Abstract
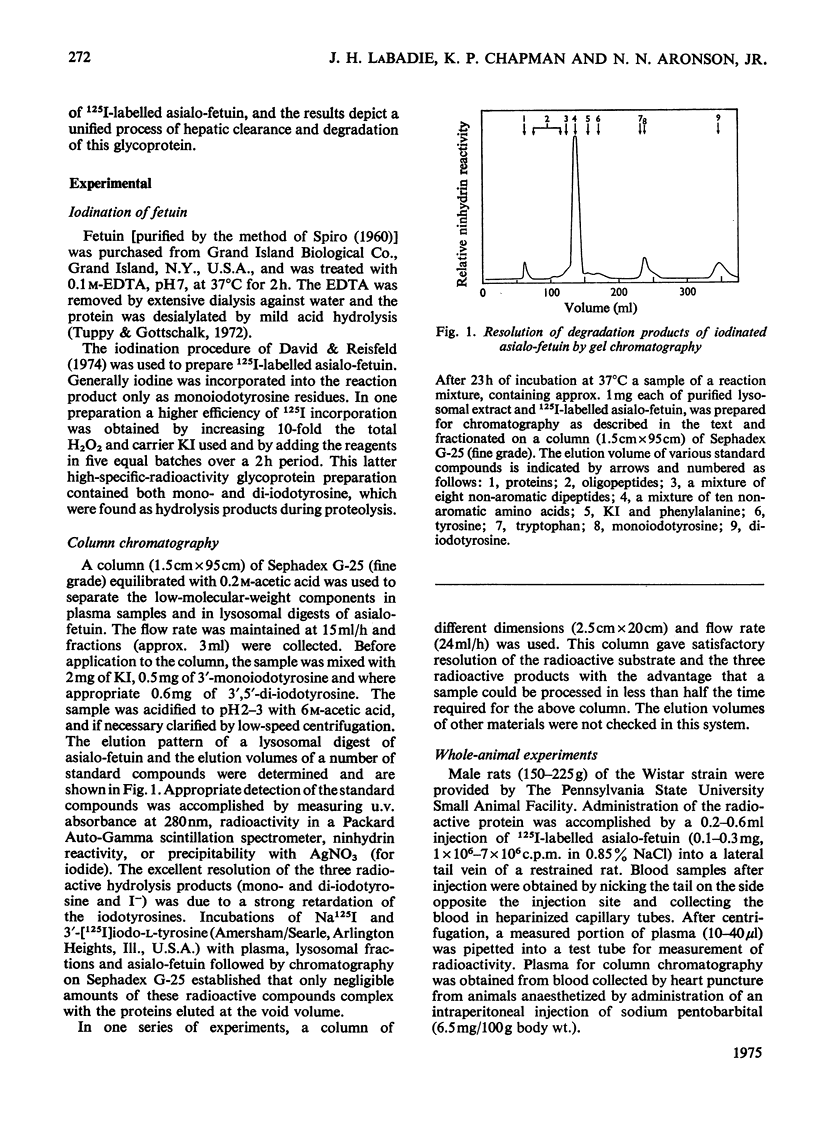
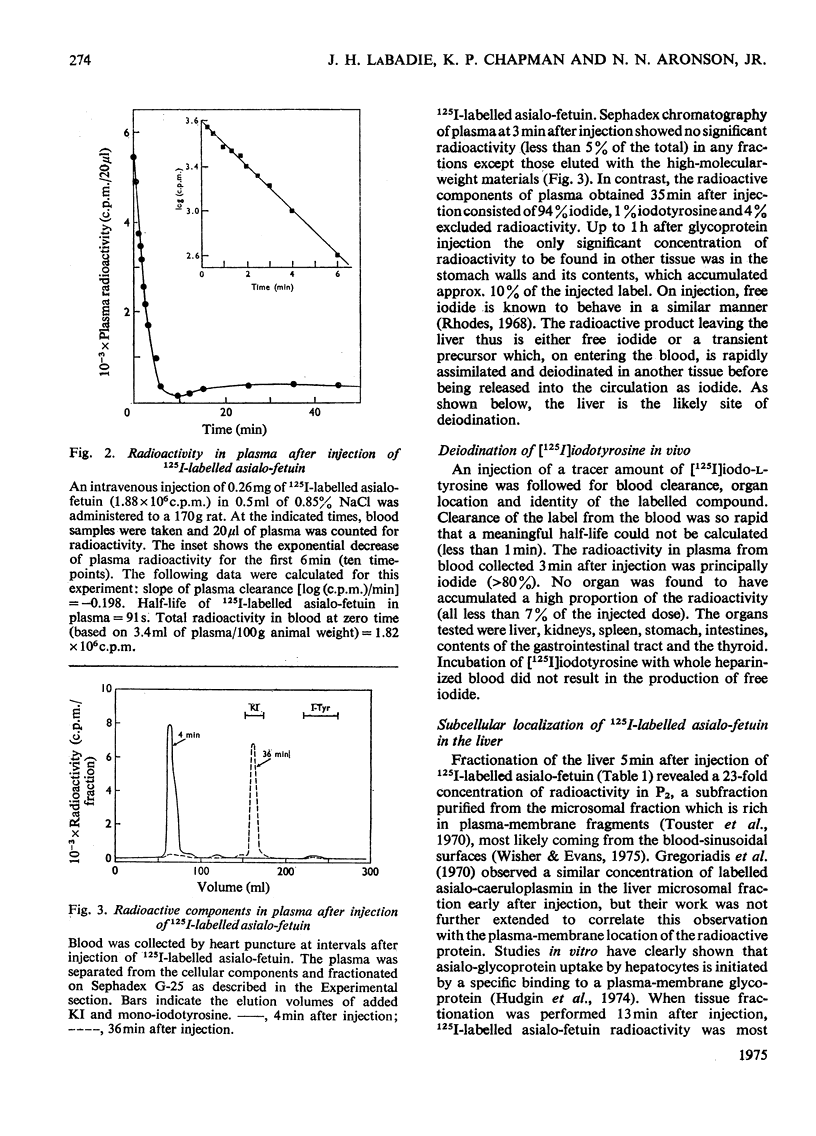
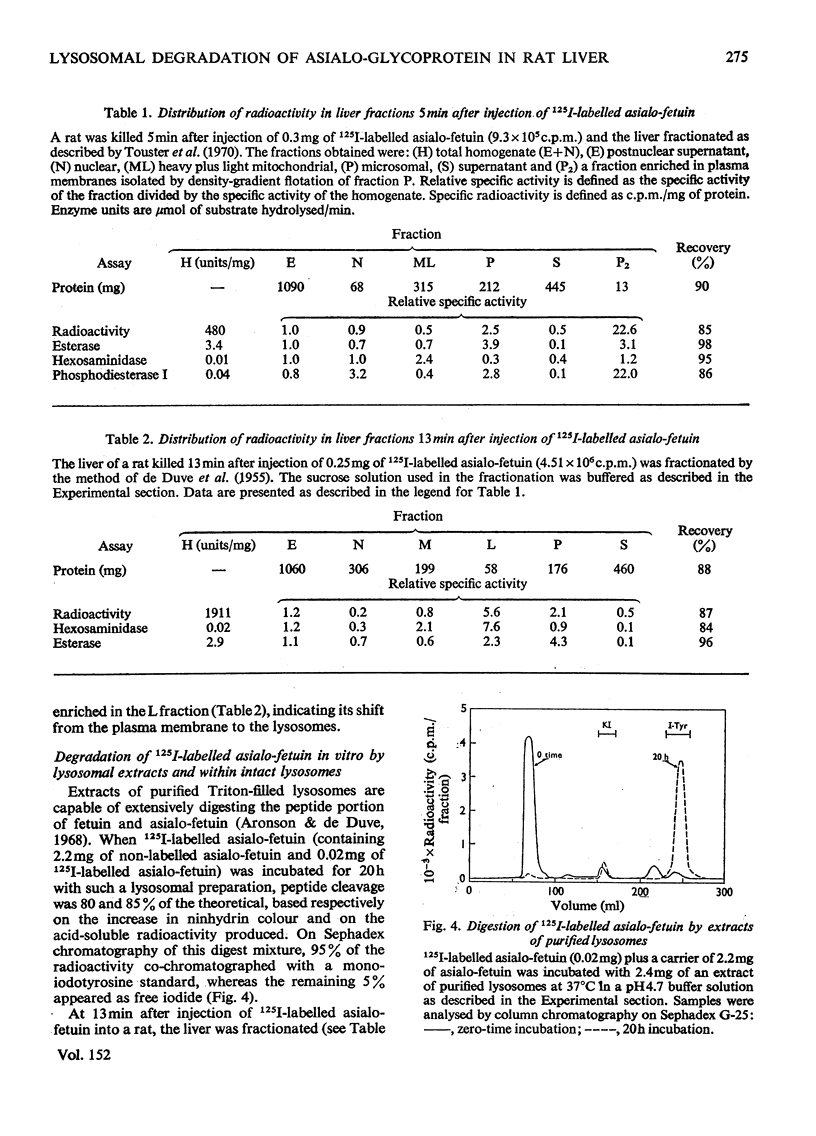
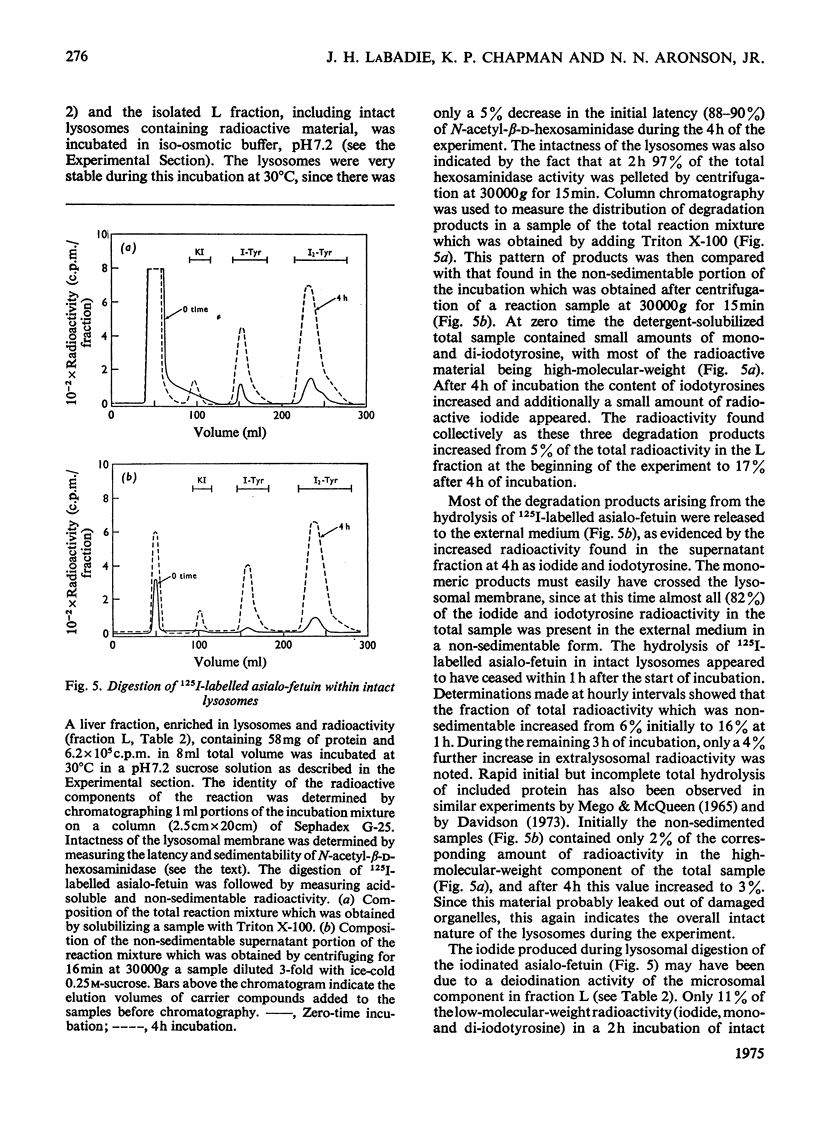
125I-labelled asialo-fetuin, administered intravenously, rapidly accumulates in rat liver and the radioactivity is subsequently cleared from the liver within 60min. Plasma radioactivity reaches a minimum between 10 and 15 min after injection and rises slightly during the period of liver clearance. Free iodide is the only radioactive compound found in plasma during this latter period. Fractionation of rat liver at 5 and 13min after injection of 125I-labelled asialo-fetuin supports the hypothesis that asialo-glycoprotein is taken into liver by pinocytosis after binding to the plasma membrane and is then hydrolysed by lysosomal enzymes. At 5min, radioactivity was concentrated 23-fold in a membrane fraction similarly enriched in phosphodiesterase I, a plasma-membrane marker enzyme, whereas at 13min the radioactivity appeared to be localized within lysosomes. Separation of three liver fractions (heavy mitochondrial, light mitochondrial and microsomal) on sucrose gradients revealed the presence of two populations of radioactive particles. One population banded in a region coincident with a lysosomal marker enzyme. The other, more abundant, population of radioactive particles had a density of 1.13 and contained some phosphodiesterase, but very little lysosomal enzyme. These latter particles appear to be pinocytotic vesicles produced after uptake of the asialo-fetuin bound by the plasma membrane. Lysosomal