Abstract
The study aimed to assess the feasibility and potential efficacy of a non-motor intervention utilizing motor imagery (MI) and transcranial direct current stimulation (tDCS) to enhance motor function. The research involved a double-blind, randomized, controlled trial with three groups: MIActive, MISham, and Control. Participants engaged in a cognitively demanding obstacle course, with time and prefrontal activation (ΔO2Hb and ΔHHb) measured across three-time points (Baseline, Post-test, 1-week follow-up). Following a pretest, active or sham tDCS was administered during an MI session, while the Control group did not receive this intervention. The MIActive group showed significant improvements in time-to-completion immediately after the intervention and one week later. Additionally, ΔO2Hb levels were lower in the MIActive group than in the other groups. These findings suggest that the combination of MI and tDCS could lead to motor improvements. The study outcomes support the feasibility and initial effectiveness of using MI and tDCS as a non-motor intervention to enhance motor outcomes in short and medium terms. Further research is recommended to explore the impact of this intervention in individuals with existing motor impairments. This study contributes to the growing body of evidence on the potential of non-motor interventions to induce neuroplastic changes that improve motor function. Clinical trial registration: ClinicalTrials.gov, identifier NCT06414213 16/05/2024.
Keywords: Motor imagery, Neuroplasticity, Rehabilitation, tDCS
Subject terms: Motor control, Rehabilitation
Introduction
Sedentary lifestyle, inactivity after injury or surgery, immobilization, and age-related changes occurring at the cortical level can lead to serious motor and cognitive dysfunctions, which may lead to motor impairments and deficient gait performance1,2. It is increasingly recognized that normal mobility requires higher-level cognitive input from the frontal cerebral circuits such as attention and executive function, especially in complex situations like obstacle course walking3. Dysfunction of the premotor cortex (PMc), located just anterior to the primary motor cortex, and dysfunction of the prefrontal cortex (PFc) are of particular interest because they have broad effects on executive functions that are important to planning, initiating and coordinating motor behaviors in simple and complex environments4. Unfortunately, evidence indicates that the PFc and other frontal lobe brain regions are specifically susceptible to age-related dysfunction demonstrated in the compensation-related utilization of neural circuits hypothesis (CRUNCH)3,5–8. CRUNCH illustrates how a dysfunctional brain adapts by utilizing other areas of the brain to compensate. This adaptation is marked by under activation of the PFc during cognitive tasks and overactivation for relatively simple tasks3,5–8. Therefore, a goal of neurorehabilitation is to promote activation of the PMc and PFc, enhance neuroplasticity, and promote healthy aging of the executive circuits.
A cornerstone of neurorehabilitation is to accelerate and boost the magnitude of functional gains in motor learning. Physical-based therapies, like complex walking or obstacle course scenarios, provide a dynamic environment where individuals must constantly adapt to navigate obstacles and adjust their locomotor strategies, thus stimulating prefrontal activation associated with motor planning and execution9. However, while these interventions offer valuable opportunities for engaging cognitive and motor processes, they may have limitations in targeting specific neural circuits or providing precise control over the intensity and focus of activation compared to other interventions. Mental imagery, particularly kinesthetic or motor imagery (MI), is integral to motor learning as it enhances cortical activity and stimulates neuroplasticity10–12. Recent research has shown that MI can increase corticospinal excitability of effector limbs and the PMc, similar to actual motor execution, aiding in learning and retention of motor skills10,12–14. In the context of motor skill acquisition, the process of observing another person and subsequently adapting one’s own actions is termed action observation (AO)15. Action observation activates, during both the observation and execution of actions, the brain’s mirror neuron system, which allows the observer to mentally simulate the observed action with more vivid imagery11, potentially enhancing learning and performance12,16,17.
To further enhance the neuroplastic effects of a MI and AO intervention and to combat the loss of motor skills due to aging, a low-level, constant-current brain stimulation known as transcranial direct current stimulation (tDCS) may be used. tDCS, applied over the frontal cerebral region, can facilitate neuromodulation of frontal and executive circuits, enhancing their receptivity to the activity-dependent neuroplasticity associated with behavioral neurorehabilitation, even within a single session13,18–21. This neuromodulatory effect has been corroborated by improvements in cortical activation, as detected by brain hemodynamic responses (O2Hb) using functional near-infrared spectroscopy (fNIRS)12,14,22. The interplay between tDCS and MI is particularly significant in this context. MI tasks are further enhanced when paired with tDCS. Anodal tDCS has been shown to increase excitability in the targeted cortical areas and extend its effects to connected regions, thereby optimizing synaptic plasticity and cognitive performance23. This broader network activation may reflect a more comprehensive brain function enhancement, suggesting that combining tDCS with MI could optimize cognitive training protocols.
This study aimed to evaluate the feasibility & preliminary efficacy of an MI intervention with elements of AO in addition to active or sham tDCS over the PFc and its effects on locomotor learning in able-bodied adults. Feasibility was evaluated in terms of recruitment, participation, and safety. Efficacy was evaluated based on time-to-completion and cerebral blood flow outcomes immediately after and one week following the intervention. We had three hypotheses: (1) intervention methods would be safe and tolerable for participants; (2) when compared to a control group, MI training would result in greater learning outcomes and retention of learning; (3) when compared to control and sham tDCS groups, Active tDCS would induce greater learning outcomes and retention of learning.
