Abstract
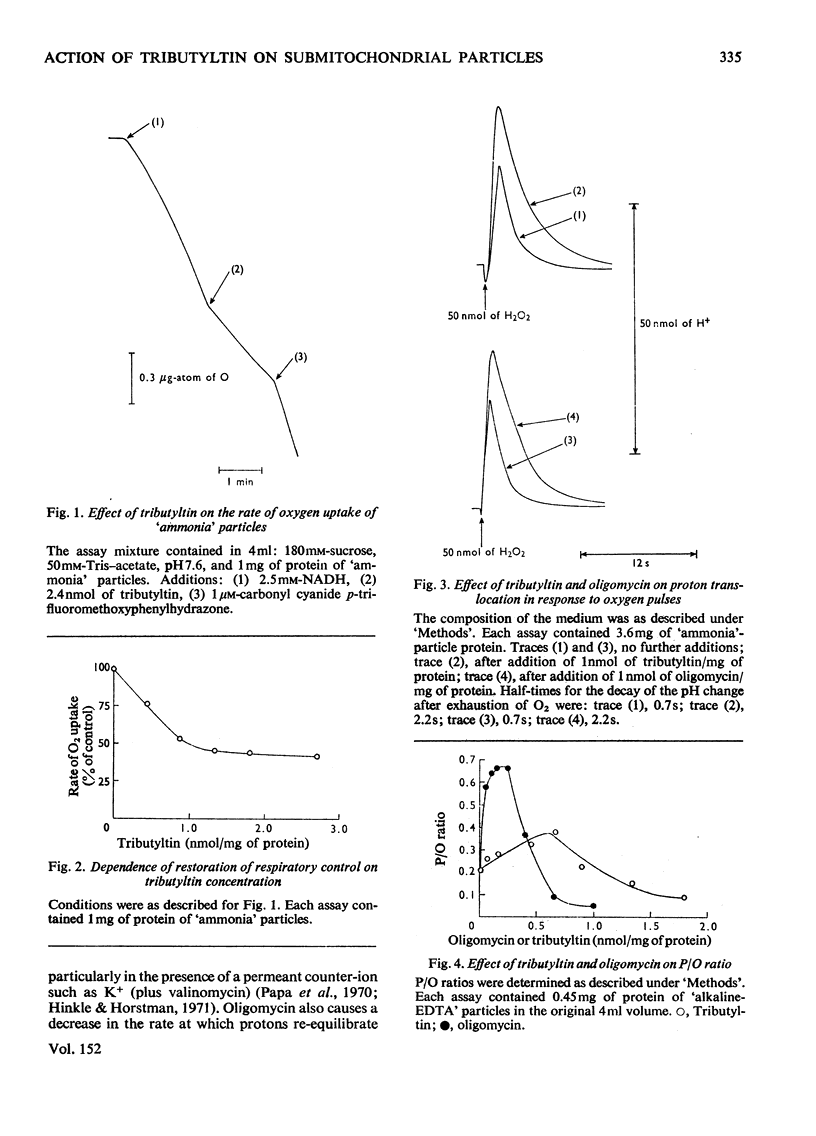
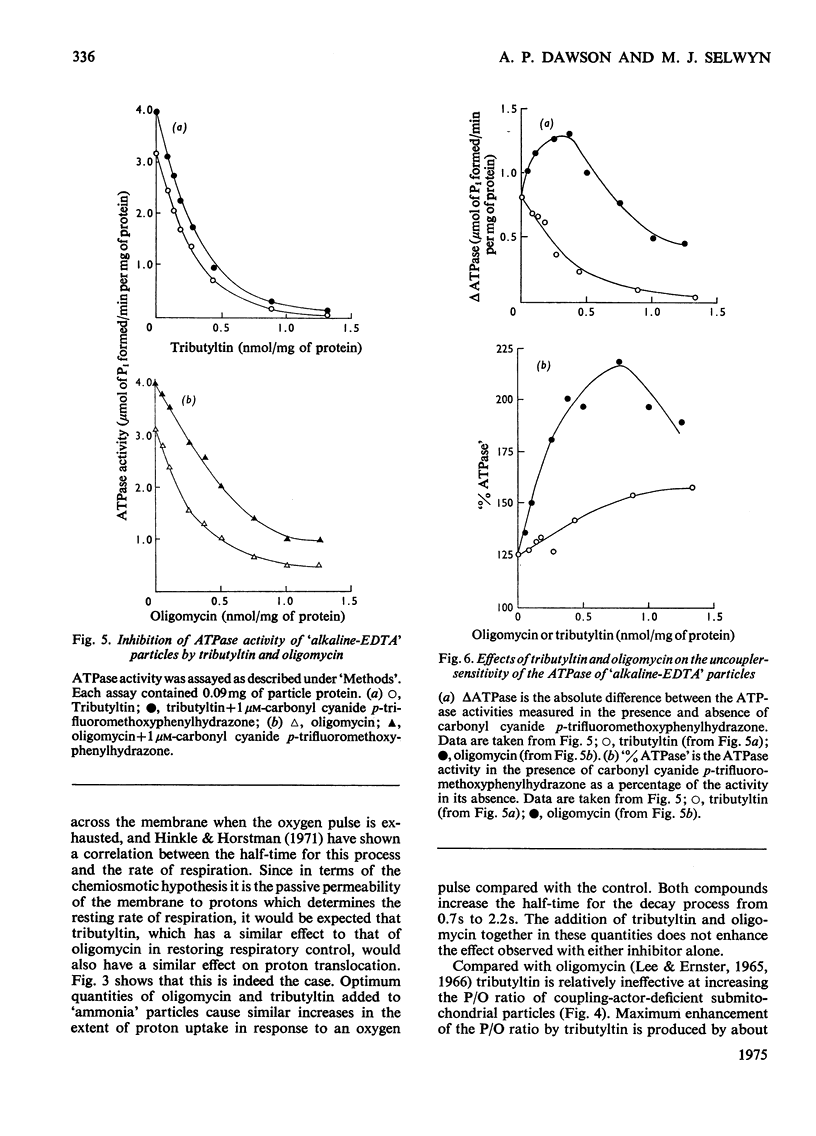
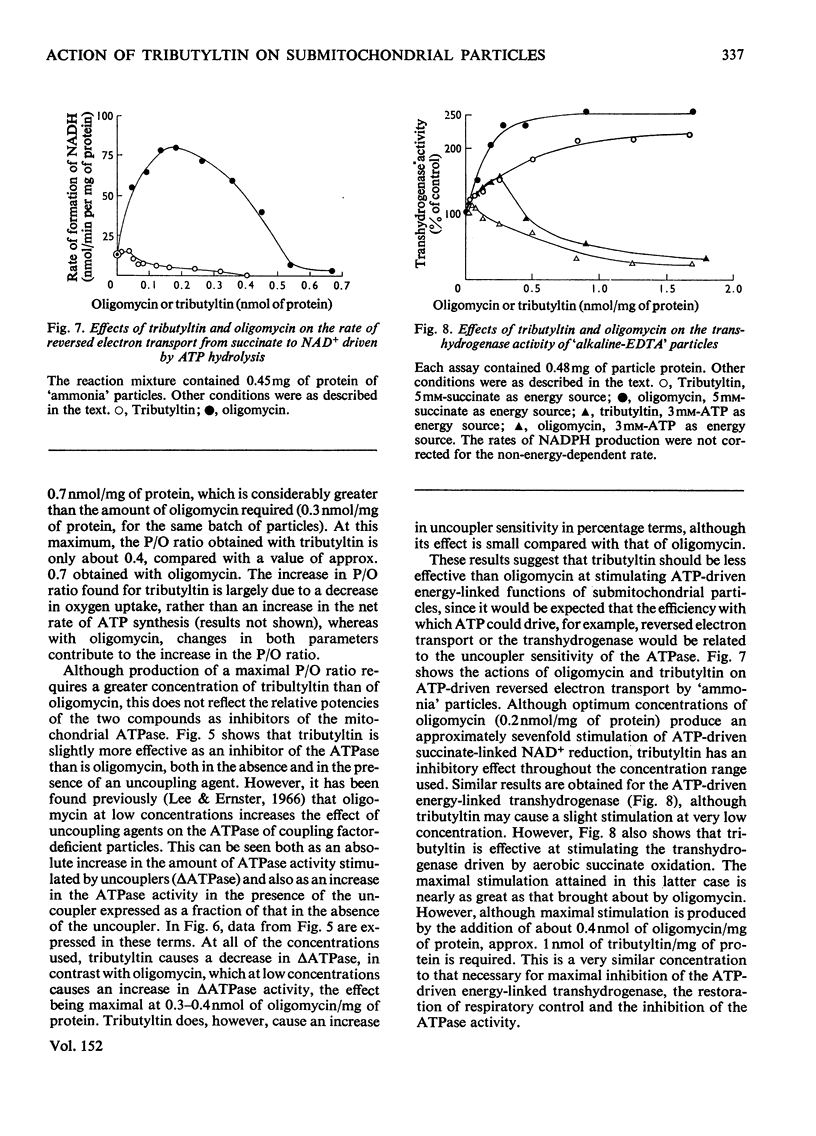
1. Tributyltin at concentrations of approx. 1nmol/mg of protein induces respiratory control and lessens the protein permeability of coupling-factor-deficient submitochondrial particles. 2. At these concentrations or lower, it increases the P/O ratio of the particles to a small extent and inhibits the adenosine triphosphatase activity without greatly increasing its sensitivity to uncoupling agents. 3. It fails to stimulate ATP-driven reversed electron transport or transhydrogenase, but stimulates the transhydrogenase driven by aerobic succinate oxidation. 4. The results indicate that, unlike oligomycin, tributyltin does not discriminate between damaged and intact ATP-synthesizing complexes. 5. The relationship between the oligomycin- and tributyltin-binding sites is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DANIELSON L., ERNSTER L. Demonstration of a mitochondrial energy-dependent pyridine nucleotide transhydrogenase reaction. Biochem Biophys Res Commun. 1963 Jan 18;10:91–96. doi: 10.1016/0006-291x(63)90274-2. [DOI] [PubMed] [Google Scholar]
- Hinkle P. C., Horstman L. L. Respiration-driven proton transport in submitochondrial particles. J Biol Chem. 1971 Oct 10;246(19):6024–6028. [PubMed] [Google Scholar]
- Kagawa Y., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. 8. Properties of a factor conferring oligomycin sensitivity on mitochondrial adenosine triphosphatase. J Biol Chem. 1966 May 25;241(10):2461–2466. [PubMed] [Google Scholar]