extracts extensively hydrolyse asialo-fetuin during incubation in vitro at pH4.7 and iodotyrosine is completely released from the iodinated glycoprotein. Protein digestion within lysosomes was demonstrated by incubating intact lysosomes containing 125I-labelled asialo-fetuin in iso-osmotic sucrose, pH7.2. The radioactive hydrolysis product, iodotyrosine, readily passed through the lysosomal membrane and was found in the external medium. These results are not sufficient to account for the presence of free iodide in plasma, but this was explained by the observation that iodotyrosines are deiodinated by microsomal enzymes in the presence of NADPH.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson N. N., Jr, De Duve C. Digestive activity of lysosomes. II. The digestion of macromolecular carbohydrates by extracts of rat liver lysosomes. J Biol Chem. 1968 Sep 10;243(17):4564–4573. [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G. S., Reisfeld R. A. Protein iodination with solid state lactoperoxidase. Biochemistry. 1974 Feb 26;13(5):1014–1021. doi: 10.1021/bi00702a028. [DOI] [PubMed] [Google Scholar]
- Davidson S. J. Protein absorption by renal cells. II. Very rapid lysosomal digestion of exogenous ribonuclease in vitro. J Cell Biol. 1973 Oct;59(1):213–222. doi: 10.1083/jcb.59.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas P., Maziere B., Autissier N., Michel R. Specificite de l'iodotyrosine desiodase des microsomes thyroïdiens et hepatiques. Biochim Biophys Acta. 1973 Jan 12;293(1):36–47. doi: 10.1016/0005-2744(73)90373-2. [DOI] [PubMed] [Google Scholar]
