of the “Village Bondeko” center in Kinshasa.
Methods
Nature and Period of the Study
The present cross-sectional and analytical study was conducted from June 4 to September 30, 2023, at the ENT department of the diocesan organization “Village Bondeko” located in the Kinshasa commune,20 whose choice was justified by its expertise in the care of patients living with disabilities (mental, auditory, and visual).21
Study Population
The study population consisted of patients aged 20 years or older, with acquired sensorineural hearing loss, who consulted the ENT department during the study period.
Sampling
The sample size calculation was performed using the Raosoft software: Approximately 150 patients were consulted each day from Monday to Friday during the study period. Included in the study were all consenting, literate patients aged ≥ 20 years; who had normal otoscopic and tympanometric examinations; who presented with hearing loss on pure tone audiometry (PTA); and who had no history of neuropsychological disorders. Patients with a history of visual disorders, chronic pain, vestibulopathy, head trauma, and HIV infections, as well as those who did not participate in all stages of the study, were excluded.
Data Collection
The collected data came from the otoscopic examination, tympanogram, PTA, and the pre-established questionnaire to gather sociodemographic information, clinical history, as well as cognitive functions.
Variables D’intérêt
The parameters of interest in the study were age, sex, education level, socio-economic status, marital status; complaints at admission, notions of alcohol and tobacco, diabetes mellitus, and hypertension.
The pure tone audiometric test (PTA) was conducted using an automated screening audiometer from the brand SHOEBOX Ottawa, Canada, equipped with a soundproof headset from the brand Radio Ear DD450 minnesota, USA. The frequency measurements from 500 to 8000 hz and intensities from −10 to 130 dB, validated according to Canadian expert guidelines, were used.22 The audiogram allowed for the quantification of hearing loss in each ear in decibels, for the frequencies 500Hz, 1000Hz, 2000Hz, 4000Hz, and 8000Hz in air conduction.23 The audiometric test was conducted in a quiet room, with the examiner positioned behind the patient, who was seated and equipped with headphones, the earpiece applied to the ear centered on the concha; the red earpiece placed on the right and the blue on the left ear. The instruction given by the examiner was to respond immediately by raising the hand each time a sound was perceived by the patient in either ear, even if the sound was faint, and to lower the hand when the sound was not heard. The results of the audiometric test were automatically presented in the form of a graph (audiogram) on which the different hearing thresholds obtained were recorded, with frequencies (Hz) on the x-axis and intensities (dB) on the y-axis directed downwards.
The evaluation of the patients’ cognitive functions was carried out using a questionnaire from the Montreal Cognitive Assessment (MoCA) scale.24–27 French Version 7.1. The MoCA scale was designed to evaluate eight sub-tasks or cognitive domains, which included immediate memory (unmarked sub-domain),28 and the seven other cognitive domains visuospatial and executive scores rated out of 5 points, language abilities (fluency) (3 points), attention (6 points), temporal-spatial orientation (6 points), delayed memory (5 points), naming (3 points), and abstraction ability (2 points); the scoring was out of a maximum of 30 points.29 During the cognitive assessment, the patient was provided with a pencil, a scratch paper, and the questionnaire to be filled out after receiving instructions from the examiner both orally and in writing. The scoring was done directly on the answer sheet by the examiner, with 1 point for a correct answer and 0 points for an incorrect answer.
Operational Definitions
The hearing levels were defined according to the WHO recommendations:30
Normal hearing, defined by an average hearing threshold ≤ 25 dB
Mild hearing loss, defined as any hearing loss of 26–40 dB
Moderate hearing loss, any hearing loss of 41–60 dB
Severe hearing loss, considered any hearing loss of 61–80 dB
Deep hearing loss, represented any hearing loss > 80 dB.
Hearing loss was considered bilateral when the initial auditory threshold in the better ear was > 25dB.
Cognitive impairment was defined by a global score on the MoCA scale ˂ 26 out of 30 points.29 The scoring was interpreted as follows:31
≥ 26/30: no cognitive impairment;
18–25/30: mild cognitive impairment;
10–17/30: moderate cognitive impairment;
<10/30: severe cognitive impairment.
Statistical Analysis of the Data
The data were initially entered using Microsoft Excel 2019, processed, and analyzed using IBM SPSS Statistics 26.0 software. The qualitative variables were presented in terms of frequencies and proportions in percentage. The quantitative data were summarized in means and standard deviations (SD). The univariate analysis allowed for the comparison of proportions between two study groups using Pearson’s chi-square test. The odds ratio and the bivariate correlation (Spearman coefficient: r) between the explanatory/independent variables such as age advancement/immunosenescence, sex, marital status, education level, socioeconomic status, hypertension, diabetes, tinnitus/hyperacusis, the notion of alcohol, tobacco, and the severity of hearing loss (r=9.288) with cognitive disorders (dependent variable) were calculated.
As for the means between the groups, they were compared using the ANOVA test. Univariate and multivariate associations and odds ratio (OR) estimates were calculated with a 95% confidence interval (CI). The multivariate logistic regression model was used with OR, 95% CI after adjusting for confounding factors to identify the independent and significant determinants of cognitive disorders in the study population. The p-value of ˂ 0.05 was considered statistically significant. Regarding potential information biases, these were eliminated, firstly by using appropriate scales as guided by Evidence Based Medicine, secondly by utilizing literature data from PubMed and Google Scholar, and thirdly through the statistical analyses conducted in this study, particularly the use of logistic regression (multivariate analysis) which excluded confounding variables.
Results
General Characteristics of the Patients
Out of a total of 150 patients, there was no overrepresentation of males or females with a sex ratio of 1 man: 1 woman. The average age of the study participants was 54.11 ± 20 years, ranging from 20 to 87 years. The advancement in age (≥60 years) represented 40.7% of the patients (n=61). The majority of patients who participated in the study were single, followed by widows with 34.7% and 27.3%, respectively. The secondary education level was more represented with 41.3%, and the socioeconomic level of the patients was relatively low for 72% of them. The majority of patients’ complaints were hearing loss at 89% (n=134), followed by tinnitus at 80% (n=120). (Table 1). A good number of patients reported a worsening of their hearing loss in less than 5 years, specifically 85%; the average duration without worsening of hearing loss was estimated at 2.3 ±1.4 years, within a range of a minimum of 1 month and a maximum of 48 months.
Table 1.