Materials and methods
Study design
The study implemented a double-blind, randomized, controlled trial design. Participants were tested three times over 7 days. After study enrollment, the participants were randomly assigned to one of three groups: MIActive (receiving active tDCS stimulation and participating in MI protocol), MISham (receiving sham tDCS stimulation and participating in MI protocol), and Control (receiving no stimulation and participating in an unrelated video-watching task) by a research member not associated with data collection. Allocation ratio was 1:1:1 and a block randomization approach with 9 per block was employed to maintain an equal distribution of participants across the three groups throughout the study. Study participants and assessors were blinded to assignment of active or sham tDCS. The independent variables were time (pre, post, and retention trials) and group (MIActive, MISham and Control), and the dependent variables were time to completion of a complex obstacle course and the amount of change in oxygenated hemoglobin (ΔO2Hb) and deoxygenated (ΔHHb) during performance of that task.
Participants and recruitment
A total of 38 participants were enrolled (MIActive n = 13, MISham n = 12, Control n = 13). G*Power 3.1.9.7 (Franz Faul, Kiel, Germany) was used to determine the sample size. The calculation was based on our initial pilot data for the pre- and post-tDCS and observational learning we used an analysis of variance (ANOVA) with repeated measures and a within-between interaction. We set a Type I error rate of 5%, aimed for a statistical power of 80%, and used an effect size of 0.73. This calculation indicated a required sample size of 10 individuals per group, with a target of 13 per group to accommodate potential attrition. The participants were young, able-bodied adults aged 18–35 years. Exclusion criteria included inability to sit, stand, or navigate obstacles without assistance, as well as having deep brain stimulators, known neurocognitive impairments or head injuries, and sensitivity to tDCS. Before starting the procedures, participants provided written informed consent, and the methods were approved by the Institutional Review Board of Appalachian State University. The research was performed in accordance with the Declaration of Helsinki and the approved protocol was followed throughout the study period. Additionally, it was registered under the identification number NCT06414213 at ClinicalTrials.gov (on 16/05/2024) and conformed to the CONSORT checklist.
Experimental procedure
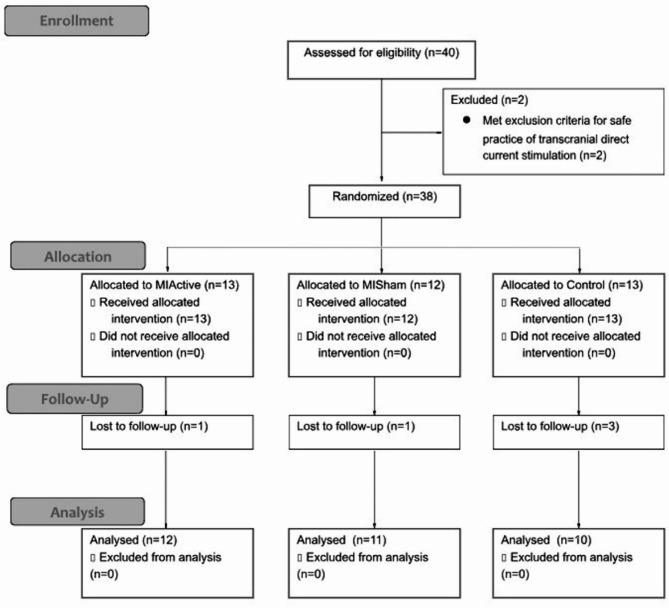
Two testing sessions were scheduled as close to 7-days apart as possible and at the same time of day, with a mean intersession interval of 7.18 ± 1.07 days. Session 1 consisted of a pre-test and a post-test, and session 2 consisted of a second post-test termed “retention.” Participants were asked not to partake in intense cognitive or physical activities 24 h before both sessions. Data were collected in a laboratory setting. A consort diagram of participant randomization and retention is shown in Fig. 1. Data collection occurred from September 2022 to December 2022 and terminated once our target recruitment was completed.
Fig. 1.
Consort diagram for participant recruitment and analysis.
Initial questionnaires and pre-intervention fNIRS Set-Up
Prior to the intervention, participants completed a tDCS inclusion criteria form to ensure the tolerability and safety of tDCS administration. Participants were instrumented with an fNIRS headpiece (Octamon, Artinis Medical Systems, Nijmegen, The Netherlands) with eight embedded light sources that emitted near-infrared light and two near-infrared light detectors. The headband was positioned approximately 1.5 cm above the nasion and the middle of the headband was aligned with the midsagittal plane of the head. Instructions for the baseline measurement and the obstacle course were provided, as well as one visual demonstration of the obstacle course.
To establish baseline levels of O2Hb, participants were seated in a chair with their back supported and facing a blank wall. Participants remained still and counted to thirty out loud at approximately a 30-second pace, focusing on each number as they said it aloud.
Once baseline fNIRS collections were completed, the participant stood on the starting point of the obstacle course and was free to begin walking.