- Goldfischer S., Novikoff A. B., Albala A., Biempica L. Hemoglobin uptake by rat hepatocytes and its breakdown within lysosomes. J Cell Biol. 1970 Mar;44(3):513–529. doi: 10.1083/jcb.44.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriadis G., Morell A. G., Sternlieb I., Scheinberg I. H. Catabolism of desialylated ceruloplasmin in the liver. J Biol Chem. 1970 Nov 10;245(21):5833–5837. [PubMed] [Google Scholar]
- Gregoriadis G., Ryman B. E. Tritiation of glycoproteins. Biochem Biophys Res Commun. 1973 Jun 19;52(4):1134–1140. doi: 10.1016/0006-291x(73)90618-9. [DOI] [PubMed] [Google Scholar]
- Hudgin R. L., Pricer W. E., Jr, Ashwell G., Stockert R. J., Morell A. G. The isolation and properties of a rabbit liver binding protein specific for asialoglycoproteins. J Biol Chem. 1974 Sep 10;249(17):5536–5543. [PubMed] [Google Scholar]
- Kussendrager K. D., de Jong Y., Bouma J. M., Gruber M. The digestion of the B chain of oxidised insulin by extracts of rat liver lysosomes. Biochim Biophys Acta. 1972 Aug 18;279(1):75–86. doi: 10.1016/0304-4165(72)90242-5. [DOI] [PubMed] [Google Scholar]
- LaBadie J. H., Aronson N. N., Jr Lysosomal beta-D-mannosidase of rat liver. Biochim Biophys Acta. 1973 Oct 10;321(2):603–614. doi: 10.1016/0005-2744(73)90203-9. [DOI] [PubMed] [Google Scholar]
- Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968 May;37(2):482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan S., Dillard C. J., Tappel A. L. Degradation of polysaccharides, mucopolysaccharides, and glycoproteins by lysosomal glycosidases. Arch Biochem Biophys. 1969 Feb;129(2):525–533. doi: 10.1016/0003-9861(69)90210-0. [DOI] [PubMed] [Google Scholar]