General Characteristics of Patients
| Variables | Total (n=150) | Percentage (%) |
|---|---|---|
| Years | ||
| < 60 years | 89 | 59.3 |
| ≥ 60 years | 61 | 40.7 |
| Sex | ||
| Female | 72 | 48 |
| Male | 78 | 52 |
| Marital status | ||
| Widow(er) | 41 | 27.3 |
| Divorced | 24 | 16 |
| Married in a couple | 33 | 22 |
| Single | 52 | 34.7 |
| Education level | ||
| Primary | 22 | 14.7 |
| Secondary | 62 | 41.3 |
| University | 34 | 22.7 |
| Postgraduate | 32 | 21.3 |
| Socio-economic level | ||
| Low | 108 | 72 |
| High | 42 | 28 |
| History | ||
| Tobacco | 67 | 44.7 |
| Alcohol | 102 | 68 |
| Diabetes | 101 | 67.3 |
| Hypertension | 104 | 69.3 |
| Complaints upon admission | ||
| Hearing loss | 134 | 89 |
| Tinnitus | 120 | 80 |
| Duration without worsening of self-reported hearing loss | ||
| ≤ 5 years | 23 | 15 |
| ˃ 5 years | 127 | 85 |
Audiometric Characteristics
SNHL Predominated in Bilateral Hearing Impairments with 80% (n=120)
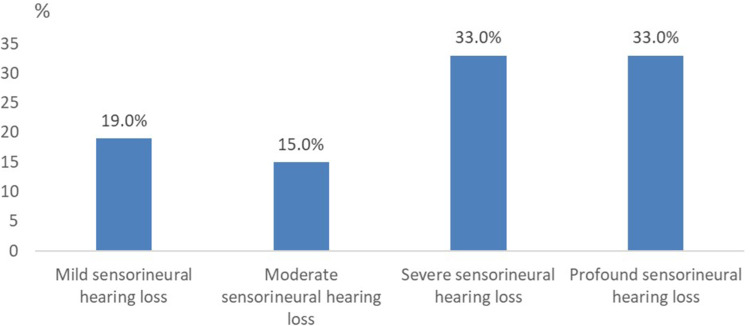
Patients with mild, moderate, severe, and profound hearing loss at PTA represented 19.3% (n=29), 14.6% (n=22), 32.6% (n=49), and 33.3% (n=50), respectively (Figure 1).
Figure 1.
Distribution of patients according to the degree of sensorineural hearing loss.
Characteristics of Cognitive Assessment
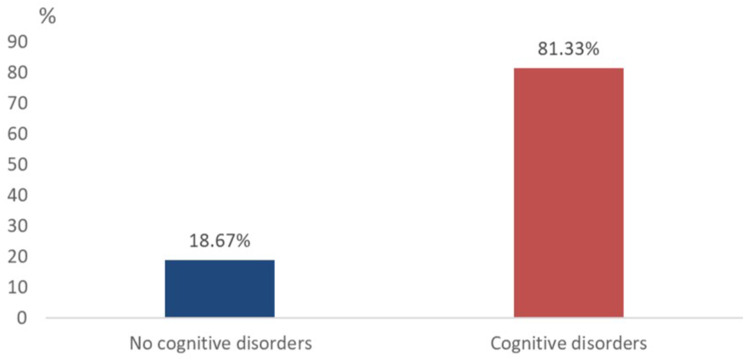
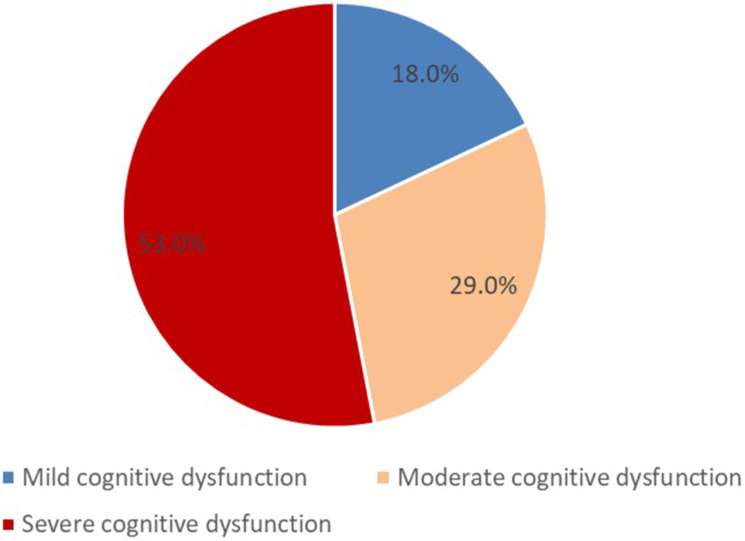
In the study population, the overall mean score on the MoCA cognitive assessment scale was 16.5±6.7 points; the median and mode being 15 and 12 points, respectively; on a range from 10 to 30 points. The different cognitive domains assessed are listed in Table 2. The majority of patients, 81% (n=122), presented with a comorbidity of cognitive disorders and sensorineural hearing loss (Figure 2) according to the following biological gradient: 18% (n=22) of patients with mild cognitive disorders, 29% (n=35) of patients with moderate cognitive disorders, and 53% (n=65) of patients with severe cognitive disorders. Thus, severe cognitive disorders were overrepresented (Figure 3).
Table 2.
Scores of the Different Cognitive Domains of Patients Obtained Using the MoCA Scale
| Variables | Mean ± SD |
|---|---|
| Visuospatial ability | 4±1 |
| Naming | 2.7±0.4 |
| Memory | 4±0.9 |
| Attention | 3.8±1.2 |
| Language | 2.5±0.7 |
| Abstraction | 1.7±0.6 |
| Temporal-spatial orientation | 5±0.6 |
Figure 2.
Frequency of cognitive disorders in the study population.
Figure 3.
Distribution of patients according to the degree of cognitive dysfunction.
Univariate Analysis of Factors Associated with Cognitive Disorders and SNHL
Socio-Demographic and Clinical Factors Associated with Cognitive Disorders
In univariate analysis, as presented in Table 3, excluding age, sex, and socio-economic status, all other socio-demographic and clinical parameters were significantly associated with cognitive disorders in the study population (p < 0.05). These include marital status, education level, smoking and alcohol consumption, patients with a history of diabetes and hypertension, as well as tinnitus (Table 3).
Table 3.