Pretest: obstacle course timed trials and fNIRS collections and analysis
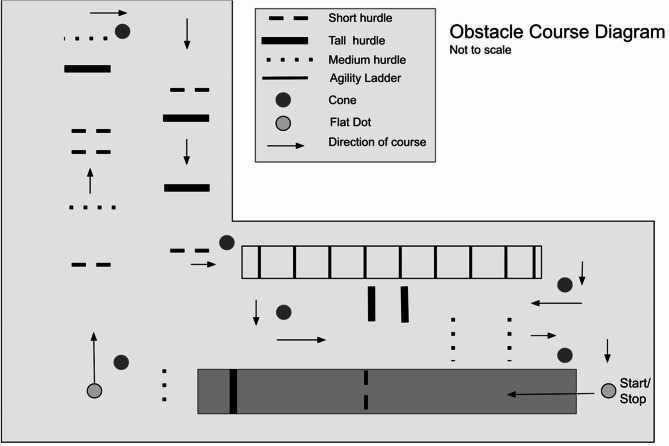
The decision to incorporate an obstacle course in this study was driven by the need to engage prefrontal circuits through challenging locomotor activities. The obstacle course consisted of a series of hurdles creating obstacles along a 44-meter walking course (Fig. 2). The hurdles were set at three different heights (15 cm, 17 cm, and 30 cm) to allow for variation of obstacles. The distribution of hurdles was randomized in a way that was not predictable. The obstacle course was created based on previous investigations to increase cognitive effort8,24. Participants were instructed to perform the course at the quickest and most efficient walking pace possible without bumping any hurdles. They were timed from the heel strike of their first step to the time they returned with both feet back to the start/finish point. Participants performed a timed trial through the obstacle course with a baseline O2Hb collection prior to each trial for a total of three baseline collections with three corresponding obstacle course trials. Whether the participant made an error (i.e. kicking, knocking over or missing a hurdle, or going the wrong direction) during a trial on the obstacle course was noted to assess the impact of errors on performance, safety, and feasibility.
Fig. 2.
Diagram of a 44-meter complex walking course. Arrows denote walking direction.
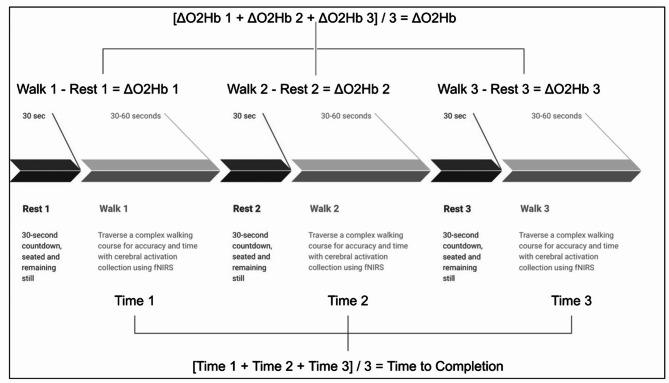
Hemoglobin density data were recorded using the fNIRS device and transmitted to a laptop via Bluetooth. The raw signals were stored with Oxysoft software (v3.2.51.4, Artinis Medical Systems, The Netherlands). Data were segmented into active walking and resting (reference) blocks for analysis. All fNIRS raw data were imported into MATLAB (The MathWorks, Inc., MA, USA) using the oxysoft2matlab function and preprocessed with the HOMER2 toolbox (Homer2 NIRS processing package). Preprocessing involved converting raw signals to optical density values [hmrIntensity2OD], which facilitated the identification and correction of motion artifacts. Motion correction was performed using a wavelet transformation (hmrMotionCorrectWavelet), and additional artifact corrections were applied (hmrMotionArtifact). All data was band-pass filtered (low-pass filter with a 0.14 Hz) cutoff to eliminate physiological confounds such as heart rate components and Mayer waves. The data were then converted to hemoglobin concentration values via the modified Beer-Lambert law. For each block, mean O2Hb and HHb concentration values were calculated, excluding the initial five seconds to account for blood flow stabilization. Task-related changes in hemoglobin concentration were determined using the following equation: (Walking O2Hb concentration) – (Baseline O2Hb Concentration) = ΔO2Hb (Fig. 3).
Fig. 3.
Diagram of test design and calculation of Time to Completion and ΔO2Hb and ΔHHb for each test and trial.
Pre-intervention protocol: MIQ-3, tDCS set-up, and tDCS sensitivity
MIActive and MISham participants completed the Movement Imagery Questionnaire 3rd Edition (MIQ-3), which assessed their ability to vividly visualize, both internally and externally, and kinesthetically imagine different tasks. The MIQ-3 has been shown to be a valid measure of one’s ability to perform a task mentally and the effectiveness of observational learning based on their ability25. The MIQ-3 was scored with a maximal score of 28 for each category, and 84 total.
Following the MIQ-3, participants in the MI groups were instrumented with tDCS. This study utilized a bilateral configuration over the DLPFC due to its ability to improve motor learning and changes in blood oxygenation in motor cortices, both at rest26 and following interventions21. 5 × 7 cm sponge electrodes were placed on a forehead strap (Soterix EASYStrap: Frontal) at the precise location of F3 (placement of cathode) and F4 (placement of anode) determined by the 10–20 system. The sponges were dampened with 4-units of saline per side. After the electrodes were connected to the tDCS stimulator (Soterix, 1 × 1 tDCS-CT clinical trial stimulator, New York, USA) the impedance was tested visually by the administrator and maintained within the optimal range as indicated by the SMARTscan-PLUS™ system to ensure acceptable contact prior to beginning stimulation and maintain blinding without compromising subject monitoring27.
Investigators and participants were blinded to the status of tDCS (active or sham) through 6-digit codes known only to a research team member which was not present for data collection. Each participant was assigned a unique code that controlled the stimulator. At the initiation of stimulation, participants were provided with a tDCS sensitivity questionnaire, addressing fourteen symptoms potentially induced by tDCS stimulation. Participants rated each symptom on a scale of 1–10 (1 = absent, 10 = severe). Scores at or above a 5 were considered a contraindication for tDCS stimulation and were to result in immediate cessation of stimulation. The total possible score for the tDCS sensitivity screen was 140.