- Mego J. L., McQueen J. D. Further studies on the degradation of injected [131-I] albumin by secondary lysosomes of mouse liver. Biochim Biophys Acta. 1965 Nov 15;111(1):166–173. doi: 10.1016/0304-4165(65)90483-6. [DOI] [PubMed] [Google Scholar]
- ROSEN H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957 Mar;67(1):10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Studies on fetuin, a glycoprotein of fetal serum. I. Isolation, chemical composition, and physiochemical properties. J Biol Chem. 1960 Oct;235(10):2860–2869. [PubMed] [Google Scholar]
- Touster O., Aronson N. N., Jr, Dulaney J. T., Hendrickson H. Isolation of rat liver plasma membranes. Use of nucleotide pyrophosphatase and phosphodiesterase I as marker enzymes. J Cell Biol. 1970 Dec;47(3):604–618. doi: 10.1083/jcb.47.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Hamer C. J., Morell A. G., Scheinberg I. H., Hickman J., Ashwell G. Physical and chemical studies on ceruloplasmin. IX. The role of galactosyl residues in the clearance of ceruloplasmin from the circulation. J Biol Chem. 1970 Sep 10;245(17):4397–4402. [PubMed] [Google Scholar]
- Wisher M. H., Evans W. H. Functional polarity of the rat hepatocyte surface membrane. Isolation and characterization of plasma-membrane subfractions from the blood-sinusoidal, bile-Canalicular and contiguous surfaces of the hepatocyte. Biochem J. 1975 Feb;146(2):375–388. doi: 10.1042/bj1460375. [DOI] [PMC free article] [PubMed] [Google Scholar]