Socio-Demographic and Clinical Parameters Associated with Cognitive Disorders
| Variables | Cognitive disorders | Risk | IC 95% | p | |
|---|---|---|---|---|---|
| Yes | No | ||||
| Years | |||||
|
51(84) | 10(16) | 0.7 | (0.3–1.8) | 0.356 |
|
71(80) | 18(20) | |||
| Sex | |||||
|
55(76) | 17(24) | 0.5 | (0.2–1.2) | 0.1 |
|
67(86) | 11(14) | |||
| Civil status | |||||
|
88 (90) | 10 (10) | 5 | (2–11) | ˂0.0001 |
|
34(65) | 18(35) | |||
| Education level | |||||
| Primary/Secondary | 62(74) | 22 (26) | 3.5 | (1.3–9.3) | 0.006 |
| University/Postgraduate | 60(91) | 6(9) | |||
| Socio-economic level | |||||
|
84(78) | 24(22) | 2,7 | (0.8–8.3) | 0.055 |
|
38(90) | 4(10) | |||
| Alcohol | |||||
|
97(95) | 5(5) | 18 | (6–52) | ˂0.0001 |
|
25 (52) | 23(48) | |||
| Smoking | |||||
|
66 (99) | 1(1) | 32 | (4–242) | ˂0.0001 |
|
56(67) | 27(33) | |||
| Diabetes | |||||
|
95(94) | 6(6) | 2 | (1.6–3) | ˂0.0001 |
|
21(43) | 28(57) | |||
| Hypertension | |||||
|
97(93) | 7(7) | 0.08 | (0.03–0.23) | ˂0.0001 |
|
25(54) | 21(46) | |||
| Tinnitus | |||||
|
92(77) | 28(23) | 0.7 | (0.6–0.8) | 0.001 |
|
30(100) | 0(0) | |||
Site, Duration, and Degree of Hearing Loss Associated with Cognitive Disorders
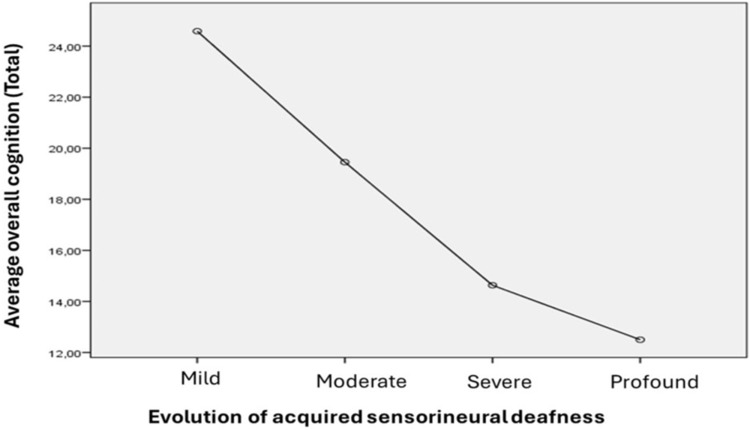
In the present study, cognitive disorders were more localized bilaterally at 93% than unilaterally at 33%, with statistical significance being very significant (P˂0.0001); the risk of developing cognitive disorders was 28 times higher in patients with bilateral hearing loss than in those with unilateral hearing loss (Table 4). Cognitive disorders were more noted in severe and profound sensorineural hearing loss than in mild and moderate sensorineural hearing loss, with a very significant statistical difference (p < 0.0001) (Table 4). The univariate ANOVA analysis demonstrated a very significant correlation (p ˂ 0.0001) between the decrease in cognitive functions on the MoCA cognitive assessment scale and the severity of auditory impairment on the PTA (Figure 4). The comparison of the mean cognitive scores on the MoCA scale between the SNHL groups showed a very significant difference (p˂0.0001) between the SNHL groups of mild (24.5±7.2), moderate (19.4±8.2), severe (14.6±3.2), and profound (12.5±2.8).
Table 4.
Site, Duration, and Degree of Hearing Loss Associated with Cognitive Disorders
| Characteristics | Cognitive disorders | Risque | IC 95% | p | |
|---|---|---|---|---|---|
| yes | No | ||||
Anatomical localization of auditory impairment
|
112 (93) 10 (33) |
8(7) 20 (67) |
28 | (10–80) | ˂0.0001 |
Duration without worsening of self-reported hearing loss≤ 5 years
|
23(100) 99(78) |
0(0) 28(22) |
1.2 | (1.1–1.4) | 0.006 |
| Degree of sensorineural hearing loss at PTA
|
9 (31) 14(64) 49 (100) 50 (100) |
20(69) 8(36) 0 (0) 0(0) |
˂0.0001 | ||
Figure 4.
Correlation between the average score on the cognitive scale (MoCA) and the severity of sensorineural hearing loss.
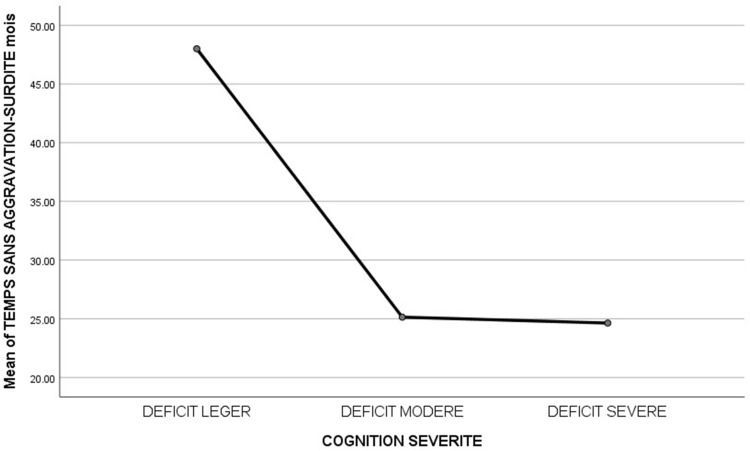
As illustrated in Figure 5, all patients whose SNHL worsened in no more than 5 years developed cognitive disorders, and according to the ANOVA analysis, patients whose hearing loss worsened suddenly developed moderate to severe cognitive disorders (Figure 5).
Figure 5.
Correlation between the duration without worsening of sensorineural hearing loss and the severity of cognitive impairment.
In bivariate analysis, the correlation between the different cognitive domains constituting the MoCA cognitive scale and SNHL, there was a significant correlation (p˂0.05) between temporospatial orientation (r =0.975), visuospatial ability (r=0.902), memory (r=0.882), attention (r=0.804), language (r=0.795), abstraction ability (r=0.777) and SNHL. Table 5, comparing the degree of hearing loss at PTA and the different cognitive domains on the MoCA scale, demonstrates a more pronounced deterioration in visuospatial ability as well as temporospatial orientation with the increase in hearing loss (p˂0.05) (Table 5).
Table 5.
Correlation Between the Degree of Hearing Loss at PTA and the Different Cognitive Domains on the MoCA Scale
| Variables | Degrees of sensorineural hearing loss | p | |||
|---|---|---|---|---|---|
| Mild | Moderate | Severe | Profound | ||
| Visuospatial ability | 4.2±0.8 | 4.6±0.5 | 4.2±1 | 3.8±1.1 | 0.009 |
| Denomination | 2.7±0.5 | 2.8±12 | 2.8±0.4 | 2.7±0.4 | 0.047 |
| Memory | 4.3±0.7 | 4.3±0.8 | 4.2±0.9 | 4.3±1.0 | 0.010 |
| Attention | 3.8±1.1 | 4.3±1.1 | 3.8±1.3 | 3.6±1.2 | 0.019 |
| Language | 2.6±0.5 | 2.5±0.7 | 2.6±0.8 | 2.4±0.9 | 0.036 |
| Abstraction | 1.7±0.4 | 2.0±0.7 | 1.6±0.5 | 1.6±0.7 | 0.011 |
| Temporal-spatial orientation | 5.2±0.8 | 5.2±1.1 | 5.2±0.9 | 4.7±1.3 | 0.003 |
Multivariate Analysis
Determinants of Cognitive Disorders
After excluding confounding factors such as marital status, education level, and diabetes; the multivariate analysis using binary logistic regression identified alcohol, tobacco, and hypertension as independent and significant determinants (p<0.05) associated with cognitive disorders in the study population (Table 6).
