Abstract
Lactones play crucial roles in various fields, such as pharmaceuticals, food, and materials science, due to their unique structures and diverse biological activities. However, certain lactones are difficult to obtain in large quantities from natural sources, necessitating their synthesis to study their properties and potential. In this study, we investigated the photocatalytic conversion of D-fructose, a biomass-derived and naturally abundant sugar, using a TiO2 photocatalyst under light irradiation in ambient conditions. The resulting products were identified using HPLC, LCMS, MALDI TOF MS, and 1H NMR. The results confirmed the successful production of D-arabino-1,4-lactone as a key product, along with the formation of other valuable compounds, including rare sugars such as erythrose and glyceraldehyde. Analysis of the reaction mechanism revealed that D-arabino-1,4-lactone can be directly produced by the α scission (C1-C2 position cleavage) of D-fructose. Furthermore, erythrose and glyceraldehyde, as rare sugars, can be produced from the decomposition of D-arabino-1,4-lactone, which means that D-arabino-1,4-lactone can be used as a source of rare sugars. Furthermore, to investigate the biological activity of D-arabino-1,4-lactone, it was administered to Bifidobacterium. The results showed that Bifidobacterium proliferated and produced more lactic acid than when cultured in a medium without D-arabino-1,4-lactone, suggesting that Bifidobacterium can utilize D-arabino-1,4-lactone.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-84921-z.
Keywords: Photocatalytic conversion; D-arabino-1,4-lactone; Biomass valorization; Prebiotic compounds; TiO2
Subject terms: Heterogeneous catalysis, Photocatalysis
Biomass is gaining attention as a renewable resource in the pursuit of a sustainable society. Bio-based materials can be converted into fuels1, pharmaceutical raw materials2, and other high-value compounds3,4, making it promising for use in a wide range of fields5,6, including the chemical and pharmaceutical industries7,8. Furthermore, plant matter has the potential to contribute to mitigating global warming by acting as a carbon dioxide sink9,10. These characteristics have led to increased interest in the research and development of biomass and its derivatives, accelerating efforts towards contributing to a sustainable society and reducing environmental impact11,12. The useful substances derived from biomass are diverse, ranging from fuels to chemical raw materials, food, pharmaceuticals, and cosmetics13–15, with expected applications in areas such as ethanol16, biodiesel17, lactic acid18, and aromatic compounds19. These substances can provide environmentally friendly and sustainable alternatives to conventional products that depend on fossil fuels.
Lactones are cyclic esters formed by the intramolecular esterification of hydroxy carboxylic acids and play important roles in various fields because of their unique structural characteristics and diverse biological activities20. For example, δ-decalactone is used as a food flavor21, whereas gluconolactone is an important component in pharmaceutical synthesis22. γ-Butyrolactone is widely used as a solvent and precursor in chemical synthesis23, and ε-caprolactone plays a crucial role as a monomer for the biodegradable polymer polycaprolactone (PCL)24,25. Moreover, the biodegradability of many lactones is an important characteristic that contributes to reducing environmental burden and developing sustainable products26,27. This feature further enhances the appeal of lactones, especially for the development of environmentally conscious products and materials. As seen in the example of PCL, lactones and their derivatives serve as the foundation for developing innovative materials that combine biocompatibility and environmental compatibility28. However, certain lactones are difficult to obtain in large quantities from natural sources. Therefore, there is an urgent need to develop technologies for synthesizing these compounds in an environmentally friendly manner to study their properties and potential. The development of sustainable processes for lactone production not only addresses increasing demand but also contributes to more environmentally responsible and economically viable industrial practices.
Lactone synthesis using photocatalysts is a promising approach to address this challenge. Photocatalysts are a group of substances that oxidize or reduce substrates near their surface under light irradiation in ambient conditions29,30. Kaplaneris et al. synthesized lactones from alcohols using photoorganocatalytic synthesis with visible light31, while Triandafillidi et al. converted alkenes to γ-lactones using a Ru(bpy)3Cl2 photocatalyst32. Wei et al. reported the formation of γ-lactones by reacting styrene and R-bromoesters using the visible light photoredox catalyst fac-Ir(ppy)333, and Özgen et al. achieved the synthesis of chiral γ-lactones from aldehydes and olefins using a combination of a tetrabutylammonium decatungstate photocatalyst and a biocatalyst34.
These previous studies have demonstrated the potential of environmentally friendly lactone synthesis using photocatalysts. However, to realize a sustainable society, not only environmentally friendly reaction processes but also the use of renewable resources as starting materials are necessary. Despite this necessity, research on methods for directly synthesizing lactones from renewable resources such as biomass has been scarce.
In this study, we demonstrated the direct conversion of D-fructose, a biomass-derived compound, to produce D-arabino-1,4-lactone as a lactone using a TiO2 photocatalyst under light irradiation in ambient condition. The products were analyzed by HPLC, LCMS, MALDI TOF MS, and 1H NMR. Additionally, we examined whether the D-arabino-1,4-lactone produced could be metabolized by bacteria beneficial to the human body. This study aims to elucidate the specific reaction pathways and intermediates involved in the photocatalytic conversion of D-fructose, and to explore the potential biological activities of the produced lactone, particularly focusing on their effects on beneficial gut bacteria.
Methods
TiO2 treatment of D-fructose and D-arabino-1,4-lactone
An aqueous solution (100 mL) containing 60 mmol L− 1 D-fructose (FUJIFILM Wako Pure Chemical Corp.) was mixed with 35 mg TiO2 photocatalyst powder (P25, Degussa). The obtained suspension was stirred at 350 rpm for 15 min in the dark using a magnetic stirrer, and then irradiated with UV light at an intensity of 10 mW cm− 2 by a Hg-Xe lamp (UV-7, U-VIX) at 20 ºC under atmospheric pressure for 144 h. The sample was collected at each 24 h. Afterward, the TiO2 powder was removed from the suspension using a 0.22 μm nylon filter to obtain the filtrate. Similarly, 2 mg of TiO2 photocatalyst powder was added to 20 mL of an aqueous solution containing 20 mmol L− 1 D-arabino-1,4-lactone (Sigma Aldrich). The obtained suspension was stirred at 350 rpm for 15 min in the dark, and then irradiated with UV light at an intensity of 5 mW cm− 2 at 20 °C under atmospheric pressure. Subsequently, the TiO2 powder was removed from the suspension using a 0.22 μm nylon filter to obtain the filtrate.