Intervention: MI with tDCS stimulation
The participants received a 20-minute session of tDCS at 2-mA (MIActive) or a 20-minute session of sham tDCS (MISham). During this 20-minute tDCS session, the participants watched a video sequence of an individual completing twenty walking trials (twenty video clips—each clip represents one trial). Participants were instructed to place their focus intently on the person performing the obstacle course and try to imagine themselves doing the skill. Periodically, a reminder would appear to help focus and redirect participant’s attention to different aspects of the video or different versions of imagery (visual or kinesthetic). Both MIActive and MISham watched the same video at the same speed.
Total MI training time was approximately 20-minutes, consistent with the stimulation duration. Prior research indicated that fifteen to thirty minutes of observation was appropriate for observational learning protocols to observe improvements18. Video perspective was chosen based on previous research which suggested that third-person perspective may lead to stronger memory representations and better retention compared to first person views28. Clips were shown on a computer screen with minimal distractions surrounding the screen and desk. Immediately following the cessation of stimulation, participants were given the tDCS sensitivity questionnaire again to assess if and to what degree symptoms had changed over the duration of stimulation. The control group watched an unrelated video29 for a duration equal to the MI groups’ pre-intervention and intervention tasks. Participants were instructed to relax and focus on the video.
Post- & retention tests
After the intervention, participants performed the obstacle course in a manner identical to the pretest utilizing the fNIRS to measure cerebral activation. Instructions were provided in the same manner as the pretest without demonstration from the primary investigator. A retention test was conducted during Session 2, following the same procedures as the posttest completed during the initial visit.
Statistical analysis
Statistical analysis was run in SPSS 27. Times of the three trials at each test were averaged to observe any learning effects during the obstacle course. Changes in cerebral blood flow at each of the three trials during each test were averaged within each time point (pre, post, and retention) to evaluate changes in cerebral blood flow specific to that time point. (Fig. 3). A 2-way factorial ANOVA was employed with a between-subjects factor of group (3 levels) and a within-subjects factor of time (3 levels).In the case of significant interactions of main effects, pairwise comparisons using Fisher’s LSD were used post-hoc. Effect size was interpreted from partial eta squared as small = 0.01, medium = 0.06, and large = 0.14. Independent samples t-tests were used to analyze any differences in the MIQ-3, and the tDCS sensitivity between groups.
Results
Participant recruitment & demographics
Thirty-eight participants were enrolled and randomized to one of three groups (MIActive = 13, MISham = 12, Control = 13). Five participants were lost to follow up, therefore data on 33 participants were analyzed. Participant demographics and the average number of days between pre-test and follow-up for each group are listed in Table 1.
Table 1.
Participant demographics and average days between session 1 and session 2.
| N | Age | Follow-up (days) | |
|---|---|---|---|
| MIActive | 12 | 22.42 ± 1.98 | 7 ± 0 |
| MISham | 11 | 22.36 ± 2.06 | 6.91 ± 0.30 |
| Control | 10 | 24.1 ± 4.04 | 6.8 ± 0.42 |
| Total | 33 | 23.06 ± 3.05 | 7.18 ± 1.07 |
Feasibility & tolerability
There was an 87% completion rate for this study, 13% of participants failed to return for the follow-up appointment for reasons unrelated to the study (i.e. sickness or unforeseen scheduling conflict). The analysis of the tDCS sensitivity questionnaires showed no statistically significant differences between groups at the pretest t(21) = 1.34, P = 0.19. However, at the posttest, the MIActive group exhibited significantly lower sensitivity t(21) = 2.136, P = 0.045, compared to the MISham group (Table 2). No participant rated tDCS sensitivity above a 4. Participant guesses on which stimulation they had were as follows: within the MIActive group ten people guessed they had active stimulation and two guessed they had sham stimulation. Further, in the MISham group nine people guessed they had active stimulation and two people guessed they had sham stimulation. Participants were able to correctly guess which stimulation they had approximately 52% of the time and participants guessed active more often.
Table 2.
Pre-intervention Survey data: relevant scores from MIQ-3 and tDCS sensitivity.
| External Imagery (mean ± SD) | Kinesthetic Imagery (mean ± SD) | Total MIQ-3 (mean ± SD) | Total pre-tDCS Sensitivity (mean ± SD) | Total post-tDCS Sensitivity (mean ± SD) | |
|---|---|---|---|---|---|
| MIActive | 21.33 ± 4.12 | 22.92 ± 4.74 | 68.5 ± 8.96 | 19.00 ± 3.16 | 16.83 ± 4.04* |
| MISham | 22.73 ± 3.00 | 21.77 ± 4.55 | 67.59± 7.61 | 21.64 ± 5.97 | 20.82 ± 4.90 |
*Significant difference between groups in sensitivity levels after tDCS stimulation (P = 0.045).
From the MIQ-3, no significant differences were detected between the MIActive and MISham groups on measures of Kinesthetic Imagery, t(21) = 0.590, P = 0.562, External Imagery t(21) = 0.920, P = 0.368, and MIQ-3 total scores t(21) = 0.989, P = 0.797 (Table 2). A higher score represents higher motor imagery ability with regards to the imagery ability tested.