- LEE C. P., AZZONE G. F., ERNSTER L. EVIDENCE FOR ENERGY-COUPLING IN NON-PHOSPHORYLATING ELECTRON TRANSPORT PARTICLES FROM BEEF-HEART MITOCHONDRIA. Nature. 1964 Jan 11;201:152–155. doi: 10.1038/201152a0. [DOI] [PubMed] [Google Scholar]
- LEE C. P., ERNSTER L. RESTORATION OF OXIDATIVE PHOSPHORYLATION IN NON-PHOSPHORYLATING SUBMITOCHONDRIAL PARTICLES BY OLIGOMYCIN. Biochem Biophys Res Commun. 1965 Feb 17;18:523–529. doi: 10.1016/0006-291x(65)90785-0. [DOI] [PubMed] [Google Scholar]
- Lancashire W. E., Griffiths D. E. Studies on energy-linked reactions: isolation, characterisation and genetic analysis of trialkyl-tin-resistant mutants of Saccharomyces cerevisiae. Eur J Biochem. 1975 Feb 21;51(2):377–392. doi: 10.1111/j.1432-1033.1975.tb03938.x. [DOI] [PubMed] [Google Scholar]
- Linnett P. E., Mitchell A. D., Beechey R. B. Changes in inhibitor sensitivity of the mitochondrial ATPase activity after detergent solubilisation. FEBS Lett. 1975 May 1;53(2):180–183. doi: 10.1016/0014-5793(75)80014-7. [DOI] [PubMed] [Google Scholar]
- Manger J. R. The effect of triethyltin on mitochondrial ion accumulation. FEBS Lett. 1969 Dec 30;5(5):331–334. doi: 10.1016/0014-5793(69)80349-2. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Evidence discriminating between the chemical and the chemiosmotic mechanisms of electron transport phosphorylation. Nature. 1965 Dec 18;208(5016):1205–1206. doi: 10.1038/2081205a0. [DOI] [PubMed] [Google Scholar]
- Papa S., Guerrieri F., Rossi Bernardi L., Tager J. M. Effect of oligomygin on proton translocation in submitochondrial particles. Biochim Biophys Acta. 1970 Jan 13;197(1):100–103. doi: 10.1016/0005-2728(70)90016-2. [DOI] [PubMed] [Google Scholar]