Table 6.
Independent Determinants of Cognitive Disorders in the Study Population
| Variables | A | E.S | Wald | ddl | Exp (OR) | IC (95%) | p |
|---|---|---|---|---|---|---|---|
| Alcohol | 2.508 | 0.621 | 16.328 | 1 | 12.286 | (4–41) | ˂0.0001 |
| Tobacco | 3.131 | 1.096 | 8.167 | 1 | 22.906 | (3–196) | 0.004 |
| High blood pressure | 1.658 | 0.611 | 7.352 | 1 | 5.246 | (1.5–17) | 0.007 |
| Constant | −0.874 | 0.717 | 1.488 | 1 | 0.417 | 0.223 |
Discussion
This article recalled the main objective of the present study, namely to establish the correlation between cognitive disorders and SNHL at the “Village Bondeko” center.
Thus, the most important results were discussed according to different mathematical models and pathophysiological bases after confronting them with the results of the literature, confirming or refuting the study’s findings.
The results of the present study corroborate (confirm) the data from the scientific literature within the framework of the exposome (set of exposures), and the influence of advancing age, gender, education level, and socio-economic status, marital status, hypertension, diabetes, tinnitus, the concept of alcohol and tobacco.32
Descriptive Approach
Age and Sex
The descriptive approach highlighted the gender neutrality in the study population of the present study. This neutrality has also been observed in other studies, notably in Iran33 and Sweden.34 However, the predominance of one sex over the other has been observed both in Africa and the rest of the world.35–37 If the small sample size (n=150), the profession of the patients, as well as the acquired origin of the SNHL in the study population, can explain the gender neutrality in the present study (men do more work related to noise exposure), it should nevertheless be noted that connexin 26 associated with the Y chromosome is linked to the onset of congenital SNHL. The presence of this protein could explain the predominance of the male sex in most studies conducted worldwide.38 Indeed, in these studies, the SNHL was mostly of congenital origin.
The selection criterion for the study population, based on the feasibility of auditory and cognitive tests, included patients with an average age of 54.11 years ±20 years. This choice is reinforced by the fact that the entire study population had acquired SNHL with various etiologies, most of which are characteristic of advanced age (immunosenescence, degenerative diseases), or are due to the patients’ professions.39,40
Education and Socio-Economic Level
The education level of the population in this study was more primary/secondary (56%) than university/post-university (44%) and explains the different professions practiced by this population (vendeurs, ouviers, taximans). The level of education can also explain why ¾ of the patients in this study (n=108) were characterized by a low socio-economic status. This economic precariousness can be explained by disabilities and early retirements caused by SNHL.41,42
Univariate Analysis
Cognitive Dysfunction and SNHL
SNHL, the third leading cause of disability in the world, the main modifiable risk factor for cognitive disorders and dementia, has been the subject of several studies worldwide, highlighting the impact of SNHL on the occurrence of cognitive disorders, especially in severe stages but also in cases of mild hearing loss, albeit to a lesser degree.43
Low cognitive scores on the MoCA cognitive assessment in patients with SNHL have been described by several recent studies, such as those by Moradi et al in Norway, in 2024, and by Hui-Fu et al in China, in 2023.44,45
The correlational and comparative approach of the present study demonstrating the significant association between the deterioration of cognitive functions on the MoCA scale and the increase in hearing loss aligns with the findings by Tongxiang et al. In China, in 2021, highlighting a linear trend between the increase in hearing thresholds at PTA and the increased risk of dementia.46
In a longitudinal study conducted in Baltimore, USA, involving adults aged 36 to 90 years, it was demonstrated that hearing loss was predictive of the risk of developing dementia, with an incidence multiplied by 2 in the case of mild hearing loss (25 to 40 dB), by 3 in the case of moderate hearing loss (41 dB to 70 dB), and by 5 in the case of severe hearing loss (>70 dB).47 This reinforces the links established by the literature indicating that SNHL can contribute to cognitive decline. Indeed, different mechanisms are involved in the case of SNHL. The absence of prolonged auditory stimulation is responsible for a structural and functional alteration of the brain, resulting in global brain atrophy, particularly a decrease in gray matter in the temporal lobe, prefrontal auditory areas, frontal areas, and the hippocampus.48 These changes occur in brain regions essential for cognitive processing (memory, language comprehension). Furthermore, the excessive use of cognitive resources to improve listening abilities makes them unavailable for other aspects of higher cognition during the listening effort; this results in an exhaustion of cognitive reserves.49
However, some results contrary to previous studies have rather demonstrated that there is no association between SNHL and cognitive disorders.50 This difference could be explained by the variability in the choice of cognitive tests, the types of audiometry used, and probably also the quality of the human resources involved in the analysis of these results, which were not better equipped.
