Abstract
The medicinal value of herbal products is often rooted in their “traditional” use, recontextualized by modern biomedical research granting them certain medical uses. Tagetes erecta L. (Asteraceae), native to Mexico, exemplifies such historical developments of a species that played a key role in developing a major pharmacologically active compound – lutein.
T. erecta (Cempasúchil in Nahuatl) has held ritual and medicinal importance in Mesoamerica and was associated with the rain god Tláloc. The species’ historical use spans ancient texts with varied medicinal applications, including treating cold-related ailments and promoting menstruation and urination.
However, the Spanish conquest redefined it culturally, medicinally, and religiously, mainly as an ornamental flower. The discovery of lutein in T. erecta marked a significant shift, emphasizing its role in macular health and preventing aging-related macular degeneration. Clinically, lutein trials reveal cognitive, visual, cardiovascular, and systemic health enhancements, substantiating its potential therapeutic benefits. Pharmacologically, it demonstrates significant anti-inflammatory, antiparasitic, and anticancer properties. Today, T. erecta is recognized globally for its rich carotenoid content. This multifunctional metabolite is also used in poultry feed and health supplements.
In contemporary culture, cempasúchil, also known as the “flower of the dead,” has been adapted for ornamental, medicinal, ceremonial, and industrial uses. However, its traditional medicinal uses in pre-Conquest Mexico remain largely unexplored, with its current applications influenced by global research.
T. erecta's evolution beyond traditional medical and ritual uses in Mesoamerica demonstrates the dynamic development of a medicinal plant's role in medicine, as well as a range of other spheres of daily life.
Keywords: Tagetes erecta L., Cempasúchil, Lutein, Traditional medicine, Medicinal plants, Macular degeneration
Graphical abstract
1. Introduction
Globally, it is estimated that of the approximately 350,000 species of vascular plants known to science, around 7 %, or approximately 26,000 species, have documented medicinal uses.1 An essential characteristic is the scientific assessment of traditional knowledge and its applications.2 Bioactive metabolites are central to the pharmacological effects, safety/toxicity, and efficacy of a medicinal plant, and the cultural and historical context influences the perception of a plant as a potential medicinal product.3 The evaluation of the therapeutic effects of plants in clinical studies is a rigorous process, and the understanding, preservation, and respect of ancestral knowledge in the era of biomedicine are an essential element of modern ethnopharmacological research.4 In integrating medicinal plants into scientific discourse, historical and ethnobotanical records are examined along with the investigation of plant metabolites, helping to develop a pharmacological profile and understand their therapeutic potential. However, there is sometimes a notable disparity, revealing a divergence between traditional and modern medicinal discourse. This article aims to explore medicinal uses from different categories of knowledge, as well as the cultural and historical context, allowing for a nuanced understanding of their medicinal value, which may or may not be determinant in the role a plant plays today.
The concept of “traditional medicine” has become a fundamental element in research and (especially medical) practice. However, what makes a medicine “traditional”? Juette et al.5,6 discuss the ambiguities of the term “traditional” and its relevance in a phytotherapeutic context (focusing on European medicines). The term “traditional medicine” came into broader use starting in the 1960s, with many of the earliest papers (Mann 1965)7 and (Croizier, 1968),8 focusing on the developments in China during the Cultural Revolution and the push for a national medical practice to support communist developments labelled as Traditional Chinese Medicine (TCM) and underwent a considerable change when it became a practice in Chinese diaspora communities and more widely in economically developed countries.9 Other studies focused on such practices, for example, in Africa (Gelfand 1964).10 Within the last 60 years, the term has been used widely, albeit often without a clear definition or an analysis of its meaning. It also links this medical practice or concept to a specific culture without recognizing cultural exchanges (or appropriation), syncretic developments, or exclusion of practices seen as being “non-native”.
The analysis presented here uses Tagetes erecta L. (Asteraceae, tribe Tagetae, TE),11 an endemic Mexican species. Its multiple uses, including as medicines, originated in Mesoamerican cultures.12 It provides a comprehensive understanding of the evolution from a reverent plant in ancient times into a globally used herbal medicine, ritual plant, ornamental, and feed product.
Mexico is considered a hotspot megadiverse country, also specifically for the Asteraceae. Similarly, nations like Brazil, Peru, the Philippines, Indonesia, Vietnam, Madagascar, and China (South-central regions), among others,13 set a compelling stage for studying medicinal plants. This biodiversity is a testament to the rich ecological tapestry and reflects the deep-rooted cultural integration of botanical resources in everyday life. Notably, the use of medicinal plants illustrates how a diverse flora contributes to a nation's cultural and health practices.14 Traditional uses have evolved, adapting to contemporary needs and knowledge in specific countries.
