Abstract
The antibiotic granaticin interferes in Bacillus subtilis with the charging process of tRNALeu causing both the arrest of protein synthesis and bacteriostasis [A. Ogilvie, K. Wiebauer & W. Kersten (1975) Biochem. J. 152, 511-515]. A concomitant inhibition of RNA synthesis is observed. This inhibition was studied with mutant strains of B. subtilis. 2. Granaticin inhibits protein and RNA synthesis in stringently controlled B. subtilis (rel+) to about the same extent. In a relaxed mutant strain (rel-) of B. subtilis, protein synthesis is also inhibited, but the accumulation of RNA continues after the addition of the drug. 3. Chloramphenicol, which is known to abolish the stringent control mechanism, added simultaneously with granaticin, allows the synthesis of RNA to proceed in the stringent strain. 4. Guanosine tetraphosphate (ppGpp) and guanosine pentaphosphate (pppGpp) accumulate in granaticin-treated stringently controlled B. subtilis but not in the rel- mutant. 5. It is concluded that the inhibition of RNA synthesis granaticin can adequately be explained as a stringent response caused by the interference by the drug with leucyl-tRNA synthetase.
Full text
PDF
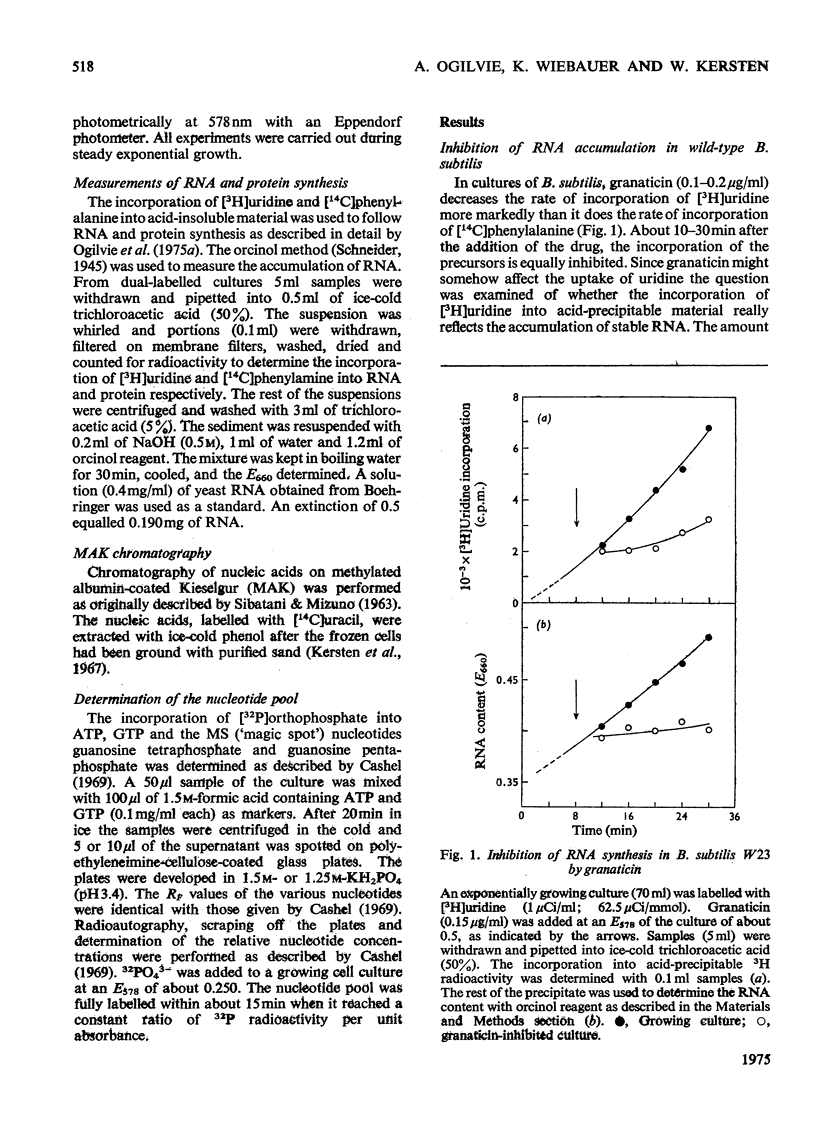
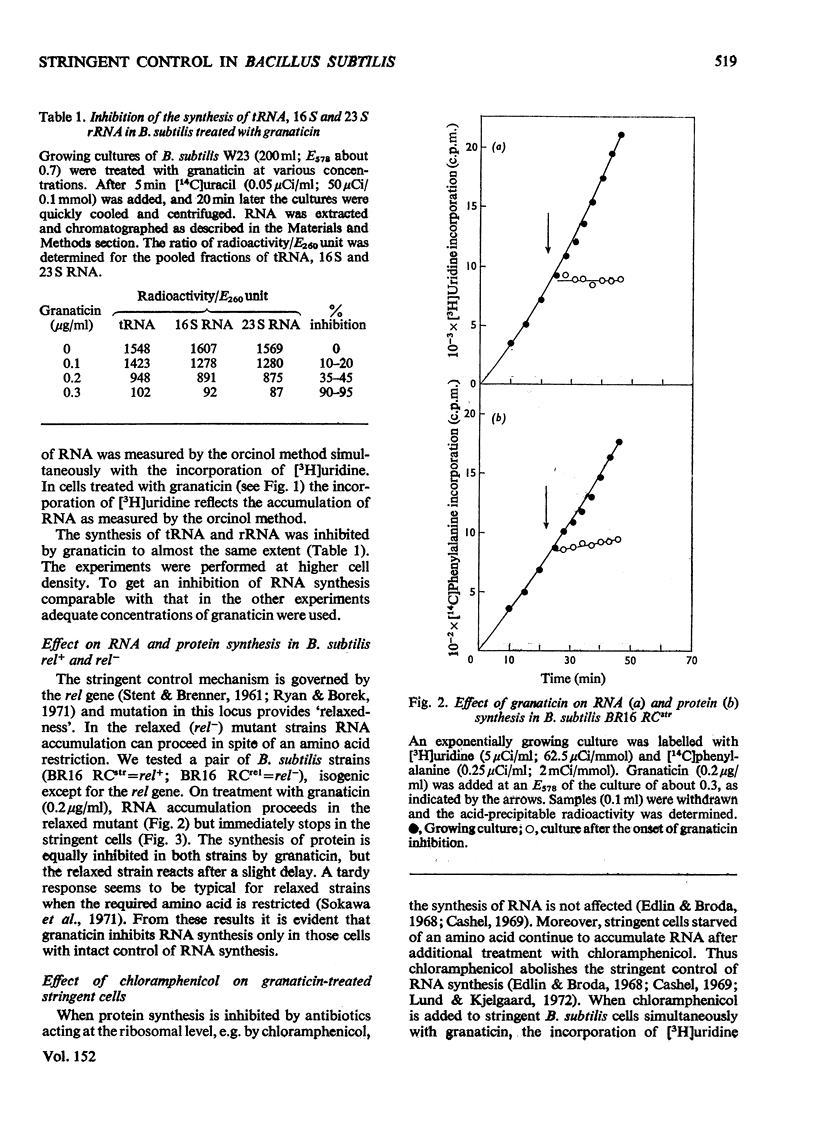