Product analysis
The analysis of the starting materials D-fructose and D-arabino-1,4-lactone in the reaction solution was performed using HPLC (Shimadzu Corp.) equipped with a refractive index detector (RI). The analytical column used was Sugar-D (250 mm × 4.6 mm, 5 μm particle size, COSMOSIL), and the mobile phase was 75% acetonitrile/25% water (v/v) at a flow rate of 1.0 mL min− 1 at 30 °C. The concentrations of D-fructose and D-arabino-1,4-lactone were calculated using the calibration curves. The reaction products arabinose, erythrose, and glyceraldehyde in the reaction solution were measured at 305 nm using HPLC equipped with a UV-VIS detector (UV-VIS). Before measurement, arabinose, erythrose, and glyceraldehyde were derivatized with p-aminobenzoic acid ethyl ester (ABEE). The procedure was as follows: An ABEE solution was prepared by mixing 332.4 mg ABEE, 31.7 mg sodium cyanoborohydride, 386.4 µL acetic acid, and 3.6 mL methanol. Then, 10 µL of the reaction solution at each irradiation time was mixed with 40 µL of the ABEE solution and vortexed for 15 s. After centrifugation for 2 min, the mixture was heated to 80 ºC. After 1 h, the mixture was cooled to room temperature and centrifuged for 2 min. Water (200 µL) and chloroform (200 µL) were then added to the mixture. After vortexing for 1 min, the mixture was centrifuged for 2 min. Subsequently, 150 µL of the upper aqueous layer of the two-layer liquid of water and chloroform was collected, and 300 µL of water was added to the aqueous solution. After vortexing for 1 min, the mixture was centrifuged for 2 min. This solution was used for HPLC analysis. The analytical column used was CAPCELL PAK C18 (150 mm × 4.6 mm, 3 μm particle size, OSAKA SODA), and the mobile phase was an 87% ammonium acetate solution (20 mmol L− 1)/ 13% acetonitrile (v/v) at a flow rate of 1.0 mL min− 1 at 40 °C. The concentrations of arabinose, erythrose, and glyceraldehyde were calculated using calibration curves. The molecular weights of the reaction products, arabinose, erythrose, and glyceraldehyde, were confirmed using LCMS (LCMS8050, Shimadzu Corp.). The analytical column used was CAPCELL PAK C18 (150 mm × 2.0 mm, 3 μm particle size, OSAKA SODA), and the mobile phase was 87% ammonium acetate solution (20 mmol L− 1)/13% acetonitrile (v/v) at a flow rate of 0.2 mL min− 1 at 40 ºC. The positive ESI mode was used.
Formaldehyde analysis was performed by measuring at 365 nm using the UV-VIS detector of HPLC. Before measurement, the products were derivatized with 2,4-dinitrophenylhydrazine (DNPH). The procedure was as follows: 30 mg of DNPH, 30 mL of methanol, 1.5 mL of hydrochloric acid, and 13.5 mL of ultrapure water were mixed by sonication for 10 min. After mixing, the solution was stored in the dark as a DNPH labeling reagent. The reaction solution was then diluted 50-fold with ultrapure water so that the concentration of formaldehyde was below 2 mM. 4 mL of the DNPH labeling reagent and 1 mL of the diluted solution were mixed and allowed to stand at 40 ºC for 10 min. After that, the mixture was left to stand in the dark at room temperature for 2 h. This solution was then used for HPLC analysis. The analytical column used was a Luna C18(2) (250 mm×4.6 mm I.D., 5 μm particle size, Shimadzu), and the mobile phase was 60% acetonitrile/ 40% water (v/v) at a flow rate of 1.0 mL min− 1 at 40 °C. The product concentration was calculated using a calibration curve. The molecular weight of formaldehyde was confirmed using LCMS (LCMS8050, Shimadzu Corp.). The analytical column used was Shim-pack VP-ODS (250 mm × 2.0 mm, 5 μm particle size, Shimadzu) with, and the mobile phase was 60% acetonitrile/40% water (v/v) at a flow rate of 0.2 mL min− 1 at 40 ºC. The positive ESI mode was used.
Formic acid and acetic acid analyses were performed by measuring at 210 nm using the UV-VIS detector of HPLC. Before the measurement, 2.5 µL of 1 mol/L sulfuric acid was added to 0.9975 mL of the solution so that the concentration of sulfuric acid was 2.5 mmol/L. This solution was used for HPLC analysis. The analytical column used was Rezex ROA-Organic Acid H+ (8%) (300 × 7.8 mm, 5 μm particle size, Phenomenex), and the mobile phase was 2.5 mmol/L sulfuric acid at a flow rate of 0.5 mL min− 1 at 60 ºC. The concentrations of formic and acetic acids were calculated from the calibration curves.
Isolation of products
To isolate D-arabino-1,4-lactone for MALDI TOF MS and 1H NMR measurements, the following experiments were conducted. The experiments were performed under the same conditions as the TiO2 treatment of D-fructose described above. After 144 h of UV irradiation, all the reaction solutions were filtered through a 0.22 μm nylon filter to remove TiO2 from the suspension. Multiple batches of the reaction solution were collected and concentrated using a rotary evaporator. D-arabino-1,4-lactone was isolated using HPLC. The column used was Sugar-D (250 mm × 4.6 mm, 5 μm particle size, COSMOSIL), and the mobile phase was 75% acetonitrile/25% water (v/v) at a flow rate of 1.0 mL min− 1 at 30 ºC. Erythrose and glyceraldehyde were labeled with ABEE using the concentrated solution and then isolated by HPLC. The column used was a CAPCELL PAK C18 (150 mm × 4.6 mm, 3 μm particle size, SHISEDO), and the mobile phase was 87% ammonium acetate solution (20 mmol L− 1) and 13% acetonitrile (v/v) at a flow rate of 1.0 mL min− 1 at 40 °C.
MALDI TOF-MS measurement
The isolated D-arabino-1,4-lactone solution was completely dried using a rotary evaporator and a centrifugal evaporator. After drying, 50 µL of 0.01 M NaCl was added and dissolved by vortexing. 10 µL of the solution was suspended with 10 µL of 2,5-dihydroxybenzoic acid as a matrix. Measurements were performed using a matrix-assisted laser desorption/ionization time-of-flight mass spectrometer (MALDI-TOF MS, AXIMA-TOF2, SHIMADZU). 10 µL of the mixture was dropped onto a plate for MALDI TOF-MS measurement. After dropping, it was dried using a dryer. Then, the measurements were performed in positive linear mode.
1H NMR measurement
For 1H NMR measurement, D2O containing DSS as an internal standard was added to the D-arabino-1,4-lactone solution isolated above and mixed 1. H NMR spectra were recorded at 500 MHz using a JNM-ECP500 (JEOL). D-arabino-1,4-lactone: 1H NMR (500 MHz, D2O): δ = 3.77 (d, 1 H, J = 4.7 Hz, H-5b), 3.97–4.01 (m, 2 H, H-2, H-5a), 4.27 (d, 1 H, J = 8.9 Hz, H-2), 4.62 (dd, 1 H, J = 1.4, 1.4 Hz, H-1).
