Abstract
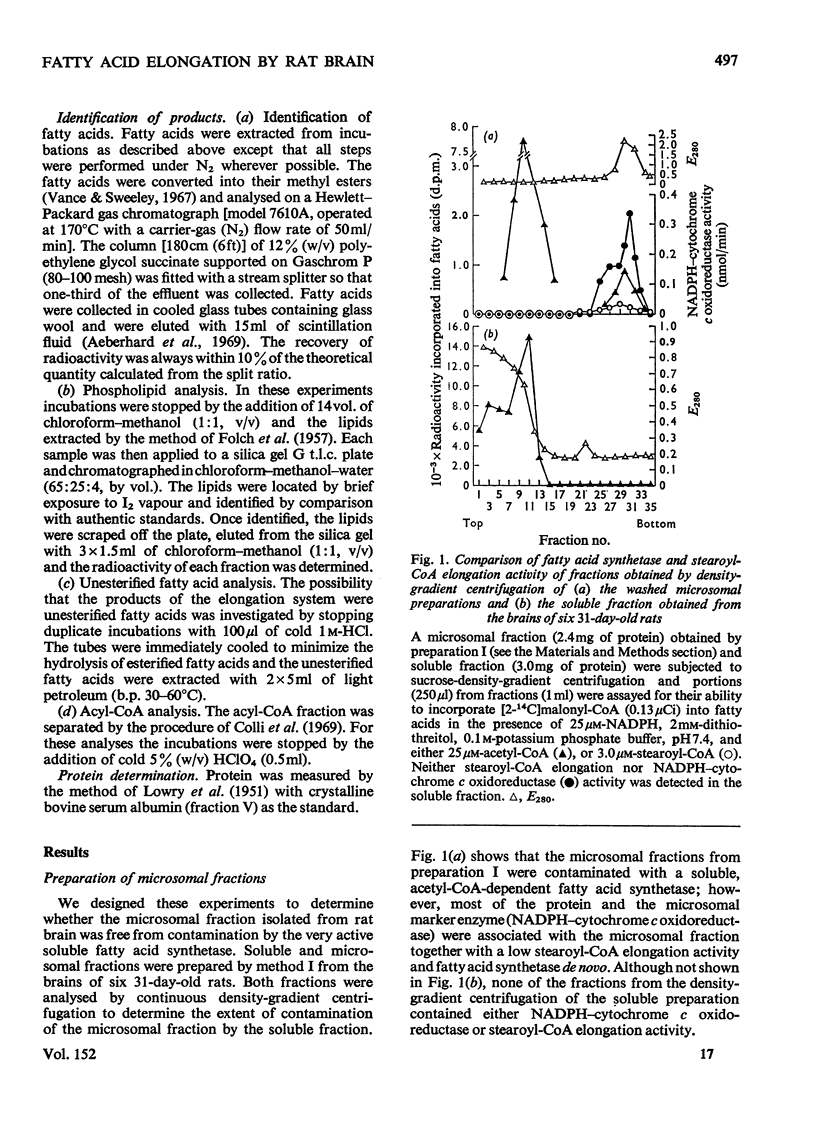
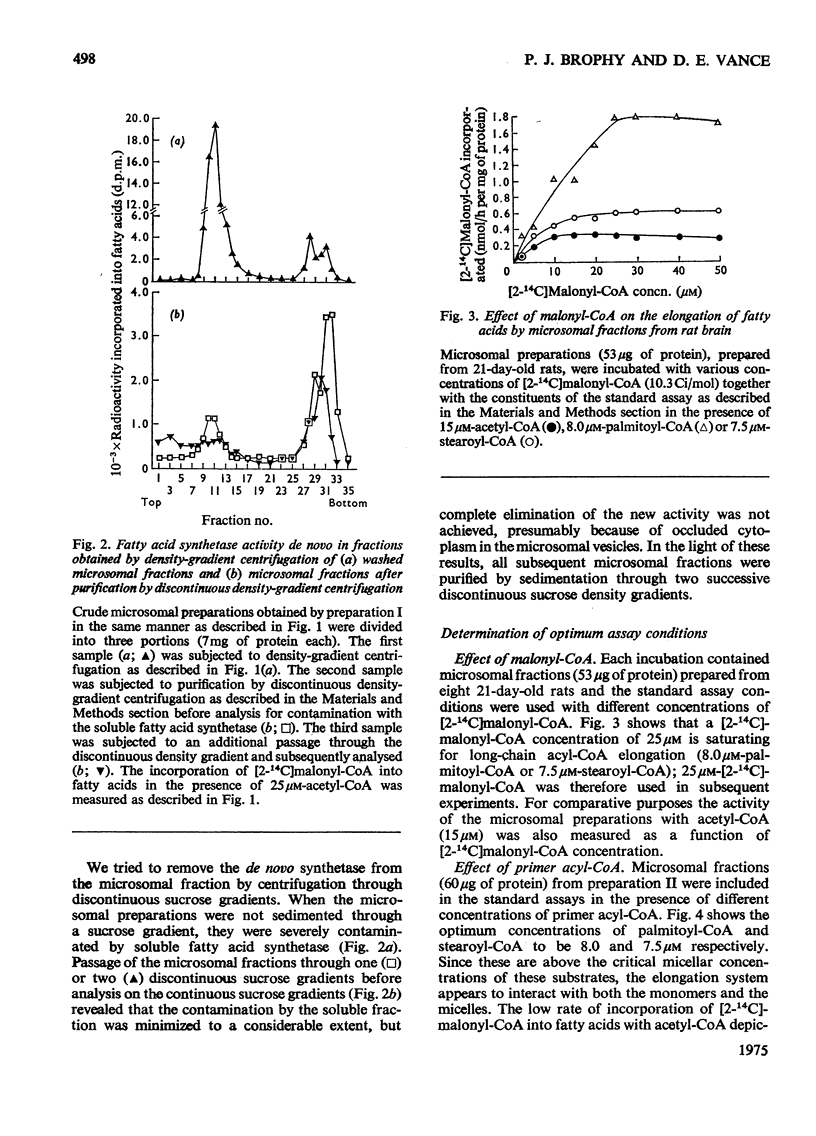
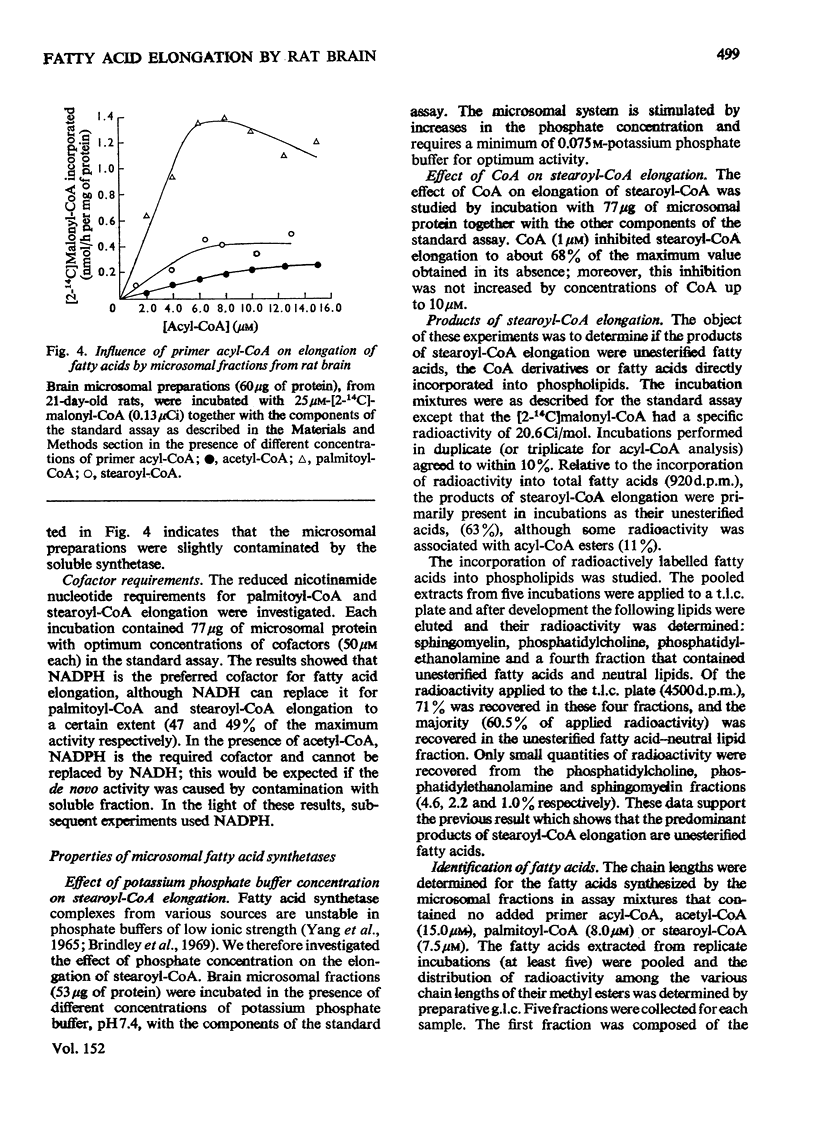
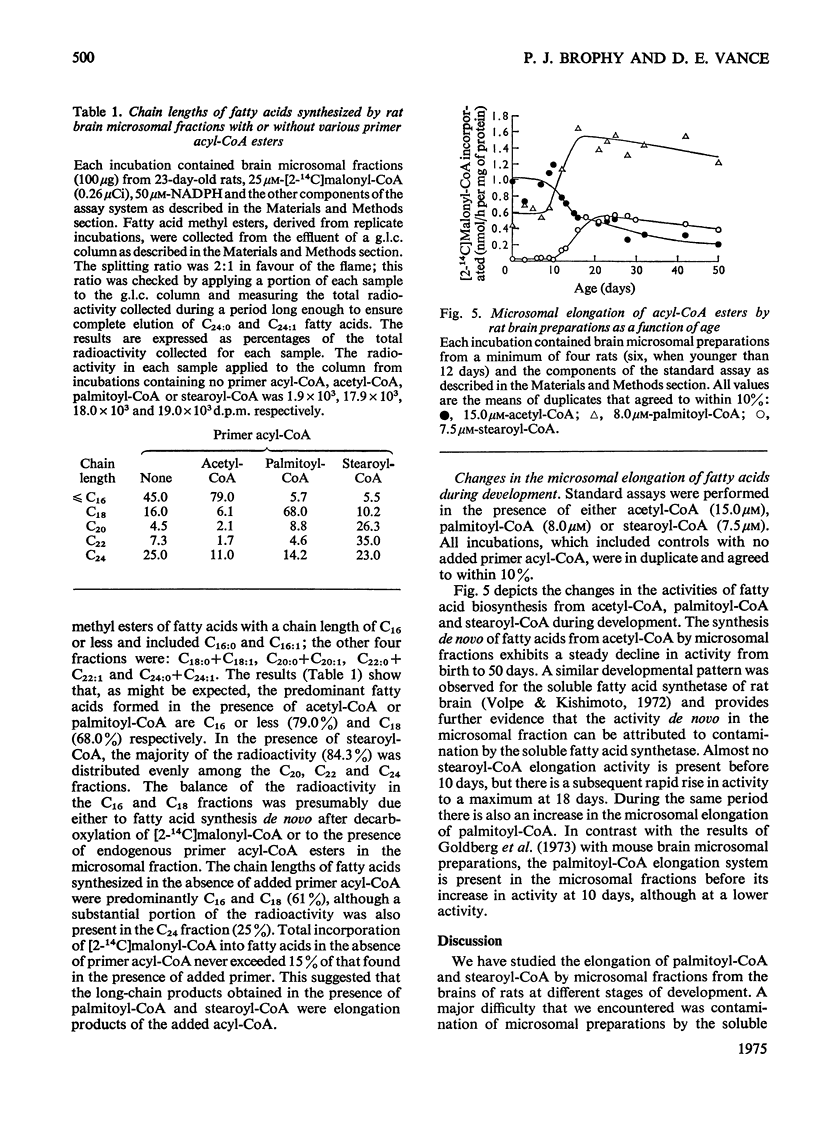
Elongation of fatty acids by microsomal fractions obtained from rat brain was measured by the incorporation of [2-14C]malonyl-CoA into fatty in the presence of palmitoyl-CoA or stearoyl-CoA. 2. Soluble and microsomal fractions were prepared from 21-day-old rats; density gradient centrifugation demonstrated that the stearoyl-CoA elongation system was localized in the microsomal fraction whereas fatty acid biosynthesis de novo from acetyl-CoA occurred in the soluble fraction. The residual activity de novo in the microsomal fraction was attributed to minor contamination by the soluble fraction. 3. The optimum concentration of [2-14C]malonyl-CoA for elongation of fatty acids was 25 mum for palmitoyl-CoA or stearoyl-CoA, and the corresponding optimum concentrations for the two primer acyl-CoA esters were 8.0 and 7.2 muM respectively. 4. Nadph was the preferred cofactor for fatty acid formation from palmitoyl-CoA or stearoyl-CoA, although NADH could partially replace it. 5. The stearoyl-CoA elongation system required a potassium phosphate buffer concentration of 0.075M for maximum activity; CoA (1 MUM) inhibited this elongation system by approx. 30%. 