The significant percentage of cognitive disorders found in the present study could also be explained by the fact that the majority of patients did not wear hearing aids. However, it is established in the literature that the use of hearing aids reduces the incidence of dementia in people with hearing loss.51,52 A recent study demonstrated that unilateral cochlear implantation in an adult population with severe to profound bilateral sensorineural hearing loss had a positive effect on cognitive functioning and quality of life one year after activation.53
In the present study, patients whose hearing loss worsened suddenly were at a higher risk of developing moderate to severe cognitive disorders. The current results corroborate those of a cohort study in China, which revealed that over a 7-year follow-up period, patients with sudden SNHL had a higher risk of dementia.54 In addition to the various characteristics of SNHL in the study population, such as the severity of SNHL, its bilateral nature, and especially associated symptoms like tinnitus, which according to some studies are responsible for an increased risk of early-onset dementia in young and middle-aged adults.55,56 These characteristics could exacerbate chronic stress, social isolation, loss of productivity, and significantly influence cognitive performance.57
Exposome and Cognitive Disorders
Various factors, including sociodemographic factors, lifestyle-related factors, environmental factors, and cardiometabolic factors, have been identified as potential contributors to the onset of part of SNHL58 and cognitive disorders.59
In the present study, patients who were married in couples and had been married (divorced and widowed) had a fivefold increased risk of developing cognitive disorders compared to singles. It is established by several longitudinal studies and meta-analyses that loneliness significantly contributes to cognitive disorders and dementia.60,61 In the D.R. Congo, divorce, and especially widowhood, constitute permanent psychological stress factors for young adults due to relatively low income and generally higher number of children.62 The overpopulation of the city of Kinshasa linked to industrialization also explains the different types of pollution, atmospheric63,64 and noise, which significantly influence SNHL and cognition.65
If the level of education as well as the socio-economic status of the population in this study could partly explain the possibility of cognitive disorders due to a feeling of incapacity; cardiovascular risk factors such as a history of diabetes, hypertension, and the notion of alcohol and tobacco highlight the important role of metabolic syndrome in SNHL and cognitive decline. The results of the present study, which state that the risk of developing cognitive disorders was twice as high in patients with diabetes, align with research conducted on the association between diabetes on one hand and SNHL and cognitive decline on the other.66,67
Numerous studies, such as the one conducted by Babarinde et al in 2021 in a Cardiology department in Nigeria, have highlighted a significant association between hypertension and SNHL (p < 0.01). Hypertension-related SNHL increased with age, severity, and duration of the disease.68 Indeed, the stria vascularis, being supplied by labyrinthine arteries without collateral circulation, is very sensitive to the impact of ischemic lesions that can lead to the death of hair cells and, ultimately, to hearing loss caused by hypoxia resulting in progressive or sudden hearing loss.69–71 However, these same microvascular causes found in cardiovascular diseases affect both cerebral vascularization in addition to that of the cochlea; they can thus be responsible for hearing loss and cognitive impairment.72 These so-called atherogenic diseases, whose incidence is increasing due to urbanization, lifestyle changes, and the nutritional transition towards a Western-type diet, also constitute a major health problem in less developed countries.73–76
The finding of an increased risk of dementia in people who drink alcohol was also demonstrated by Fitzpatrick et al in England, in 2023.77 Yet some studies continue to show results consistent with the protective effects of low to moderate alcohol consumption on dementia and cognitive functions.78 A cohort study found that abstaining from alcohol and consuming more than 14 units of alcohol per week are associated with an increased risk of dementia. However, the risk of dementia among abstainers could be partly attributable to cardiometabolic disease.79
Multivariate Analysis
Independent Determinants of Cognitive Disorders in Multivariate Analysis
In the present study, multivariate logistic regression identified alcohol, tobacco, and hypertension as independent and significant determinants of cognitive disorders. In addition to the studies by Gareth-Hagger et al in the United Kingdom in 201380 and by H. Hendriks et al in the Netherlands in 2020,81 several other studies have been conducted to establish the deleterious effects of chronic alcohol and tobacco consumption on cognitive functions. Indeed, chronic alcohol consumption would increase the risk of dementia by causing structural and functional brain damage, responsible for a decline in cognitive functions, particularly a decrease in learning abilities, memory, attention, and problem-solving capacity.82,83 Regarding smoking and hypertension, their influences on the decline in cognitive performance and the occurrence of SNHL are well established.84–88
Strengths and Limitations of the Study
The present study was limited to a certain extent. The small sample size, the lack of genetic information from patients which did not allow for the consideration of all types of sensorineural hearing loss, as well as the unavailability of certain equipment for the assessment of hearing loss in very high-frequency auditory impairments. Indeed, only tonal audiometry was used in the present study. This study only used the MoCA cognitive assessment test, allowing for the evaluation of overall cognition; however, there are other tests that are much more specific for evaluating each cognitive domain separately. Therefore, it is difficult to accurately demonstrate which specific cognitive domain is most affected by SNHL. In addition, the absence of a control group without SNHL also constitutes a limitation of the study. Furthermore, although the “Bondeko Village” Center serves as a tertiary reference in the organization of primary care within the Catholic network, the results of this study cannot be generalized.
However, this study has the merit of being the first to have investigated the association between cognitive disorders and SNHL in the DRC. In addition to this, the use of odds ratios measured with confidence intervals (CI) for univariate and multivariate analyses has strengthened the credibility of our results. The relevance of the obtained results remains a major asset in the development of subsequent research projects.
Conclusion and Perspectives
This study confirmed, based on a very significant association, the link between cognitive disorders and SNHL. The present study also emphasized the risk factors significantly associated with cognitive disorders such as low education level, marital status, hypertension, diabetes, tinnitus, alcohol consumption, and tobacco use, but could not demonstrate an association between aging, gender, and socioeconomic status with cognitive disorders. Other studies, such as cohort studies and meta-analyses, would be necessary in order to contribute, within this multidisciplinary approach, to the improvement of the management of sensorineural hearing loss by integrating the neuropsychological aspect.
The negative impact of SNHL on cognitive aspects should be a public health priority for policymakers and public health leaders, with the aim of improving the availability and quality of care for individuals with SNHL, especially in developing countries where audiology and otorhinolaryngology care are limited, and where the lack of effective health coverage reduces access to hearing rehabilitation services (hearing aids, cochlear implants). Moreover, patients with SNHL should benefit from cognitive support and psychological counseling. In the absence of effective interventions on hearing health in SSA, and in particular in DRC, disabling SNHL and its consequences on cognition would further increase unproductivity, and thus the economic burden on health systems.
Abbréviations
SSA, Sub-Saharan Africa; PTA, PTA: Pur tone audiometrydB, Decibel; MoCA, Montreal cognitive assessment; MMSE, Mini-Mental State Exam; WHO, World Health Organization; ENT, Otolaryngology; DRC, Democratic Republic of Congo; SNHL, Sensorineural hearing loss; SPSS, Statistical Package for the social sciences; US, United States.
Ethical Considerations
The protocol of this study had obtained approval from the National Ethics Committee under the number 446/CNES/BN/PMMF/2023. All participants were informed of the objective of the study, in accordance with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflict of interest.