Preparation of Bifidobacterium suspension
Bifidobacterial strains (Bifidobacterium catenulatum (B. catenulatum), JCM1194T; Bifidobacterium pseudocatenulatum (B. pseudocatenulatum), JCM1200T; and Bifidobacterium breve (B. breve), JCM7016) were obtained from the Microbe Division of the RIKEN BioResource Research Center, a National Research and Development Agency in Japan. Each Bifidobacterium was cultured as follows. First, it was anaerobically cultured at 37 °C for 24 h using modified GAM broth (NISSUI 05433) and an Anaeropack (Sugiyamagen Co., Ltd.). The culture solution was centrifuged at 4 °C, 3,500 rpm for 15 min, the supernatant was discarded, and the pellet was washed with 1 mL of physiological saline. This washing process was repeated four times, and then the suspension was adjusted to an optical density of 0.7 at 660 nm, using a medium for the sugar assimilation test.
Preparation of E. coli suspension
Escherichia coli (E. coli, NBRC106373) was obtained from the NITE Biological Resource Center. E. coli was cultured as follows. First, E. coli was shake-cultured at 30 °C for 16 h using NB medium (05514, NISSUI). Then, the culture solution was centrifuged at 4 °C, 3,500 rpm for 10 min, the supernatant was discarded, and the pellet was washed with 1 mL of medium for the sugar assimilation test. This washing process was repeated 3 times, and then the E. coli suspension was adjusted to an optical density of 0.5 at 660 nm, using the medium for the sugar assimilation test.
Sugar assimilation test
To prepare the medium for the sugar assimilation tests, 1 g of peptone (KISHIDA chemical), 0.5 g of sodium chloride (FUJIFILM Wako Pure Chemical Corp.), 1.2 mL of 0.2% bromothymol blue solution (FUJIFILM Wako Pure Chemical Corp.), and 1 g of yeast extract (Nacalai Tesque, Inc.) were added to 100 mL of ultrapure water. The mixture was then thoroughly mixed and sterilized using an autoclave. 30 mg each of glucose (FUJIFILM Wako Pure Chemical Corp.), sodium gluconate (FUJIFILM Wako Pure Chemical Corp.), or D-arabino-1,4-lactone (Sigma Aldrich) was added to 2.97 mL of the prepared medium for the sugar assimilation test and dissolved using a vortex. Then, 30 µL of the bacterial solution prepared above was added and suspended using a vortex. After anaerobic culture using an Anaeropack for 48 h, optical density at 660 nm was measured.
The optical density at 660 nm positively correlates with the bacterial concentration. Therefore, using the following equation, the relative value of the optical density of bacteria in the medium containing each sugar to the optical density of bacteria in the medium containing glucose was calculated as the normalized optical density. This value was used as the relative bacterial concentration.
 |
Quantification of lactic acid
The bacterial culture was subjected to anaerobic cultivation for 48 h and heated at 80 °C for 15 min. After heating, the culture was centrifuged at 12,000 × g for 10 min and the supernatant was collected. The supernatant was used to quantify the lactic acid. The F-kit D-lactic acid/L-lactic acid (J. K. International) was employed for the quantitative analysis of lactic acid.
Results and discussion
TiO2 treatment of D-fructose and product analysis
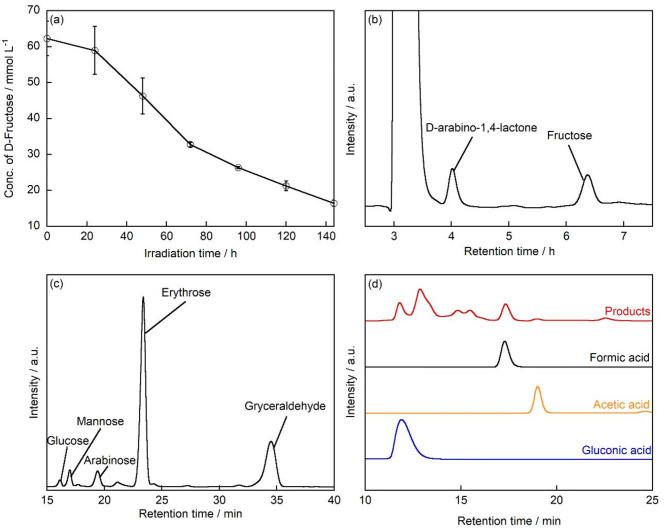
TiO2 was used to treat an aqueous solution of D-fructose under UV irradiation at room temperature in air. Figure 1a shows the concentration of D-fructose as a function of UV irradiation time. The concentration of D-fructose gradually decreased from 60 mmol L− 1 before UV irradiation to 16.4 mmol L− 1 after 144 h of UV irradiation. This suggests that D-fructose was decomposed by the photocatalytic reaction of TiO2 under UV irradiation, resulting in a decrease in its concentration.
Fig. 1.
(a) D-fructose concentration as a function of UV irradiation time. HPLC chromatograms for obtained sample after TiO2 treatment of D-fructose after 144 h of UV irradiation: (b) D-fructose: 60 mmol L− 1, TiO2: 35 mg, UV light: 10 mW cm− 2, temperature: 25 ºC, Column: Sugar-D, detector: RI; (c) Products are functionalized with ABEE. The unreacted ABEE-labeled fructose derivative is not displayed in the chromatogram due to its short retention time in the HPLC analysis. Column: CAPCELL PAK C18, detector: UV-VIS; (d) Column: Rezex ROA-Organic Acid H+ (8%), detector: UV-VIS.
Figure 1b shows the chromatogram obtained from the HPLC analysis with an RI detector for the products formed during the treatment of D-fructose with TiO2 after 144 h of light irradiation. The peaks with retention time (R.T.) of 6.4 min and 3.9 min corresponded to those of D-fructose and D-arabino-1,4-lactone standards, respectively. There is a larger peak observed at R.T.=3.3 min which exceeds the intensities of both D-fructose and D-arabino-1,4-lactone peaks. Since this peak was detected prior to initiating the photocatalytic reaction, it can be suggested that it does not originate from any reaction products. In HPLC analysis, such peaks are typically attributed to system-related phenomena, such as dissolved gases present in the carrier solvent. D-arabino-1,4-lactone was further isolated and measured by MALDI TOF MS, and an ion at m/z = 171 was detected (Fig. S1), consistent with the molecular weight of D-arabino-1,4-lactone when Na was added to D-arabino-1,4-lactone. D-arabino-1,4-lactone was further measured by 1H NMR. The results showed that the 1H NMR spectrum of D-arabino-1,4-lactone matched that of the standard compound (Fig. S2). Therefore, it is suggested that D-arabino-1,4-lactone was produced when D-fructose was decomposed by the photocatalytic reaction of TiO2 under UV irradiation. The other products generated after the treatment of D-fructose with TiO2 after 144 h of light irradiation were then analyzed by LC/MS. Figure 1c shows the HPLC chromatogram of the ABEE-labeled products. The peaks at R.T. = 16.0, 16.8, 19.2, 23.1, and 34.0 min corresponded to the R.T. of the standard compounds of glucose, mannose, arabinose, erythrose, and glyceraldehyde, respectively. Furthermore, ions with m/z = 330, 330, 300, 270, and 240 were detected (Fig. S3a-e), which matched the molecular weights of each sugar when ABEE-derivatized and protonated. Notably, glucose and mannose were also confirmed by HPLC before the photocatalytic reaction (Fig. S3f), suggesting that they were not produced by the photocatalytic reaction. It is known that glucose and mannose can be produced in aqueous solutions through the isomerization of D-fructose35. Therefore, it is considered that a small portion of the D-fructose may have been converted into these sugars via isomerization.