6. The fatty acids formed from malonyl-CoA and palmitoyl-CoA had a predominant chain length of C18 whereas stearoyl-CoA elongation resulted in an even distribution of fatty acids with chain lengths of C20, C22 and C24. 7. The products of stearoyl-CoA elongation were identified as primarily unesterified fatty acids. 8. The developmental pattern of fatty acid biosynthesis by rat brain microsomal preparations was studied and both the palmitoyl-CoA and stearoyl-CoA elongation systems showed large increases in activity between days 10 and 18 after birth.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aeberhard E., Grippo J., Menkes J. H. Fatty acid synthesis in the developing brain. Pediatr Res. 1969 Nov;3(6):590–596. doi: 10.1203/00006450-196911000-00009. [DOI] [PubMed] [Google Scholar]
- BEN GEREN B. The formation from the Schwann cell surface of myelin in the peripheral nerves of chick embryos. Exp Cell Res. 1954 Nov;7(2):558–562. doi: 10.1016/s0014-4827(54)80098-x. [DOI] [PubMed] [Google Scholar]
- Baumann N. A., Harpin M. L., Bourré J. M. Long chain fatty acid formation: key step in myelination studied in mutant mice. Nature. 1970 Aug 29;227(5261):960–961. doi: 10.1038/227960a0. [DOI] [PubMed] [Google Scholar]
- Bourre J. M., Pollet S., Chaix G., Daudu O., Baumann N. Etude "in vitro" des acides gras synthétisés dans les microsomes de cerveaux de souris normales et "quaking". Biochimie. 1973;55(11):1473–1479. [PubMed] [Google Scholar]