Time-to-completion
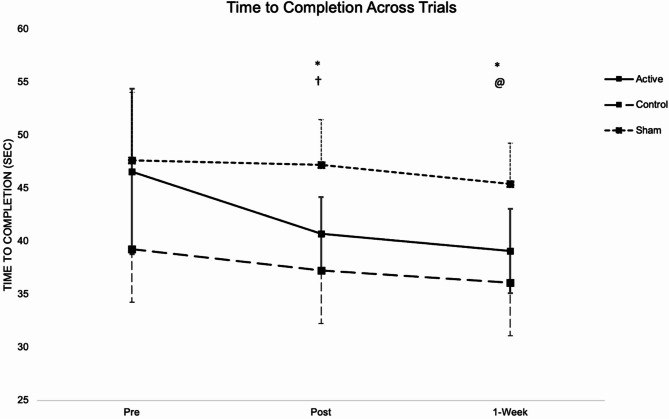
Data for time-to-completion violated assumptions of sphericity (χ2 = 10.02, P = 0.001), therefore ANOVA results were interpreted with a Huynh-Feldt correction. The analysis revealed a significant group-by-time interaction effect (F[3.45, 51.75] = 4.322, P = 0.006, η2 = 0.224) (Table 3). Post hoc tests were conducted to further explore the interaction. Post hoc analysis showed that the MIActive Group had significantly faster times at the posttest (P < 0.001) when compared to the pretest and at 1-Week when compared to the pretest (P < 0.001) and posttest (P = 0.042). MISham showed a significant decrease in time from posttest to retention (P = 0.033) and the Control group had a significant decrease from pretest to retention (P = 0.033) (Fig. 4). 95% confidence intervals for these differences are presented in Table 3. Group comparisons at each time point revealed significant differences in time-to-completion. At the pretest, the Control group demonstrated significantly faster completion times than the Sham groups (P = 0.04). During the posttest, the Control group again outperformed the Sham group (P = 0.001), and the Active group showed significantly faster times than the Sham group (P = 0.01). At the 1-week retention session, the Control group continued to have significantly faster times than the Sham group (P = 0.001), with the Active group also being significantly faster than the Sham group (P = 0.03).
Table 3.
Mean time to completion of all groups across all time points.
| Group | Session | Time (sec) | 95% CI |
|---|---|---|---|
| MIActive | Pre | 46.60 ± 7.80 | 42.20, 50.99 |
| Post | 40.72 ± 3.45* | 37.54, 43.90 | |
| Week | 39.10 ± 3.98@@† | 35.78, 42.42 | |
| MISham | Pre | 47.64 ± 6.42 | 43.05, 52.23 |
| Post | 47.21 ± 4.30 | 43.90, 50.53 | |
| Week | 45.43 ± 3.86† | 41.96, 48.90 | |
| Control | Pre | 39.27 ± 8.07 | 34.45, 44.08 |
| Post | 37.28 ± 7.85 | 33.81, 40.76 | |
| Week | 36.10 ± 8.37@ | 32.46, 39.74 |
*Significant decrease from pre to post P < 0.001.
@Significant decrease from pre to week P < 0.05; @@ P < 0.001.
†Significant decrease from post to week p < 0.05.
Fig. 4.
Time-to-completion across all trials, pretest (1), posttest (2), and retention test (3) of a 44-meter complex walking course. *MIActive had a significant decrease in time from Pre to Post (P < 0.001), Post to Retention(P < 0.05), and Pre to Retention (P < 0.001). †Control had significant decrease in time from Pre to Post (P < 0.05). @MISham had a significant decrease in time from Post to Retention (P < 0.05).
Change in oxygenated and deoxygenated hemoglobin
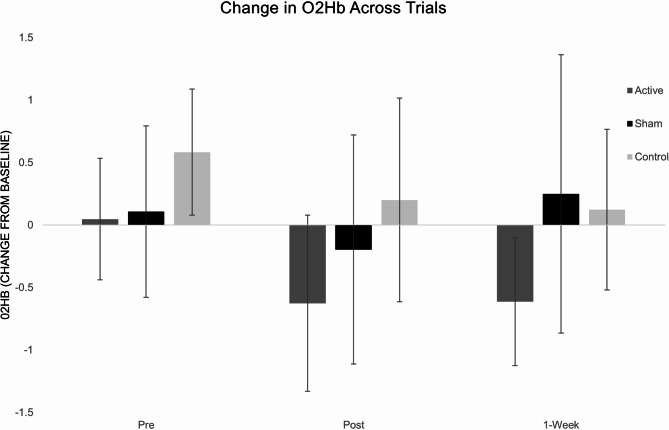
Figure 5 shows the averaged time course of O2Hb concentrations in the PFC during the rest period and obstacle course from one participant. For ΔO2Hb, there was no significant group-by-time interaction (F[4,60] = 1.34, P = 0.26, η2 = 0.082). ANOVA revealed a main effect of time (F[2,60] = 4.43, P = 0.013, η2 = 0.129) and a main effect of group (F[2,30] = 5.198, P = 0.012, η2 = 0.257). Post hoc analysis indicated that there was a significant decrease from pretest to posttest across all groups (mean difference = 0.453; 95% CI [0.104, 0.802]; P = 0.013) and that the MIActive group had a greater magnitude decrease than MISham (mean difference= -0.451; 95% CI [-0.893, -0.009]; P = 0.046) and MIControl (mean difference= -0.699; 95% CI [-1.153, -0.246]; P = 0.004). (Fig. 6) Analysis of ΔHHb revealed no significant group-by-time interaction and no effects for time or group.
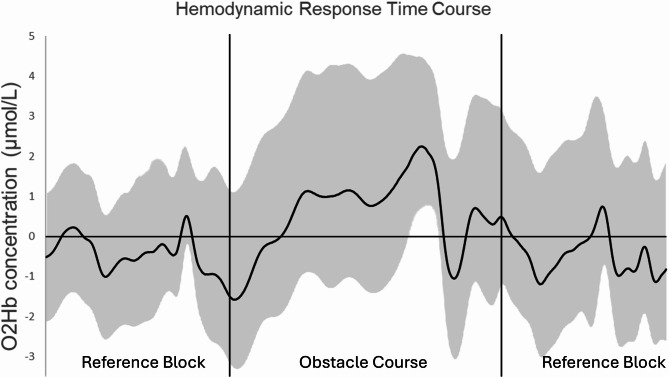
Fig. 5.
fNIRS signal time courses during obstacle course. Average time course of oxygenated hemoglobin (O2Hb: dark, solid line) for one individual. Mean ± SD. Vertical black lines indicate the start and end of the obstacle course.