Next, to confirm whether aldehydes were produced after TiO2 treatment of D-fructose under UV irradiation, the DNPH-labeled samples were analyzed by LCMS. As a result, the R.T. of the detected peak matched the R.T. of the formaldehyde standard (Fig. S4a). Furthermore, ions with m/z = 209 were detected from the formaldehyde peak, which corresponded to the molecular weight of formaldehyde when DNPH-labeled and deprotonated (Fig. S4b). Additionally, the organic acids generated after the treatment of D-fructose with TiO2 after 144 h of light irradiation were measured, the R.T. of the detected peaks matched the R.T. of the formic acid, acetic acid and gluconic acid standards (Fig. 1d). A peak at R.T.=13 min exhibits larger intensity than those of formic acid and acetic acid. This peak was observed prior to initiating the photocatalytic reaction, indicating that it does not originate from reaction products. Moreover, since the peak appeared exclusively after fructose addition, it may be attributed to residual high-concentration fructose in the system.
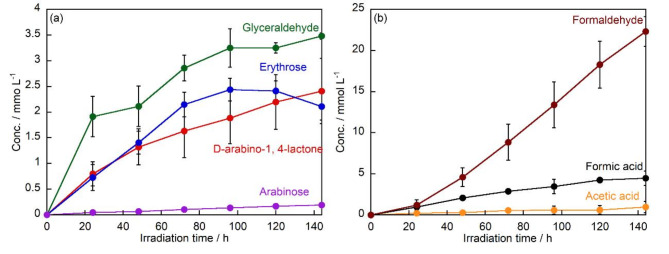
The amount of products produced after the TiO2 treatment of D-fructose under UV irradiation was compared for each time (Fig. 2a). The concentrations of the arabinose and D-arabino-1,4-lactone, increased to 0.19 mmol L− 1 and 2.4 mmol L− 1 after 144 h of UV irradiation, respectively. The concentration of erythrose increased to 2.4 mmol L− 1 after 96 h of UV irradiation, but decreased after 96 h. After 144 h of UV irradiation, glyceraldehyde was the major product throughout all the reaction times, and the concentration of glyceraldehyde increased to 3.5 mmol L− 1 after 144 h of UV irradiation. The production of D-arabino-1,4-lactone from fructose may continue to increase beyond 144 h of irradiation due to the presence of residual starting material. However, as the fructose concentration decreases, the decomposition rate of D-arabino-1,4-lactone is expected to exceed its photocatalytic production rate. Consequently, a decrease in D-arabino-1,4-lactone concentration should be observed, similar to the behavior observed with erythrose.
Fig. 2.
Changes in the concentrations of (a) D-arabino-1,4-lactone, arabinose, erythrose, and glyceraldehyde, and (b) formaldehyde, formic acid, and acetic acid produced by TiO2 treatment of D-fructose under UV irradiation.
In addition, the amounts of formaldehyde, formic acid and acetic acid formed were compared for each time (Fig. 2b). The concentration of formaldehyde increased to 22.3 mmol L− 1 after 144 h of UV irradiation. Formaldehyde is thought to be formed during the conversion of D-fructose to D-arabino-1,4-lactone. In addition, the oxidative degradation of glyceraldehyde to formaldehyde has been reported36. Since glyceraldehyde is the major product of D-fructose degradation, it is considered that a large amount of formaldehyde, its degradation product, was also produced. The organic acids, formic acid and acetic acid, increased to 4.5 mmol L− 1 and 0.97 mmol L− 1, respectively, after 144 h of UV irradiation. It has been reported that formic acid is produced during the degradation reaction of sugars37. Thus, the amount of formic acid produced continued to increase because formic acid is produced during the further degradation of the sugar produced by the degradation of D-fructose.
Reaction mechanisms
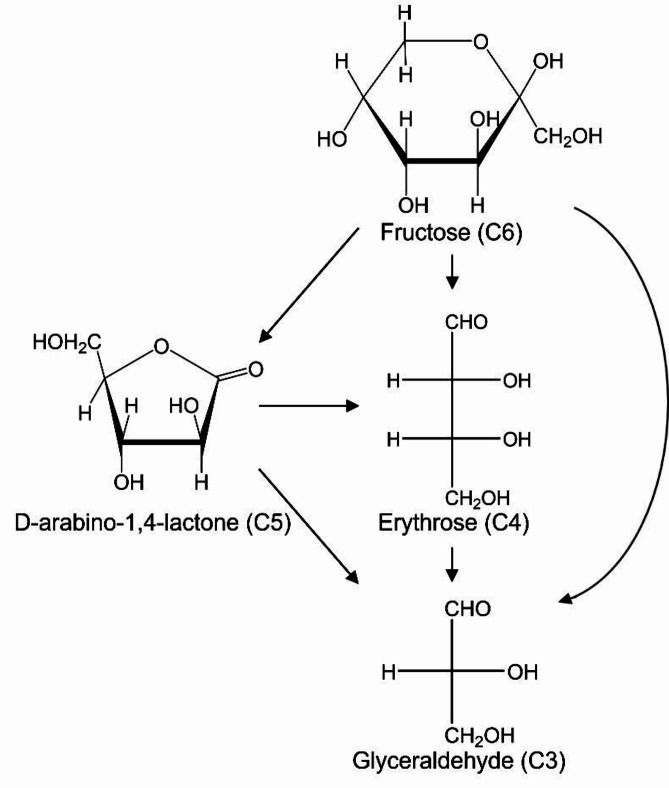
On the basis of the above results, the reaction pathways by which each product was formed from D-fructose are discussed. In the TiO2 photocatalytic reaction of D-fructose (having six carbon atoms, C6), D-arabino-1,4-lactone and arabinose (both having five carbon atoms, C5) were formed. However, the concentration of arabinose was much lower than that of D-arabino-1,4-lactone, thus, the main C5 product obtained from D-fructose by TiO2 photocatalysis is D-arabino-1,4-lactone. Comparing the structures of D-fructose and D-arabino-1,4-lactone, it is assumed that D-arabino-1,4-lactone is formed by α scission (C1-C2 position cleavage) of D-fructose.