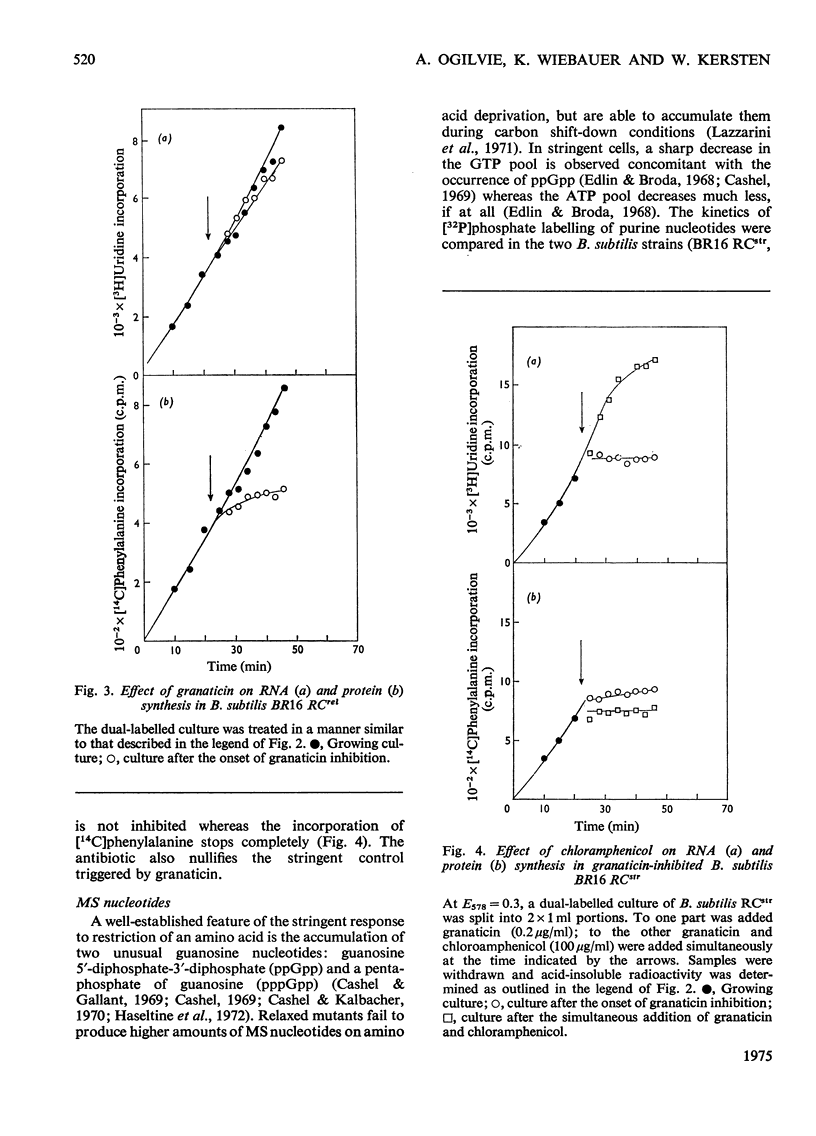
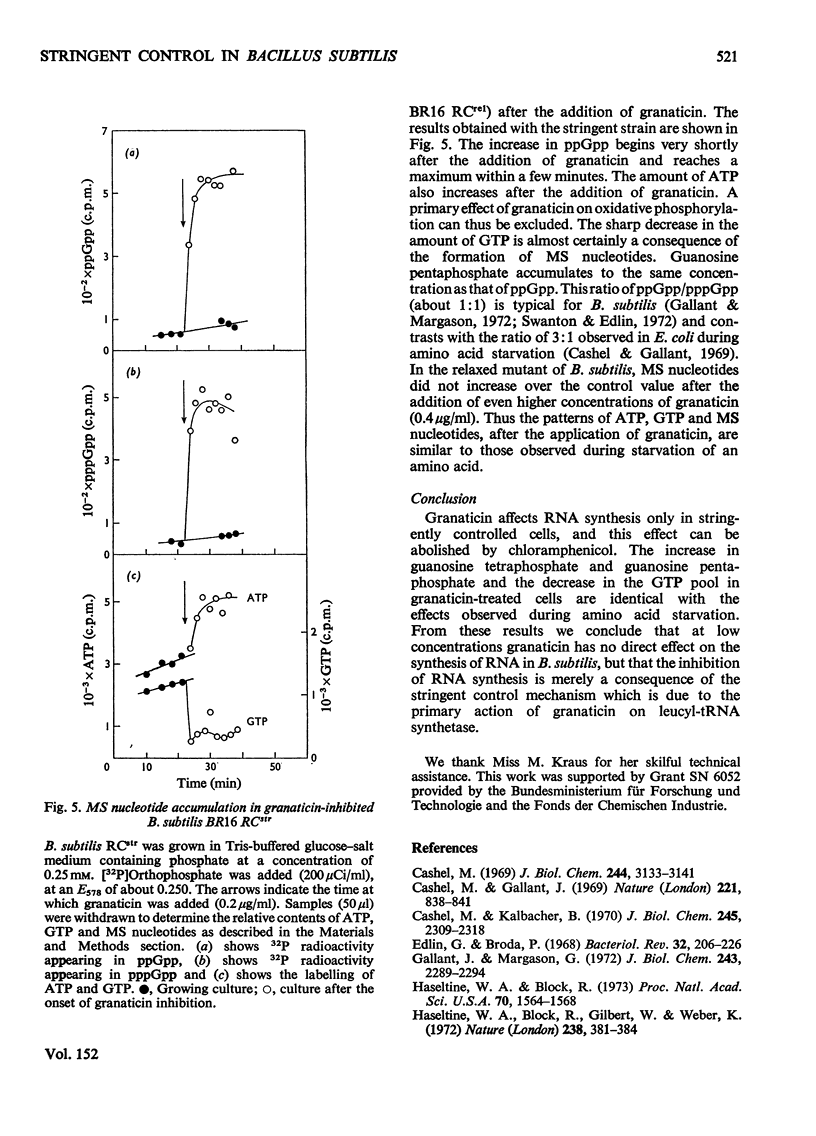

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cashel M., Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969 Mar 1;221(5183):838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- Cashel M., Kalbacher B. The control of ribonucleic acid synthesis in Escherichia coli. V. Characterization of a nucleotide associated with the stringent response. J Biol Chem. 1970 May 10;245(9):2309–2318. [PubMed] [Google Scholar]
- Cashel M. The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J Biol Chem. 1969 Jun 25;244(12):3133–3141. [PubMed] [Google Scholar]
- Edlin G., Broda P. Physiology and genetics of the "ribonucleic acid control" locus in escherichia coli. Bacteriol Rev. 1968 Sep;32(3):206–226. doi: 10.1128/br.32.3.206-226.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant J., Margason G. Amino acid control of messenger ribonucleic acid synthesis in Bacillus subtilis. J Biol Chem. 1972 Apr 25;247(8):2289–2294. [PubMed] [Google Scholar]
- Haseltine W. A., Block R., Gilbert W., Weber K. MSI and MSII made on ribosome in idling step of protein synthesis. Nature. 1972 Aug 18;238(5364):381–384. doi: 10.1038/238381a0. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Block R. Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged transfer ribonucleic acid in the acceptor site of ribosomes. Proc Natl Acad Sci U S A. 1973 May;70(5):1564–1568. doi: 10.1073/pnas.70.5.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten W., Kersten H., Steiner F. E., Emmerich B. The effect of chromomycin and methramycin on the synthesis of deoxyribonucleic acid and ribonucleic acids. Hoppe Seylers Z Physiol Chem. 1967 Nov;348(11):1415–1423. doi: 10.1515/bchm2.1967.348.1.1415. [DOI] [PubMed] [Google Scholar]
- Lazzarini R. A., Cashel M., Gallant J. On the regulation of guanosine tetraphosphate levels in stringent and relaxed strains of Escherichia coli. J Biol Chem. 1971 Jul 25;246(14):4381–4385. [PubMed] [Google Scholar]
- Lund E., Kjeldgaard N. O. Metabolism of guanosine tetraphosphate in Escherichia coli. Eur J Biochem. 1972 Jul 24;28(3):316–326. doi: 10.1111/j.1432-1033.1972.tb01916.x. [DOI] [PubMed] [Google Scholar]
- Ogilvie A., Lämmerman M., Wiebauer K., Kersten W. Quinone induced stringent control. Accumulation of ppGpp and inhibition of RNA synthesis in stringent Escherichia coli by 5,8-dioxo-6-amino-7-chloroquinoline. Biochim Biophys Acta. 1975 Jun 16;395(2):136–145. [PubMed] [Google Scholar]