References
- 1.Lisan Q, Goldberg M, Lahlou G, et al. Prevalence of Hearing Loss and Hearing Aid Use Among Adults in France in the CONSTANCES Study. JAMA Network Open. 2022;5(6):e2217633. doi: 10.1001/jamanetworkopen.2022.17633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haile LM, Kamenov K, Briant PS, et al. Hearing loss prevalence and years lived with disability, 1990–2019: findings from the Global Burden of Disease Study 2019. Lancet. 13(10278):996‑1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulwafu W, Kuper H, Ensink RJH. Prevalence and causes of hearing impairment in Africa. Trop Med Int Health. 2016;21(2):158‑65. doi: 10.1111/tmi.12640 [DOI] [PubMed] [Google Scholar]
- 4.Choi JE, Hong SH, Moon IJ. Academic Performance, Communication, and Psychosocial Development of Prelingual Deaf Children with Cochlear Implants in Mainstream Schools. J Audiol Otol Avr. 2020;24(2):61‑70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shukla A, Harper M, Pedersen E, et al. Hearing Loss, Loneliness, and Social Isolation: a Systematic Review. Otolar Head Neck Surg off J Am Acad Otolar Head Neck Surg. 2020;162(5):622‑33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA. Association of Age-Related Hearing Loss With Cognitive Function, Cognitive Impairment, and Dementia: a Systematic Review and Meta-analysis. JAMA Otolar Head Neck Surg. 144(2):115‑26. doi: 10.1001/jamaoto.2017.2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Livingston G, Huntley J, Liu KY, et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet. 2024;404(10452):572‑628. [DOI] [PubMed] [Google Scholar]
- 8.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet Lond Engl. 2020;396(10248):413‑46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cejas I, Barker DH, Petruzzello E, Sarangoulis CM, Quittner AL. Cochlear Implantation and Educational and Quality-of-Life Outcomes in Adolescence. JAMA Otolar Head Neck Surg. 2023;149(8):708‑15. doi: 10.1001/jamaoto.2023.1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Convertino C, Borgna G, Marschark M, Durkin A. Word and world knowledge among deaf learners with and without cochlear implants. J Deaf Stud Deaf Educ. 2014;19(4):471‑83. doi: 10.1093/deafed/enu024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaumbu Nsapu I M, Luwa E-Andjafono D O, Matanda Nzanza R, et al. Effects of Environmental Noise on School Performance among Hearing-Impaired Students. Int J Otolaryngol Head Amp Neck Surg. 2022;11(05):242‑57. [Google Scholar]
- 12.Deal JA, Betz J, Yaffe K, et al. Hearing Impairment and Incident Dementia and Cognitive Decline in Older Adults: the Health ABC Study. J Gerontol a Biol Sci Med Sci. 72(5):703‑9. doi: 10.1093/gerona/glw069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ying G, Zhao G, Xu X, Su S, Xie X. Association of age-related hearing loss with cognitive impairment and dementia: an umbrella review. Front Aging Neurosci. 2023;15:1241224. doi: 10.3389/fnagi.2023.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deafness and hearing loss [Internet]. Available from: https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss. Accessed December 25, 2024
- 15.AlMalki FA, Albukhaty S, Alyamani AA, Khalaf MN, Thomas S. The relevant information about the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) using the five-question approach (when, where, what, why, and how) and its impact on the environment. Environ Sci Pollut Res Int. 2023;30(22):61430‑54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.OMS | Bureau régional pour l’Afrique [Internet]. 2024. Available from: Situation reports on COVID-19 outbreak, 18 March 2020. https://www.afro.who.int/publications/situation-reports-covid-19-outbreak-18-march-2020. Accessed December 25, 2024
- 17.Juma CA, Mushabaa NK, Abdu Salam F, Ahmadi A, Lucero-Prisno DE. COVID-19: the Current Situation in the Democratic Republic of Congo. Am J Trop Med Hyg. 2020;103(6):2168‑70. doi: 10.4269/ajtmh.20-1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng X, Wang J, Sun J, Zhu K. COVID-19 and Sudden Sensorineural Hearing Loss: a Systematic Review. Front Neurol. 2022;13:883749. doi: 10.3389/fneur.2022.883749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ceban F, Ling S, Lui LMW, et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: a systematic review and meta-analysis. Brain Behav Immun. 2022;101:93‑135. doi: 10.1016/j.bbi.2021.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinshasa Available from: https://www.google.com/maps/place/Kinshasa/@-4.3223442,15.3118448,15z/data=!3m1!4b1!4m6!3m5!1s0x1a6a3154d96a44bb:0xb0b25789df22586f!8m2!3d-4.3251555!4d15.3128644!16s%2Fm%2F03m9z25?entry=ttu. Accessed December 25, 2024
- 21.Association Bumba. Available from: VILLAGE BONDEKO | association Bumba: https://www.assobumba.org/village-bondeko. Accessed December 25, 2024
- 22.Frank A, Goldlist S, Mark Fraser AE, Bromwich M. Validation of SHOEBOX QuickTest Hearing Loss Screening Tool in Individuals With Cognitive Impairment. Front Digit Health. 13(3):724997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masson E EM-Consulte: Available from: https://www.em-consulte.com/article/658989/audiometrie-tonale. Accessed December 25, 2024
- 24.The longitudinal effect of sensory loss on depression among Chinese older adults – PubMed. Available from: https://pubmed.ncbi.nlm.nih.gov/33561802/. Accessed December 25, 2024 [DOI] [PubMed]
- 25.Version Papier Disponible sur. Available from: https://mocacognition.com/fr/version-papier/. Accessed December 25, 2024
- 26.Video. Disponible sur. Available from: https://mocacognition.com/video#remote_moca_video. Accessed December 25, 2024
- 27.Kang JM, Cho YS, Park S, et al. Montreal cognitive assessment reflects cognitive reserve. BMC Geriatr. 2018;18(1):261. doi: 10.1186/s12877-018-0951-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pirani A, Nasreddine Z, Neviani F, et al. MoCA 7.1: multicenter Validation of the First Italian Version of Montreal Cognitive Assessment. J Alzheimers Dis Rep. 2022;6(1):509‑20. doi: 10.3233/ADR-210053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc Avr. 2005;53(4):695‑9. [DOI] [PubMed] [Google Scholar]
- 30.Olusanya BO, Davis AC, Hoffman HJ. Hearing loss grades and the International classification of functioning, disability and health. Bull World Health Organ. 2019;97(10):725‑8. doi: 10.2471/BLT.19.230367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Julayanont P, Tangwongchai S, Hemrungrojn S, et al. The Montreal Cognitive Assessment—Basic: a Screening Tool for Mild Cognitive Impairment in Illiterate and Low-Educated Elderly Adults. J Am Geriatr Soc. 2015;63(12):2550‑4. doi: 10.1111/jgs.13820 [DOI] [PubMed] [Google Scholar]
- 32.Hendriks S, Ranson JM, Peetoom K, et al. Risk Factors for Young-Onset Dementia in the UK Biobank. JAMA Neurol. 1(2):134‑42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.ASGHARI A, FARHADI M, DANESHI A, et al. The Prevalence of Hearing Impairment by Age and Gender in a Population-based Study. Iran J Public Health. 2017;46(9):1237‑46. [PMC free article] [PubMed] [Google Scholar]