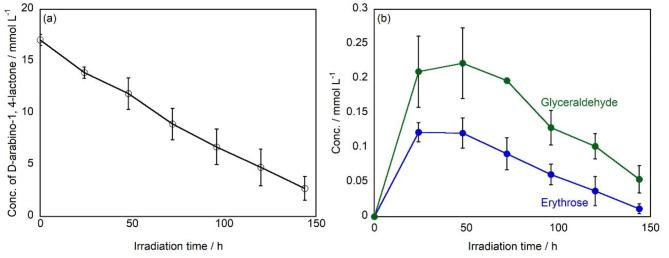
In previous reports, when glucose (C6) was decomposed using a TiO2 photocatalyst, arabinose (C5), erythrose (C4), and glyceraldehyde (C3) are produced, with the order of yield being C5 > C4 > C338. This is because glucose undergoes a stepwise reaction, first being degraded to arabinose, and then arabinose being further degraded to erythrose (C6◊C5◊C4◊C3). However, in this study, when D-fructose (C6) was decomposed, glyceraldehyde (C3) was produced in the greatest amount, followed by erythrose (C4), and arabinose (C5) had the lowest yield (C3 > C4 > C5). This result suggests that erythrose and glyceraldehyde are produced not only via the aforementioned stepwise reaction but also through different reaction pathways involving C-C bond cleavage. On the other hand, to further investigate the reaction mechanism, we studied how D-arabino-1,4-lactone (C5), produced from D-fructose (C6), is further decomposed by the TiO2 photocatalytic reaction (Fig. 3a). As a result, the concentration of D-arabino-1,4-lactone gradually decreased and was completely degraded after 144 h using TiO2 under UV irradiation. Figure 3b shows a comparison of the amount of each product formed by the photocatalytic degradation of D-arabino-1,4-lactone. The formation of erythrose and glyceraldehyde was confirmed. Glyceraldehyde was the main product throughout all reaction times, and the concentration increased and reached to 0.22 mmol L− 1 after 48 h of UV irradiation, followed by a gradual decrease, ultimately declining to 0.05 mmol L− 1 at 144 h. The concentration of erythrose exhibited a maximum of 0.12 mmol L− 1 at 24 h of UV irradiation, after which it decreased continuously, reaching 0.01 mmol L− 1 at 96 h.
Fig. 3.
(a) Changes in the concentration of D-arabino-1,4-lactone, and (b) products as a function of UV irradiation time.
Therefore, when D-arabino-1,4-lactone was decomposed using a TiO2 photocatalyst, more glyceraldehyde (C3) was produced than erythrose (C4). This suggests that, similar to the case with D-fructose, both erythrose (C4) and glyceraldehyde (C3) are generated not only through the stepwise reaction from D-arabino-1,4-lactone (C5) but also via reaction pathways involving C-C bond cleavage.
From the above results, not only D-arabino-1,4-lactone as a lactone, but also erythrose and glyceraldehyde were produced by the TiO2 photocatalytic reaction of D-fructose. The reaction pathway suggests that D-arabino-1,4-lactone is formed through the degradation reaction of D-fructose, while erythrose and glyceraldehyde are mainly produced by C-C bond cleavage. Additionally, the decomposition of D-arabino-1,4-lactone also resulted in the formation of erythrose and glyceraldehyde. The reaction pathways are summarized in Fig. 4. Erythrose and glyceraldehyde are rare sugars. In recent years, rare sugars have been expected to be used as pharmaceutical agents and functional food additives39–41. However, they are difficult to obtain because they rarely exist in nature. It has been revealed that D-arabino-1,4-lactone can also be used as a raw material to produce the rare sugars erythrose and glyceraldehyde.
Fig. 4.
Presumed reaction mechanism of decomposition of D-fructose and generation of products using TiO2 photocatalyst under light irradiation.
Biological activity tests of D-arabino-1,4-lactone
In this study, we investigated D-arabino-1,4-lactone, a lactone newly confirmed to be produced by the photocatalytic decomposition of D-fructose. D-arabino-1,4-lactone has been reported to be the substrate for arabino-1,4-lactone oxidase, an enzyme involved in the final stage of ascorbic acid biosynthesis42. This enzyme catalyzes the conversion of D-arabino-1,4-lactone to ascorbic acid. Ascorbic acid is an important antioxidant for many organisms. D-arabino-1,4-lactone can be a raw material for synthesizing ascorbic acid. However, this compound has been reported to be produced in limited amounts, and its biological activity has not been extensively studied. Therefore, we decided to explore the biological activity of D-arabino-1,4-lactone. Gluconolactone has been reported to be assimilated by Bifidobacterium and is expected to be used as a prebiotic43. We hypothesized that D-arabino-1,4-lactone may also be assimilated by Bifidobacterium. Bifidobacterium has also been reported to have effects such as the production of B vitamins44, the suppression of the formation of carcinogenic substances and intestinal putrefactive products45,46, the prevention of constipation47, and the enhancement of the body’s immune system48. In particular, breastfed infants develop an intestinal flora composed almost entirely of Bifidobacterium at an early stage. The formation of this specific intestinal flora is thought to play a crucial role in preventing infectious diseases in infants49. In this study, we used three species of Bifidobacterium for the sugar assimilation test: B. catenulatum and B. pseudocatenulatum, which are known to have growth-promoting effects with sodium gluconate50, and B. breve, which are resistant to gastric acid and can easily reach the intestine alive51 and have also been reported to have anti-obesity effects52. B. infantis and B. breve are known as important Bifidobacterium species that have been reported to become dominant in the intestines of breastfed infants due to priority effects. Among these, B. infantis possesses the ability to degrade all human milk oligosaccharides (HMOs)53. In contrast, despite its very low HMO utilization capacity, B. breve has been reported to become dominant in the intestines of many breastfed infants53. Additionally, B. breve has been widely reported to be effective from a probiotics perspective54. For these reasons, we decided to investigate the effects on B. breve. For comparison, we also used E. coli.