- Ogilvie A., Wiebauer K., Kersten W. Inhibition of leucyl-transfer ribonucleic acid synthetasymol. Biochem J. 1975 Dec;152(3):511–515. doi: 10.1042/bj1520511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen F. S., Lund E., Kjeldgaard N. O. Codon specific, tRNA dependent in vitro synthesis of ppGpp and pppGpp. Nat New Biol. 1973 May 2;243(122):13–15. [PubMed] [Google Scholar]
- Ryan A. M., Borek E. The relaxed control phenomenon. Prog Nucleic Acid Res Mol Biol. 1971;11:193–228. doi: 10.1016/s0079-6603(08)60328-1. [DOI] [PubMed] [Google Scholar]
- SIBATANI A., MIZUNO N. SYNTHESIS OF RIBONUCLEIC ACIDS IN ESCHERICHIA COLI IRRADIATED WITH ULTRAVIOLET LIGHT. Biochim Biophys Acta. 1963 Oct 15;76:188–200. [PubMed] [Google Scholar]
- STENT G. S., BRENNER S. A genetic locus for the regulation of ribonucleic acid synthesis. Proc Natl Acad Sci U S A. 1961 Dec 15;47:2005–2014. doi: 10.1073/pnas.47.12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokawa Y., Sokawa J., Kaziro Y. Function of the rel gene in Escherichia coli. Nat New Biol. 1971 Nov 3;234(44):7–10. doi: 10.1038/newbio234007a0. [DOI] [PubMed] [Google Scholar]
- Swanton M., Edlin G. Isolation and characterization of an RNA relaxed mutant of B. subtilis. Biochem Biophys Res Commun. 1972 Jan 31;46(2):583–588. doi: 10.1016/s0006-291x(72)80179-7. [DOI] [PubMed] [Google Scholar]