Fig. 6.
Changes in the concentration of O2Hb of the PFC across pretest (1), posttest (2), and retention test (3) during the complex walking course.
Discussion
This study examined the feasibility and efficacy of a multi-modal MI and brain stimulation intervention by investigating how tDCS combined with MI affected the learning and performance of a complex obstacle walking course. Our findings provide evidence that an MI intervention with brain stimulation is feasible and may be efficacious for improving motor performance. However, given the lack of cerebral blood flow changes, the mechanisms behind this improvement are not entirely clear.
Motor adaptations
The primary finding of this study was that a non-motor intervention – 20 min of active tDCS combined with MI – was effective in producing motor adaptations across all post-tests. The MIActive group significantly improved time-to-completion after a single session with a 12.93% decrease in time. Additionally, the MIActive group maintained those motor improvements and experienced further significant improvements in time by 4.39% at the 1-week retention timepoint. The total baseline to retention change was significant as well, with overall improvements of 16.75%. These results indicate that a multi-layered approach of active tDCS in combination with MI was effective at improving motor outcomes.
These findings suggest that the use of tDCS helped facilitate motor learning and skill acquisition in a way that MI alone could not, as improvements were not observed in the MISham group. These results align with previous research which was summarized in two recent meta-analyses. In these studies the use of MI and AO in rehabilitation of lower limb injuries only proved effective at improving function when used in conjunction with another traditional rehabilitation technique30,31. These reviews explored other physical rehabilitation techniques, such as low-intensity strengthening, balance, and mobility exercises. The results of our study suggest that MI with brain stimulation, in the absence of traditional physical rehabilitation, can lead to robust motor improvements, potentially offering benefits for patients who are unable to conduct such rehabilitative motor tasks.
The precise cause of motor improvements after tDCS stimulation is not yet entirely understood. Previous investigations stated that placement of electrical stimulation over F3 and F4 resulted in broad field effects in the frontal region32. While our study targeted the PFc with F3 and F4 placements, it is plausible that the electric field extended beyond this region, affecting adjacent prefrontal areas associated with various cognitive functions33. These areas encompass executive functioning, response selection, working memory, attention, habit formation, and spatial memory, all of which play integral roles in motor skill acquisition and execution34.
Cerebral level adaptations
While motor outcomes were a major focus of this study, we were also interested in the potential neural mechanistic changes that underlie the observed motor improvements. This was examined by measuring cerebral blood flow changes. The results for ΔO2Hb showed no significant effects of the intervention, with all groups displaying a significant decrease in ΔO2Hb from pretest to posttest. This decrease in frontal cortex blood flow suggests an increased ease of the task, reflected by decreased activation, indicative of a practice effect, not influenced by MI or tDCS. We did however note a moderate effect size for the group-by-time effect of ΔO2Hb (η2 = 0.082), with the largest magnitude decrease in ΔO2Hb in the MIActive group. This observation aligns with prior research where trends in cerebral blood flow responses were noted without achieving statistical significance, potentially due to limited sample sizes8. Sample sizes were chosen based off motor outcomes rather than cerebral blood flow responses which were secondary outcome interests.
One possibility no differences in cerebral blood flow were observed across interventions is that ΔO2Hb is reflective of the cognitive demand of a task. This study occurred in a healthy young adult population as opposed to a neurologically-impaired or aging population, that may respond differently to task demands3,8,12. This is illustrated by the CRUNCH hypothesis, which says the aging or dysfunctional brain compensates for lack of cognitive energy by over activating the prefrontal area in simple tasks and under-activating in complex tasks. This compensation leads to diminished capacity for adaptation, and motor deficits. Conversely, younger adults have a broader capacity for adaptations to task environment and complexity and therefore may have had less room for improvement in our study3,7,8.
Additionally, it is important to acknowledge that our current investigation relied on frontal cortex fNIRS alone to assess changes in cerebral blood flow changes, which provides valuable but limited insight into neural activity. While fNIRS is sensitive to cerebral blood flow changes associated with cognitive processes, it does not directly measure neuronal activity or connectivity. Therefore, it is possible that some neural adaptations, such as enhanced synchronization or connectivity between brain regions, were not captured by our measurements, as we measured activity in the frontal cortex only. Despite not detecting substantial cerebral blood flow changes, this raises intrigue about the potential neural mechanisms at play that influenced our observed changes in time to completion. It is possible that further investigation into more diverse populations, and utilization of more advanced neuroimaging techniques, such as functional magnetic resonance imaging (fMRI) or electroencephalography (EEG), could offer more comprehensive insights into the neural dynamics that may occur with this intervention.
Questionnaires
Results of the Kinesthetic, Visual 3rd person, and total scores from the MIQ-3 (Table 2) indicate that there was no significant difference between each group’s ability to participate in mental imagery. Therefore, we can assume that both groups were evenly matched in this regard and ability to mentally imagine likely did not play a role in the outcomes of the intervention. Additionally, the tDCS sensitivity questionnaire results (Table 2) showed that while both groups had similar pre-tDCS sensitivity levels, the MIActive group had lower sensitivity (< 3%) at post-training compared to the MISham group. When these findings are juxtaposed to participant stimulation guesses, there was no greater ability for MIActive over MISham to correctly guess the type of stimulation they received. Therefore, mildly reduced sensitivity in the MIActive group did not seem to influence the outcome.