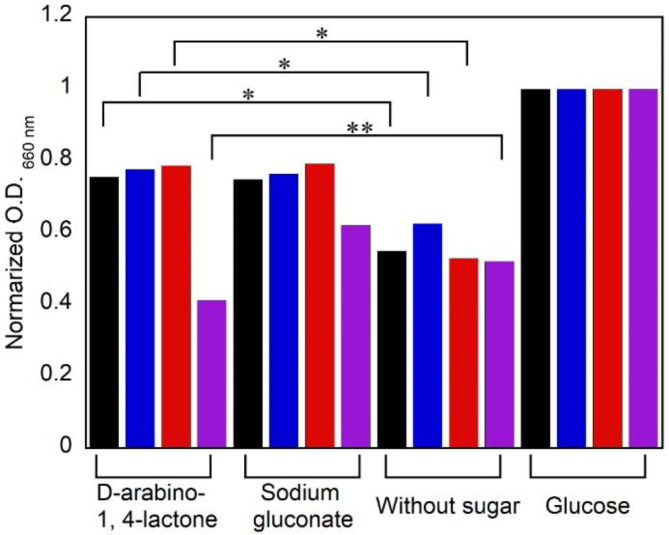
The relative value of the optical density of bacteria in the medium containing each sugar to the optical density of bacteria in the medium containing glucose was calculated as the normalized optical density. Additionally, since the optical density positively correlates with the bacterial concentration, the normalized optical density represents the relative value of the bacterial concentration in the medium under each condition compared to the bacterial concentration cultured in the medium containing glucose. The addition of sodium gluconate to the medium served as a positive control, as this compound has been reported to be assimilated by Bifidobacterium50, and the case where no sugar was added to the medium was used as a negative control. For B. catenulatum, B. pseudocatenulatum, and B. breve, the relative bacterial concentrations were as follows: 0.76, 0.78, and 0.79, respectively, when D-arabino-1,4-lactone was added; 0.75, 0.76, and 0.79, respectively, when sodium gluconate was added; and 0.55, 0.63, and 0.53, respectively, when no sugar was added (Fig. 5). From the above results, although the relative values of the bacterial concentration when D-arabino-1,4-lactone was added were lower than 1 in all Bifidobacterium species, indicating a lower bacterial concentration than when only glucose was used, a significant increase in the bacterial concentration was observed compared with that when no sugar was added (p < 0.05). Additionally, these values were similar to those of sodium gluconate, and no significant differences were observed. These results confirm that B. catenulatum, B. pseudocatenulatum, and B. breve can proliferate in the presence of D-arabino-1,4-lactone to a similar extent as in the presence of sodium gluconate.
Fig. 5.
Assimilation test by bacteria using each sugar after 48 h incubation. Black: B. catenulatum, blue: B. pseudocatenulatum, red: B. breve, purple: E. coli (n = 3, *p < 0.05, **p < 0.01).
On the other hand, we also conducted a sugar assimilation test using E. coli, which can have a negative impact on the human body if it is present in excess in the intestine. When E. coli was used, the relative values of the bacterial concentration were 0.41 when D-arabino-1,4-lactone was added, 0.62 when sodium gluconate was added, and 0.52 when no sugar was added. When D-arabino-1,4-lactone was used, the relative value of the bacterial concentration significantly decreased from 1, and there were significant differences compared to when sodium gluconate was used or when no sugar was added (p < 0.01). In glucose-free medium, D-arabino-1,4-lactone was found to inhibit the growth of E. coli. Since E. coli lacks D-arabinono-1,4-lactone oxidase55, the D-arabino-1,4-lactone added to the medium remains unmetabolized. This D-arabino-1,4-lactone exists in equilibrium with arabonic acid56, which causes the pH of the medium to decrease. Indeed, after 48 h of cultivation, the pH of the medium decreased to 4, which significantly deviates from the optimal pH range for E. coli (7.0-7.5)57. This pH reduction is considered to be the primary factor responsible for the inhibition of E. coli growth58.
Bifidobacterium produces lactic acid during the process of assimilating sugars such as glucose59. Therefore, lactic acid was measured to investigate whether the sugars added to the medium were assimilated. Table 1 shows the lactic acid concentration in the medium when the sugar assimilation test was conducted using B. breve, which had the highest relative bacterial concentration in the presence of D-arabino-1,4-lactone. The D-lactic acid concentrations were 1.57, 0.871, 18.6, and 17.4 mmol/L, and the L-lactic acid concentrations were 0.0610, 0.154, 0.466, and 1.40 mmol/L, respectively, for media containing D-arabino-1,4-lactone, no sugar, sodium gluconate, or glucose. The total lactic acid concentrations were 1.63, 1.02, 18.8, and 19.1 mmol/L for media containing D-arabino-1,4-lactone, no sugar, sodium gluconate, or glucose, respectively. The amount of lactic acid produced with D-arabino-1,4-lactone was lower than that produced with sodium gluconate or glucose, which served as positive controls. Although the amount of L-lactic acid was lower with D-arabino-1,4-lactone than without sugar, the total amount of D, L-lactic acid was greater with D-arabino-1,4-lactone. These results suggest that B. breve assimilates D-arabino-1,4-lactone.
Table 1.
Concentration of lactic acid in medium after assimilation test using B. breve (mmol L− 1).
| D-arabino-1,4-lactone | No sugar | Sodium gluconate | Glucose | |
|---|---|---|---|---|
| D-lactic acid | 1.57 | 0.871 | 18.6 | 17.4 |
| L-lactic acid | 0.0610 | 0.154 | 0.466 | 1.40 |
| Total | 1.63 | 1.02 | 18.8 | 19.1 |
Conclusions
In this study, the production of D-arabino-1,4-lactone, as a lactone, from D-fructose using TiO2 photocatalyst was confirmed. The reaction mechanism involves α scission (C1-C2 position cleavage) of D-fructose to produce D-arabino-1,4-lactone, as well as degradation and C-C bond cleavage of D-fructose and D-arabino-1,4-lactone to produce erythrose and glyceraldehyde. The results of the sugar assimilation test and lactic acid measurement using D-arabino-1,4-lactone revealed that Bifidobacterium proliferates by assimilating D-arabino-1,4-lactone. Additionally, the growth of E. coli was inhibited by adding D-arabino-1,4-lactone. Therefore, it can be expected that D-arabino-1,4-lactone can be used for the growth of Bifidobacterium and as a new food additive.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
S.U. conducted the synthesis, performed data acquisition, and wrote the original draft. P.B.P. carried out synthesis and data acquisition. T.J., N.T., Y.U., and H.T. contributed to data acquisition. S.S.L. and S.L. developed the methodology and participated in writing, review, and editing. K.Y. designed the project, contributed to writing, review, and editing, and provided co-supervision. K.N. designed the project, contributed to writing, review, and editing, supervised the research, and provided resources. All authors reviewed and approved the final manuscript.
Data availability
All data included in this study is available upon request by contact with the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kenji Yamatoya, Email: yamatoya_k@meiji.ac.jp.