- Roberton A. M., Holloway C. T., Knight I. G., Beechey R. B. A comparison of the effects of NN'-dicyclohexylcarbodi-imide, oligomycin A and aurovertin on enrgy-linked reactions in mitochondria and submitochondrial particles. Biochem J. 1968 Jul;108(3):445–456. doi: 10.1042/bj1080445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. S., Aldridge W. N. Oxidative phosphorylation. The effect of anions on the inhibition by triethyltin of various mitochondrial functions, and the relationship between this inhibition and binding of triethyltin. Biochem J. 1972 Mar;127(1):51–59. doi: 10.1042/bj1270051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selwyn M. J., Dawson A. P., Stockdale M., Gains N. Chloride-hydroxide exchange across mitochondrial, erythrocyte and artificial lipid membranes mediated by trialkyl- and triphenyltin compounds. Eur J Biochem. 1970 May 1;14(1):120–126. doi: 10.1111/j.1432-1033.1970.tb00268.x. [DOI] [PubMed] [Google Scholar]
- Selwyn M. J., Dunnett S. J., Philo R. D., Dawson A. P. Factors affecting the inhibition of mitochondrial adenosine triphosphatase by trialkyltin compounds. Biochem J. 1972 Apr;127(3):66P–67P. doi: 10.1042/bj1270066pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockdale M., Dawson A. P., Selwyn M. J. Effects of trialkyltin and triphenyltin compounds on mitochondrial respiration. Eur J Biochem. 1970 Aug;15(2):342–351. doi: 10.1111/j.1432-1033.1970.tb01013.x. [DOI] [PubMed] [Google Scholar]