Feasibility
Our study was essential in preparing for full-scale trials by identifying potential challenges that could affect the success of the research protocol. Feasibility in the study was supported by a high recruitment rate and low attrition, with 95% of contacted participants enrolling and less than 14% attrition rate. Furthermore, no participants withdrew from the study due to adverse events related to our protocol, with only mild discomfort during tDCS application reported, which is a common occurrence35 Therefore, we concluded this method should also be considered safe when used with proper precautions.
Conclusion
The preliminary data obtained from our multimodal, non-motor intervention, using a 20-minute MI training paired with 2 mA of prefrontal tDCS proved feasible with high recruitment and retention rates. Further, our intervention lends support for improving motor performance in healthy adults. The positive performance outcomes observed in our study offer a compelling rationale for considering this intervention as a valuable addition to the repertoire of traditional rehabilitation methods for individuals facing motor dysfunctions. Moreover, this non-motor intervention might serve as a valuable tool for risk reduction among individuals at risk of falls or injuries due to limitations in functional capacity, as illustrated by the CRUNCH model. This approach could offer a preventive strategy, helping individuals maintain or regain their motor function and potentially reducing the risk of accidents related to mobility issues. Additionally, there was no evidence of any disadvantages to this approach with proper intensity and duration dosage.
While our study presents promising results for the application of an MI intervention with aspects of AO and tDCS in rehabilitation, it is essential to recognize that these findings are preliminary, and it is important to continue building upon this foundation. Future research should consider avenues for further investigation and development of our intervention, exploring various configurations and intensities of the intervention. For instance, incorporating multiple training sessions per week over an extended period or implementing long-term follow-up assessments could provide valuable insights into the durability and long-term effects of the observed gains. Such adjustments to the protocol could offer a more comprehensive understanding of the intervention’s efficacy and its potential applicability. Additionally, future research directions could involve different populations and employ more advanced imaging techniques to probe cerebral level changes. By utilizing advanced imaging modalities like unenhanced and enhanced magnetic resonance angiography, researchers can non-invasively assess cerebral adaptations with greater precision and detail, refining treatment approaches and improving patient care.
NCT06414213 Improving Locomotor Learning With Brain Stimulation (ELLMITS).
Author contributions
In accordance with the International Committee of Medical Journal Editors (ICMJE) guidelines, authorship was based on substantial contributions to the research work. All authors contributed significantly to the conception and design of the study. Dr. Skinner, Mrs. Muscarello, Dr. Needle, and Dr. Meucci contributed to the analysis and interpretation of data. Furthermore, all authors were involved in drafting the article and critically revising it for important intellectual content. All authors have approved the final version of the manuscript to be published, indicating their agreement with the content and conclusions presented.
Data availability
The data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wheeler, M. J. et al. Sedentary behavior as a risk factor for cognitive decline? A focus on the influence of glycemic control in brain health. Alzheimers Dement. (N Y)3, 291–300. 10.1016/j.trci.2017.04.001 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Binotto, M. A., Lenardt, M. H. & Rodríguez-Martínez, M. D. C. Physical frailty and gait speed in community elderly: a systematic review. Rev. Esc Enferm USP52, e03392. 10.1590/S1980-220X2017028703392 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Clark, D. J. et al. Multimodal Imaging of Brain Activity to investigate walking and mobility decline in older adults (mind in Motion Study): hypothesis, theory, and methods. Front. Aging Neurosci.11, 358. 10.3389/fnagi.2019.00358 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Purves, D. et al. (eds), e. Neuroscience. 2nd Edition edn. (Sinauer Associates, 2001).
- 5.McDonough, I. M., Wong, J. T. & Gallo, D. A. Age-related differences in prefrontal cortex activity during retrieval monitoring: testing the compensation and dysfunction accounts. Cereb. Cortex23, 1049–1060. 10.1093/cercor/bhs064 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson, M. K., Mitchell, K. J., Raye, C. L. & Greene, E. J. An age-related deficit in prefrontal cortical function associated with refreshing information. Psychol. Sci.15, 127–132. 10.1111/j.0963-7214.2004.01502009.x (2004). [DOI] [PubMed] [Google Scholar]
- 7.Balasubramanian, C. K., Clark, D. J. & Gouelle, A. Validity of the gait variability index in older adults: effect of aging and mobility impairments. Gait Posture41, 941–946. 10.1016/j.gaitpost.2015.03.349 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark, D. J. et al. Combining Frontal Transcranial Direct Current Stimulation with walking Rehabilitation to Enhance mobility and executive function: a Pilot Clinical Trial. Neuromodulation24, 950–959. 10.1111/ner.13250 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erni, T. & Dietz, V. Obstacle avoidance during human walking: learning rate and cross-modal transfer. J. Physiol.534, 303–312. 10.1111/j.1469-7793.2001.00303.x (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermes, D. et al. Functional MRI-based identification of brain areas involved in motor imagery for implantable brain-computer interfaces. J. Neural Eng.8, 025007. 