Kazuya Nakata, Email: nakata@go.tuat.ac.jp.
References
- 1.Gnanasekaran, L., Priya, A., Thanigaivel, S., Hoang, T. K. & Soto-Moscoso, M. The conversion of biomass to fuels via cutting-edge technologies: explorations from natural utilization systems. Fuel331, 125668 (2023). [Google Scholar]
- 2.Espro, C. et al. Sustainable production of pharmaceutical, nutraceutical and bioactive compounds from biomass and waste. Chem. Soc. Rev.50, 11191–11207 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Llatance-Guevara, L., Flores, N. E., Barrionuevo, G. O. & Mullo Casillas, J. L. Waste Biomass Selective and sustainable photooxidation to high-added-Value products: a review. Catalysts12, 1091 (2022). [Google Scholar]
- 4.Cruz-Santos, M. M. et al. Production and applications of pullulan from lignocellulosic biomass: challenges and perspectives. Bioresour Technol.385, 129460 (2023). [DOI] [PubMed] [Google Scholar]
- 5.Ummalyma, S. B. et al. Sustainable microalgal biomass production in food industry wastewater for low-cost biorefinery products: a review. Phytochem Rev.22, 969–991 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dharmaraja, J. et al. Lignocellulosic biomass conversion via greener pretreatment methods towards biorefinery applications. Bioresour Technol.369, 128328 (2023). [DOI] [PubMed] [Google Scholar]
- 7.de Vries, J. G. Industrial implementation of chemical biomass conversion. Curr. Opin. Green. Sustainable Chem.39, 100715 (2023). [Google Scholar]
- 8.Zhou, T. et al. A review on microalgae-mediated biotechnology for removing pharmaceutical contaminants in aqueous environments: occurrence, fate, and removal mechanism. J. Hazard. Mater.443, 130213 (2023). [DOI] [PubMed] [Google Scholar]
- 9.Keller, F., Lee, R. P. & Meyer, B. Life cycle assessment of global warming potential, resource depletion and acidification potential of fossil, renewable and secondary feedstock for olefin production in Germany. J. Clean. Prod.250, 119484 (2020). [Google Scholar]
- 10.Cho, H. H., Strezov, V. & Evans, T. J. A review on global warming potential, challenges and opportunities of renewable hydrogen production technologies. Sustain. Mater. Technol.35, e00567 (2023). [Google Scholar]
- 11.Osman, A. I. et al. Cost, environmental impact, and resilience of renewable energy under a changing climate: a review. Environ. Chem. Lett.21, 741–764 (2023). [Google Scholar]
- 12.Zuiderveen, E. A. et al. The potential of emerging bio-based products to reduce environmental impacts. Nat. Commun.14, 8521 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev.41, 1538–1558 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Wu, X. et al. Photocatalytic transformations of lignocellulosic biomass into chemicals. Chem. Soc. Rev.49, 6198–6223 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Osman, A. I., Farrell, C., Al-Muhtaseb, A. H., Harrison, J. & Rooney, D. W. The production and application of carbon nanomaterials from high alkali silicate herbaceous biomass. Sci. Rep.10, 2563 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zabed, H., Sahu, J., Boyce, A. N. & Faruq, G. Fuel ethanol production from lignocellulosic biomass: an overview on feedstocks and technological approaches. Renew. Sustainable Energy Rev.66, 751–774 (2016). [Google Scholar]
- 17.Chua, S. Y. et al. Biodiesel synthesis using natural solid catalyst derived from biomass waste—A review. J. Ind. Eng. Chem.81, 41–60 (2020). [Google Scholar]
- 18.John, R. P., Nampoothiri, K. M. & Pandey, A. Fermentative production of lactic acid from biomass: an overview on process developments and future perspectives. Appl. Microbiol. Biotechnol.74, 524–534. 10.1007/s00253-006-0779-6 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Zhang, B. et al. Catalytic fast co-pyrolysis of biomass and food waste to produce aromatics: Analytical Py–GC/MS study. Bioresour Technol.189, 30–35 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Sartori, S. K. & Diaz, M. A. N. Diaz-Muñoz, G. Lactones: classification, synthesis, biological activities, and industrial applications. Tetrahedron84, 132001 (2021). [Google Scholar]
- 21.Leksrisompong, P., Barbano, D. M., Foegeding, A. E., Gerard, P. & Drake, M. The roles of fat and Ph on the detection thresholds and partition coefficients of three compounds: Diacetyl, δ-decalactone and furaneol. J. sens. stud.25, 347–370 (2010). [Google Scholar]
- 22.Singh Aidhen, I., Thoti, N. Natural products & bioactivity inspired synthetic pursuits interfacing with Carbohydrates: ongoing journey with C-Glycosides. Chem. Rec.21, 3131–3177 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Mao, B., Fañanás-Mastral, M. & Feringa, B. L. Catalytic asymmetric synthesis of butenolides and butyrolactones. Chem. Rev.117, 10502–10566 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sisson, A. L., Ekinci, D. & Lendlein, A. The contemporary role of ε-caprolactone chemistry to create advanced polymer architectures. Polym54, 4333–4350 (2013). [Google Scholar]
- 25.Kaluzynski, K., Pretula, J., Lewinski, P., Kaźmierski, S. & Penczek, S. Synthesis and properties of functionalized poly (ε-caprolactone); chain polymerization followed by polycondensation in one pot with initiator and catalyst in one molecule. Synthesis and molecular structures. Macromolecules55, 2210–2221 (2022). [Google Scholar]
- 26.Lecomte, P. & Jérôme, C. Recent developments in ring-opening polymerization of lactones. Adv. Polym. Sci.245, 173–217 (2012). [Google Scholar]
- 27.Li, Y. T., Yu, H. Y., Li, W. B., Liu, Y. & Lu, X. B. Recyclable polyhydroxyalkanoates via a regioselective ring-opening polymerization of α, β-disubstituted β-lactone monomers. Macromolecules54, 4641–4648 (2021). [Google Scholar]
- 28.Mandal, P. & Shunmugam, R. Polycaprolactone: a biodegradable polymer with its application in the field of self-assembly study. J. Macromol. Sci. Part. Pure Appl. Chem.58, 111–129 (2020). [Google Scholar]
- 29.Nakata, K. & Fujishima, A. TiO2 photocatalysis: design and applications. J. Photochem. Photobiol C13, 169–189 (2012). [Google Scholar]
- 30.Nakata, K., Ochiai, T., Murakami, T. & Fujishima, A. Photoenergy conversion with TiO2 photocatalysis: new materials and recent applications. Electrochim. Acta84, 103–111 (2012). [Google Scholar]