- Brophy P. J., Gower D. B. Studies on the inhibition by 5alpha-pregnane-3,20-dione of the biosynthesis of 16-androstenes and dehydroepiandrosterone in boar testis preparations. Biochim Biophys Acta. 1974 Sep 19;360(3):252–259. doi: 10.1016/0005-2760(74)90054-x. [DOI] [PubMed] [Google Scholar]
- Carey E. M., Cantrill R. C. The relationship between fatty acid activation and elongation in rabbit brain microsomes. J Neurochem. 1975 Apr;24(4):807–809. [PubMed] [Google Scholar]
- Carey E. M., Parkin L. Fatty acid metabolism in the microsomal fraction of developing rabbit brain. Biochim Biophys Acta. 1975 Feb 20;380(2):176–189. doi: 10.1016/0005-2760(75)90004-1. [DOI] [PubMed] [Google Scholar]
- Colli W., Hinkle P. C., Pullman M. E. Characterization of the fatty acid elongation system in soluble extracts and membrane preparations of rat liver mitochondria. J Biol Chem. 1969 Dec 10;244(23):6432–6443. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- GERSTL B., TAVASTSTJERNA M. G., HAYMAN R. B., SMITH J. K., ENG L. F. LIPID STUDIES OF WHITE MATTER AND THALAMUS OF HUMAN BRAINS. J Neurochem. 1963 Dec;10:889–902. doi: 10.1111/j.1471-4159.1963.tb11916.x. [DOI] [PubMed] [Google Scholar]