10.1088/1741-2560/8/2/025007 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Meers, R., Nuttall, H. E. & Vogt, S. Motor imagery alone drives corticospinal excitability during concurrent action observation and motor imagery. Cortex126, 322–333. 10.1016/j.cortex.2020.01.012 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Iso, N. et al. Hemodynamic Signal Changes during Motor Imagery Task Performance are Associated with the degree of Motor Task Learning. Front. Hum. Neurosci.15, 603069. 10.3389/fnhum.2021.603069 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sobierajewicz, J., Jaśkowski, W. & Van der Lubbe, R. H. J. Does Transcranial Direct Current Stimulation affect the learning of a fine sequential Hand Motor Skill with Motor Imagery? J. Mot Behav.51, 451–465. 10.1080/00222895.2018.1513395 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Iso, N. et al. Monitoring local Regional hemodynamic Signal changes during motor execution and motor imagery using Near-Infrared Spectroscopy. Front. Physiol.6, 416. 10.3389/fphys.2015.00416 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buccino, G. Action observation treatment: a novel tool in neurorehabilitation. Philosophical Trans. Royal Soc. B Biol. Sci.369, 20130185 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Catmur, C., Thompson, E. L., Bairaktari, O., Lind, F. & Bird, G. Sensorimotor training alters action understanding. Cognition171, 10–14. 10.1016/j.cognition.2017.10.024 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Cui, X., Jeter, C. B., Yang, D., Montague, P. R. & Eagleman, D. M. Vividness of mental imagery: individual variability can be measured objectively. Vis. Res.47, 474–478. 10.1016/j.visres.2006.11.013 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moghadas, T. Y., Yavari, K. M. & Shahrbanian, S. Effects of a single Session Transcranial Direct Current Stimulation (tDCS) on Hand Mental Rotation and visuo–spatial Working Memory. Neuropsychology5, 37–54. 10.30473/CLPSY.2019.43737.1389 (2019). [Google Scholar]
- 19.Buch, E. R. et al. Effects of tDCS on motor learning and memory formation: a consensus and critical position paper. Clin. Neurophysiol.128, 589–603. 10.1016/j.clinph.2017.01.004 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Wade, S. & Hammond, G. Anodal transcranial direct current stimulation over premotor cortex facilitates observational learning of a motor sequence. Eur. J. Neurosci.41, 1597–1602. 10.1111/ejn.12916 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Chatterjee, S. et al. (ed, A.) Effects of Prefrontal Transcranial Direct Current Stimulation on Retention of Performance gains on an obstacle negotiation Task in older adults. Neuromodulation: Technol. Neural Interface.10.1016/j.neurom.2022.02.231 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ono, Y. et al. Motor learning and modulation of prefrontal cortex: an fNIRS assessment. J. Neural Eng.12, 066004. 10.1088/1741-2560/12/6/066004 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Chan, M. M. Y. & Han, Y. M. Y. Effects of Transcranial Direct Current Stimulation (tDCS) in the normalization of brain activation in patients with neuropsychiatric disorders: a systematic review of Neurophysiological and Neuroimaging studies. Neural Plast.2020(8854412). 10.1155/2020/8854412 (2020). [DOI] [PMC free article] [PubMed]
- 24.Hawkins, K. A. et al. Prefrontal over-activation during walking in people with mobility deficits: interpretation and functional implications. Hum. Mov. Sci.59, 46–55. 10.1016/j.humov.2018.03.010 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams, S. E. et al. Further validation and development of the movement imagery questionnaire. J. Sport Exerc. Psychol.34, 621–646. 10.1123/jsep.34.5.621 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Bouchard, A. E., Renauld, E. & Fecteau, S. Changes in resting-state functional MRI connectivity during and after transcranial direct current stimulation in healthy adults. Front. Hum. Neurosci.17, 1229618. 10.3389/fnhum.2023.1229618 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charvet, L. E. et al. Remotely supervised transcranial direct current stimulation for the treatment of fatigue in multiple sclerosis: results from a randomized, sham-controlled trial. Mult Scler.24, 1760–1769. 10.1177/1352458517732842 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marre, Q., Huet, N. & Labeye, E. Embodied mental imagery improves memory. Q. J. Exp. Psychol. (Hove)74, 1396–1405. 10.1177/17470218211009227 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Smith, B. in Bob Ross - One Hour Special - The Grandeur of Summer (ed Sally Schenk) 59:42. PBS, (1987).
- 30.Nanbancha, A., Mawhinney, C. & Sinsurin, K. The effect of motor imagery and action observation in the rehabilitation of lower limb injuries: a scoping review. Clin. Rehabil.37, 145–161. 10.1177/02692155221123546 (2023). [DOI] [PubMed] [Google Scholar]
- 31.Rannaud Monany, D., Papaxanthis, C., Guillot, A. & Lebon, F. Motor imagery and action observation following immobilization-induced hypoactivity: a narrative review. Ann. Phys. Rehabil Med.65, 101541. 10.1016/j.rehab.2021.101541 (2022). [DOI] [PubMed] [Google Scholar]
- 32.Indahlastari, A. et al. Modeling transcranial electrical stimulation in the aging brain. Brain Stimul.13, 664–674. 10.1016/j.brs.2020.02.007 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunoni, A. R. & Vanderhasselt, M. A. Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: a systematic review and meta-analysis. Brain Cogn.86, 1–9. 10.1016/j.bandc.2014.01.008 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Jobson, D. D., Hase, Y., Clarkson, A. N. & Kalaria, R. N. The role of the medial prefrontal cortex in cognition, ageing and dementia. Brain Commun.3, fcab125. 10.1093/braincomms/fcab125 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bikson, M. et al. Safety of Transcranial Direct Current Stimulation: evidence based Update 2016. Brain Stimul.9, 641–661. 10.1016/j.brs.2016.06.004 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request.