- 31.Kaplaneris, N., Bisticha, A., Papadopoulos, G. N., Limnios, D. & Kokotos, C. G. Photoorganocatalytic synthesis of lactones via a selective C–H activation–alkylation of alcohols. Green. Chem.19, 4451–4456 (2017). [Google Scholar]
- 32.Triandafillidi, I., Kokotou, M. G. & Kokotos, C. G. Photocatalytic synthesis of γ-lactones from alkenes: high-resolution mass spectrometry as a tool to study photoredox reactions. Org. Lett.20, 36–39 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Wei, X. J. et al. A novel intermolecular synthesis of γ-lactones via visible-light photoredox catalysis. Org. Lett.15, 6054–6057 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Özgen, F. F. et al. The synthesis of Chiral γ-Lactones by merging Decatungstate Photocatalysis with Biocatalysis. ChemCatChem14, e202200855 (2022). [Google Scholar]
- 35.Harris, D. W. & Feather, M. S. Mechanism of the interconversion of D-glucose, D-mannose, and D-fructose in acid solution. J. Am. Chem. Soc.97, 178–181 (1975). [Google Scholar]
- 36.Estahbanati, M. K. et al. Photocatalytic conversion of alcohols to hydrogen and carbon-containing products: a cleaner alcohol valorization approach. J. Clean. Prod.318, 128546 (2021). [Google Scholar]
- 37.Chong, R. et al. Selective conversion of aqueous glucose to value-added sugar aldose on TiO2-based photocatalysts. J. Catal.314, 101–108 (2014). [Google Scholar]
- 38.Rao, V. N. et al. Light-driven transformation of biomass into chemicals using photocatalysts–vistas and challenges. J. Environ. Manage.284, 111983 (2021). [DOI] [PubMed] [Google Scholar]
- 39.Ahmed, A., Khan, T. A., Dan Ramdath, D., Kendall, C. W. & Sievenpiper, J. L. Rare sugars and their health effects in humans: a systematic review and narrative synthesis of the evidence from human trials. Nutr. Rev.80, 255–270 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sawettanun, S. & Ogawa, M. Physicochemical parameters, volatile compounds and organoleptic properties of bread prepared with substituted sucrose with rare sugar D-allulose. Int. J. Food Sci. Technol.57, 5931–5942 (2022). [Google Scholar]
- 41.Zhang, W. et al. D-allulose, a versatile rare sugar: recent biotechnological advances and challenges. Crit. Rev. Food Sci. Nutr.63, 5661–5679 (2023). [DOI] [PubMed] [Google Scholar]
- 42.Biyani, N. & Madhubala, R. Leishmania Donovani encodes a functional enzyme involved in vitamin C biosynthesis: arabino-1, 4-lactone oxidase. Mol. Biochem. Parasitol.180, 76–85 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Canete-Rodriguez, A. M. et al. Gluconic acid: Properties, production methods and applications—An excellent opportunity for agro-industrial by-products and waste bio-valorization. Process. Biochem.51, 1891–1903 (2016). [Google Scholar]
- 44.Kleerebezem, M. & Vaughan, E. E. Probiotic and gut lactobacilli and bifidobacteria: molecular approaches to study diversity and activity. Annu. Rev. Microbiol.63, 269–290 (2009). [DOI] [PubMed] [Google Scholar]
- 45.Dos Reis, S. A. et al. Review of the mechanisms of probiotic actions in the prevention of colorectal cancer. Nutr. Res.37, 1–19 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Wong, C. B., Odamaki, T. & Xiao, J. Beneficial effects of Bifidobacterium longum subsp. longum BB536 on human health: modulation of gut microbiome as the principal action. J. Fucnt Foods54, 506–519 (2019). [Google Scholar]
- 47.Wang, L. et al. Bifidobacterium longum relieves constipation by regulating the intestinal barrier of mice. Food Funct.13, 5037–5049 (2022). [DOI] [PubMed] [Google Scholar]
- 48.Dong, P., Yang, Y. & Wang, W. The role of intestinal bifidobacteria on immune system development in young rats. Early Hum. Dev.86, 51–58 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Fanaro, S., Chierici, R., Guerrini, P. & Vigi, V. Intestinal microflora in early infancy: composition and development. Acta Paediatr.92, 48–55 (2003). [DOI] [PubMed] [Google Scholar]
- 50.Asano, T., Yuasa, K., Kunugita, K., Teraji, T. & Mitsuoka, T. Effects of gluconic acid on human faecal bacteria. Microb. Ecol. Health Dis.7, 247–256 (1994). [Google Scholar]
- 51.Liu, Z. et al. Screening of bifidobacteria with acquired tolerance to human gastrointestinal tract. Anaerobe13, 215–219 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Kondo, S. et al. Antiobesity effects of Bifidobacterium breve strain B-3 supplementation in a mouse model with high-fat diet-induced obesity. Biosci. Biotechnol. Biochem.74, 1656–1661 (2010). [DOI] [PubMed] [Google Scholar]
- 53.Ojima, M. N. et al. Priority effects shape the structure of infant-type Bifidobacterium communities on human milk oligosaccharides. ISME J.16, 2265–2279 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Derrien, M., Turroni, F., Ventura, M. & van Sinderen, D. Insights into endogenous Bifidobacterium species in the human gut microbiota during adulthood. Trends Microbiol.30, 940–947 (2022). [DOI] [PubMed] [Google Scholar]
- 55.Lee, B. H., Huh, W. K., Kim, S. T., Lee, J. S. & Kang, S. O. Bacterial production of d-Erythroascorbic acid andl-ascorbic acid through functional expression of Saccharomyces cerevisiae d-Arabinono-1, 4-Lactone oxidase in Escherichia coli. Appl. Environ. Microbiol.65, 4685–4687 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fricke, P. M., Hartmann, R., Wirtz, A., Bott, M. & Polen, T. Production of L-arabinonic acid from L-arabinose by the acetic acid bacterium Gluconobacter oxydans. Bioresour Technol. Rep.17, 100965 (2022). [Google Scholar]
- 57.Adler, J. & Templeton, B. The effect of environmental conditions on the motility of Escherichia coli. Microbiol46, 175–184 (1967). [DOI] [PubMed] [Google Scholar]
- 58.Hudson, P. L., Hung, K. J., Bergerat, A. & Mitchell, C. Effect of vaginal Lactobacillus species on Escherichia coli growth. Female Pelvic Med. Reconstr. Surg.26, 146–151 (2020). [DOI] [PubMed] [Google Scholar]
- 59.Amaretti, A. et al. Kinetics and metabolism of Bifidobacterium adolescentis MB 239 growing on glucose, galactose, lactose, and galactooligosaccharides. Appl. Environ. Microbiol.73, 3637–3644 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data included in this study is available upon request by contact with the corresponding author.