- 34.Severe-to-profound hearing impairment: demographic data, gender differences and benefits of audiological rehabilitation [Internet]. Available from: https://www.tandfonline.com/doi/epdf/10.1080/09638288.2018.1477208?needAccess=true. Accessed December 25, 2024 [DOI] [PubMed]
- 35.Villavisanis DF, Berson ER, Lauer AM, Cosetti MK, Schrode KM. Sex-Based Differences in Hearing Loss: perspectives from Non-Clinical Research to Clinical Outcomes. Otol Neurotol off Publ Am Otol Soc Am Neurotol Soc Eur Acad Otol Neurotol. 2020;41(3):290‑8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sa O, So A, D A. Burden of hearing loss in Subsaharan Africa: snapshot from an ENT clinic in Nigeria. Heighpubs Otolaryngol Rhinol. 2019;3(1):001‑5. [Google Scholar]
- 37.Khanal P, Acharya S, Lageju N. Pattern of Hearing Loss Among Patients Attending ENT Department of a Tertiary Hospital in Nepal: a Retrospective Study. Ind J Otolaryngol Head Neck Surg. 2022;74(Suppl 1):559. doi: 10.1007/s12070-021-02392-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-janabi AM, Ahmmed HS, Al-Khafaji SM. Connexin 26 (GJB2) gene mutations linked with autosomal recessive non-syndromic sensor neural hearing loss in the Iraqi population. J Med Life. 2021;14(6):841‑6. doi: 10.25122/jml-2021-0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang W, Zhao X, Chai R, Fan J. Progress on mechanisms of age-related hearing loss. Front Neurosci. 2023;17:1253574. doi: 10.3389/fnins.2023.1253574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Dong C, Han Y, Gu Z, Sun C. Immunosenescence, aging and successful aging. Front Immunol. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsimpida D, Kontopantelis E, Ashcroft D, Panagioti M. Socioeconomic and lifestyle factors associated with hearing loss in older adults: a cross-sectional study of the English Longitudinal Study of Ageing (ELSA). BMJ Open. 9(9):e031030. doi: 10.1136/bmjopen-2019-031030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malcolm KA, Suen JJ, Nieman CL. Socioeconomic position and hearing loss: current understanding and recent advances. Curr Opin Otolaryngol Head Neck Surg. 30(5):351‑7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin FR, Ferrucci L, Metter EJ, An Y, Zonderman AB, Resnick SM. Hearing Loss and Cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology. 2011;25(6):763‑70. doi: 10.1037/a0024238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moradi S, Engdahl B, Johannessen A, Selbæk G, Aarhus L, Haanes GG. Hearing loss, hearing aid use, and performance on the Montreal cognitive assessment (MoCA): findings from the HUNT study in Norway. Front Neurosci. 2024;17:1327759. doi: 10.3389/fnins.2023.1327759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu X, Eikelboom RH, Liu B, Wang S, Jayakody DMP. The longitudinal relationship between hearing loss and cognitive decline in tonal language-speaking older adults in China. Front Aging Neurosci. 15:1122607. doi: 10.3389/fnagi.2023.1122607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diao T, Ma X, Zhang J, Duan M, Yu L. The Correlation Between Hearing Loss, Especially High-Frequency Hearing Loss and Cognitive Decline Among the Elderly. Front Neurosci. 2021;15:750874. doi: 10.3389/fnins.2021.750874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin FR, Metter EJ, O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing Loss and Incident Dementia. Arch Neurol. 2011;68(2):214‑20. doi: 10.1001/archneurol.2010.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Croll PH, Vernooij MW, Reid RI, et al. Hearing loss and microstructural integrity of the brain in a dementia-free older population. Alzheimers Dement J Alzheimers Assoc. 2020;16(11):1515‑23. [DOI] [PubMed] [Google Scholar]
- 49.Griffiths TD, Lad M, Kumar S, et al. How Can Hearing Loss Cause Dementia? Neuron. 2020;108(3):401‑12. doi: 10.1016/j.neuron.2020.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bucks RS, Dunlop PD, Taljaard DS, et al. Hearing loss and cognition in the Busselton Baby Boomer cohort: an epidemiological study. Laryngoscope. 2016;126(10):2367‑75. doi: 10.1002/lary.25896 [DOI] [PubMed] [Google Scholar]
- 51.Yeo BSY, HJJMD S, Toh EMS, et al. Association of Hearing Aids and Cochlear Implants With Cognitive Decline and Dementia. JAMA Neurol. 2023;80(2):134‑41. doi: 10.1001/jamaneurol.2022.4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dawes P, Völter C. Do hearing loss interventions prevent dementia? Z Gerontol Geriatr. 2023;56(4):261‑8. doi: 10.1007/s00391-023-02178-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vandenbroeke T, Andries E, Lammers MJ, et al. Cognitive Changes Up to 4 Years After Cochlear Implantation in Older Adults: a Prospective Longitudinal Study Using the RBANS-H. Ear Hear. 2024. doi: 10.1097/AUD.0000000000001583 [DOI] [PubMed] [Google Scholar]
- 54.de Araújo PIMP, PLES D, Ramos HVL, et al. Impact of hearing impairment on cognitive performance. Braz J Otorhinolaryngol. 2024;91(2):101521. doi: 10.1016/j.bjorl.2024.101521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Butt WW, Wieland DR, Wang H, Lin CH, Wang JJ, Weng CH. Tinnitus and Dementia Risk: a Nationwide Population-based Case-control Study. J Laryngol Otol. 2024;1‑25. [DOI] [PubMed] [Google Scholar]
- 56.Cheng YF, Xirasagar S, Yang TH, Wu CS, Kao YW, Lin HC. Risk of early-onset dementia among persons with tinnitus: a retrospective case-control study. Sci Rep. 2021;11(1):13399. doi: 10.1038/s41598-021-92802-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Impact of social isolation on grey matter structure and cognitive functions: a population-based longitudinal neuroimaging study - PMC Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC10281670/. Accessed December 25, 2024 [DOI] [PMC free article] [PubMed]
- 58.Sokolo Gedikondele J, Longo-Mbenza B, Matanda Nzanza R, et al. Critical Review of Extreme Effects of Exposome/. Interaction Between Genetics and Environment on Hearing Disorders Reclassified. 2020. [Google Scholar]
- 59.Byrd DR, Martin DA, Joseph RP. Environmental, Sociocultural, Behavioral, and Biological Factors Associated with Cognitive Decline, Alzheimer’s Disease, and Other Types of Dementia in Black Americans. Curr Epidemiol Rep. 2023;10(4):252‑63. doi: 10.1007/s40471-023-00337-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harrington KD, Vasan S, Eun KJ, Sliwinski MJ, Lim MH. Loneliness and cognitive function in older adults without dementia: a systematic review and meta-analysis. J Alzheimers Dis JAD. 2023;91(4):1243‑59. doi: 10.3233/JAD-220832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lara E, Martín-María N, De la Torre-Luque A, et al. Does loneliness contribute to mild cognitive impairment and dementia? A systematic review and meta-analysis of longitudinal studies. Ageing Res Rev Juill. 2019;52:7‑16. [DOI] [PubMed] [Google Scholar]
- 62.Joseph-antoine MN, Benjamin L-M, Semo Christian M, Mbenza Ben L. Longo Maxime. causes and consequences of spoliation in the city of Kinshasa, the Democratic Republic of the Congo. Sci Rep. [Google Scholar]
- 63.Causes and Consequences of the Destruction of Green Spaces in the City of Kinshasa, the Democratic Republic of Congo. 2021.