- Goldberg I., Shechter I., Bloch K. Fatty acyl-coenzyme A elongation in brain of normal and quaking mice. Science. 1973 Nov 2;182(4111):497–499. doi: 10.1126/science.182.4111.497. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- O'BRIEN J. S. STABILITY OF THE MYELIN MEMBRANE. Science. 1965 Mar 5;147(3662):1099–1107. doi: 10.1126/science.147.3662.1099. [DOI] [PubMed] [Google Scholar]
- PETERS A. FURTHER OBSERVATIONS ON THE STRUCTURE OF MYELIN SHEATHS IN THE CENTRAL NERVOUS SYSTEM. J Cell Biol. 1964 Feb;20:281–296. doi: 10.1083/jcb.20.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollet S., Bourre J. M., Daudu O., Baumann N. Biosynthèse des acides gras dans les microsomes de cerveau de souris. C R Acad Sci Hebd Seances Acad Sci D. 1971 Oct 18;273(16):1426–1429. [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance D. E., Esders T. W., Bloch K. On the role of a palmityl thioesterase in fatty acid elongation. J Biol Chem. 1973 Apr 10;248(7):2310–2316. [PubMed] [Google Scholar]
- Vance D. E., Sweeley C. C. Quantitative determination of the neutral glycosyl ceramides in human blood. J Lipid Res. 1967 Nov;8(6):621–630. [PubMed] [Google Scholar]
- Volpe J. J., Kishimoto Y. Fatty acid synthetase of brain: development, influence of nutritional and hormonal factors and comparison with liver enzyme. J Neurochem. 1972 Mar;19(3):737–753. doi: 10.1111/j.1471-4159.1972.tb01389.x. [DOI] [PubMed] [Google Scholar]