- 64.Nkanga MSN, Longo-Mbenza B, Adeniyi OV, et al. Ageing, exposure to pollution, and interactions between climate change and local seasons as oxidant conditions predicting incident hematologic malignancy at Kinshasa University clinics, Democratic Republic of Congo (DRC). BMC Cancer. 2017;17(1):559. doi: 10.1186/s12885-017-3547-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ju MJ, Park SK, Kim SY, Choi YH. Long-term exposure to ambient air pollutants and hearing loss in Korean adults. Sci Total Environ. 2022;820:153124. doi: 10.1016/j.scitotenv.2022.153124 [DOI] [PubMed] [Google Scholar]
- 66.HK V, MN J, NL A, et al. Prévalence et facteurs associes des déficits neurocognitifs chez les patients diabétiques admis au centre neuro-psychopathologique de Kinshasa. Rev Afr Médecine Santé Publique. 2024;7(1):260‑71. [Google Scholar]
- 67.Doo JG, Kim D, Kim Y, et al. Biomarkers Suggesting Favorable Prognostic Outcomes in Sudden Sensorineural Hearing Loss. Int J Mol Sci. 2020;21(19):7248. doi: 10.3390/ijms21197248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Babarinde JA, Adeyemo AA, Adeoye AM. Hearing loss and hypertension: exploring the linkage. Egypt J Otolaryngol. 2021;37(1):98. doi: 10.1186/s43163-021-00162-1 [DOI] [Google Scholar]
- 69.Longo-Mbenza B, Muaka MM, Mokondjimobe E, Ndembe DK, Mona DT, Buassabu-Bu-Tsumbu B. Oxidative stress-elevated high gamma glutamyl transferase levels, and aging, intake of tropical food plants, migration and visual disability in Central Africans. Int J Ophthalmol. 2012;5(4):493‑8. doi: 10.3980/j.issn.2222-3959.2012.04.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gavriilaki E, Eftychidis I, Papassotiriou I. Update on endothelial dysfunction in COVID-19: severe disease, long COVID-19 and pediatric characteristics. J Lab Med. 2021;45(6):293‑302. doi: 10.1515/labmed-2021-0134 [DOI] [Google Scholar]
- 71.Teraoka M, Hato N, Inufusa H, You F. Role of Oxidative Stress in Sensorineural Hearing Loss. Int J Mol Sci. 2024;25(8):4146. doi: 10.3390/ijms25084146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wayne RV, Johnsrude IS. A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. Ageing Res Rev. 2015;23(Pt B):154‑66. doi: 10.1016/j.arr.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 73.Risasi Etutu Junior R, Lukusa MA, Amisi CM. Profil épidémiologique, clinique et facteurs de risque de diabète sucré. Cas de l’Hôpital Provincial Général de Référence de Kinshasa. Congo Res Pap. 2021;1(7):98‑115. [Google Scholar]
- 74.Tongo SY, Longo-Mbenza B, Aundu AM, et al. Are Any Changes in Carotid Intima–Media Thickness Associated with Cardiometabolic Risk Among Adult Bantu Central African Hypertensive Patients from Monkole and Biamba Marie Mutombo Hospitals? Vasc Health Risk Manag. 18:453‑61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mbala FK, Mbangi JM, Nkodila AN, et al. Determinants of Regional Obesity (Visceral and Subcutaneous Obesity) within Cardiovascular Risk Factors in the Cardiology Department of the University Clinics of Kinshasa. World J Cardiovasc Dis. 12(9):444‑56. [Google Scholar]
- 76.Kouvari M, D’Cunha NM, Travica N, et al. Metabolic Syndrome, Cognitive Impairment and the Role of Diet: a Narrative Review. Nutrients. 2022;14(2):333. doi: 10.3390/nu14020333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fitzpatrick L, Mortimore G. Alcohol-related dementia. Br J Nurs Mark Allen Publ. 2023;32(20):972‑7. [DOI] [PubMed] [Google Scholar]
- 78.Visontay R, Rao RT, Mewton L. Alcohol use and dementia: new research directions. Curr Opin Psychiatry. 2021;34(2):165‑70. doi: 10.1097/YCO.0000000000000679 [DOI] [PubMed] [Google Scholar]
- 79.Sabia S, Fayosse A, Dumurgier J, et al. Alcohol consumption and risk of dementia: 23 year follow-up of Whitehall II cohort study. BMJ. 2018;362:k2927. doi: 10.1136/bmj.k2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hagger-Johnson G, Sabia S, Brunner EJ, et al. Combined impact of smoking and heavy alcohol use on cognitive decline in early old age: Whitehall II prospective cohort study. Br J Psychiatry Août. 2013;203(2):120‑5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hendriks H, van de Rest O, Snippe A, Kieboom J, Hogenelst K. Alcohol Consumption, Drinking Patterns, and Cognitive Performance in Young Adults: a Cross-Sectional and Longitudinal Analysis. Nutrients. 12(1):200. doi: 10.3390/nu12010200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yalcin EB, Delikkaya BN, Pelit W, Tong M, La Monte SM D, Rounds S. The Differential Effects of Chronic Alcohol and Cigarette Smoke Exposures on Cognitive-Behavioral Dysfunction in Long Evans Rats. J Behav Brain Sci. 2022;12(9):413‑32. doi: 10.4236/jbbs.2022.129024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de la Monte SM, Kril JJ. Human alcohol-related neuropathology. Acta Neuropathol. 2014;127(1):71‑90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Campos MW, Serebrisky D, Castaldelli-Maia JM. Smoking and Cognition. Curr Drug Abuse Rev. 2016;9(2):76‑9. doi: 10.2174/1874473709666160803101633 [DOI] [PubMed] [Google Scholar]
- 85.Wattamwar K, Qian ZJ, Otter J, et al. Association of Cardiovascular Comorbidities With Hearing Loss in the Older Old. JAMA Otolar Head Neck Surg. 144(7). doi: 10.1001/jamaoto.2018.0643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hou Y, Liu B. Relationship Between Hypertension and Hearing Loss: analysis of the Related Factors. Clin Interv Aging. 2024;19:845‑56. doi: 10.2147/CIA.S458869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Gennip ACE, van Sloten TT, Fayosse A, et al. Age at cardiovascular disease onset, dementia risk, and the role of lifestyle factors. Alzheimers Dement. 2023;20(3):1693‑702. doi: 10.1002/alz.13562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ma’an ND, Turaki I, Shwe D, et al. Analysis of sensorineural hearing loss in patients attending an otolaryngology clinic in North Central Nigeria. PLOS Glob Public Health. 2023;3(4):e0000685. doi: 10.1371/journal.pgph.0000685 [DOI] [PMC free article] [PubMed] [Google Scholar]