Before the arrival of the Spaniards, Mesoamerica was a web of flourishing and competing societies that developed a city-state comparable to those of Rome, Egypt, or China – México-Tenochtitlan. This civilization featured a political structure and remarkable inventions such as the wheel, the concept of zero (0), and ideographic writing. It had distinct social stratification. A structured religion with rituals and sacrifices existed alongside a magic/religious medicine that necessitated using plants and ritual procedures to treat diseases. The medicine of that era was deeply intertwined with rituals and religion, attributing the origin of diseases to divine punishments or induced evils. It is documented that plants, including TE, were believed to incarnate the physical presence of some gods, indicating the potential of such plants to alleviate conditions attributed to divine punishment during that period.
The Spanish Conquest marked a significant turning point in the history of medicinal plants such as TE. This era was marked by extensive cultural and religious exchanges, resulting in a syncretism of beliefs and practices (mestizaje). The introduction of Christianity in the “New World” often conflicted with indigenous rituals, especially those involving plants like cempasúchil. This research delves into how, amidst these cultural clashes, TE as a medicinal and ritual plant became a symbol of resistance and adaptation, embodying the complex process of botanical and cultural transformation.
TE acquired a new identity in Europe, transforming it into a symbol of beauty and ornamental value. It was even described as toxic and poisonous by some authors, in contrast to other American plants that gained widespread acceptance as medicinal plants, such as Palo Santo (Guaiacum sanctum L.), Sarsaparilla (Smilax aristolochiifolia Mill.), and Cinchona (Cinchona succirubra Vahl and others.), known for their effectiveness in treating conditions prevalent among the European population at the time.
TE and other species like Zea mays L, Solanum lycopersicum L. (syn: Lycopersicum esculentum Mill. or Persea americana Mill.), to name only three, with their origins in Mexico, have a history shaped by the conquest. Their journey, characterized by variations in usage, naming, and perception across cultures, underscores the role of language, trade routes, and classifications in shaping their global narratives. This analysis situates TE within a broader historical and botanical context, raising fundamental questions about traditional and complementary medicine. Most notably, it addresses the question of what constitutes traditional medical use.
In the contemporary era, TE has transcended its traditional uses, assuming new roles in ritualistic practices in various countries and becoming an essential resource in industries like poultry and dietary supplements. Its transition from a widely used medicinal plant to a globally recognized “flower of the dead” epitomizes the dynamic evolution of botanical history and cultural adaptation.
2. Methodology
This paper is based on data gathered from different sources, examining historical text and related sources (Table A1). This analysis aims to trace TE's medical historical usage and cultural significance, exploring its evolution. In the medical-bioscientific context, a systematic literature review was conducted across Web of Science, PubMed, and Google Scholar, as well as hand searchers, especially of historical documents, focusing on the ethnobotanical, phytochemical, pharmacological in vivo, in vitro data, and clinical trials of TE. The inclusion and exclusion criteria (Table A2) include specific filters and Boolean operators (Table A3). The management of references and the identification of duplicates were facilitated by ENDNOTE X21 software, while Office 365 was used to organize the data into coherent tables.
This methodology enables a nuanced exploration of TE, bridging historical perspectives with modern scientific findings and offering a broader view of the plant, uncovering the contextual foundations that have shaped its tradition and cultural, medicinal, and scientific relevance. Ethical considerations focused on the responsible use of published data throughout our investigation.
3. Results and discussion
3.1. Tagetes erecta L. In the modern medical context
3.1.1. Phytochemistry/pharmacology and clinical trials
Chemically, TE is rich in flavonoids, phenolic metabolites, terpenoids, hydrocarbons, and carotenoids like lutein, among others (Table 1), which play a crucial role in the plant's protective adaptations and can serve as an argument to justify medicinal applications. The concentration of these bioactive metabolites positions TE as an important topic in pharmacological studies, highlighting TE as a rich source of lutein15 (for more details about the discovery and historical development of lutein, see Section A2.), justifying its current use in treating various diseases and emphasizing the chemical basis for its pharmacological effects and therapeutic efficacy of its chemical extracts. Flavonoids and phenolics, like syringic and gallic acids, offer health protection through more non-specific antioxidant and anti-inflammatory effects. Terpenoids and hydrocarbons contribute to the plant's medical significance and protective adaptations, while metabolites like thiophenes and quinolines offer antimicrobial benefits.
Table 1.
Phytochemistry in Tagetes erecta L.
| Functional Group | Metabolites |
|---|---|
| Hydrocarbons16 | Alkanes |
| N-hexadecane | |
| Ketones and Esters16 | Ketones |
| 7-megastigmadien-9-one | |
| Esters | |
| Palmitin, Ethylene glycol linoleate | |
| Aromatic Compounds16,17 | Thiphenes |
| D-terthienyl, terthieny1, 5-butyl-2,2-bithienyl, 5,5-dimethyl-2,2-bithienyl, 5-vinyl-2,2-bithiophene, 1-[5-(1-propyn-1-yl)-[2,2-bithiophen]-5-yl]- ethanone | |
| Quinolines | |
| 4-dimethylquinoline | |
| Phenolic Compounds16,18 | Syringic acid, Gallic acid, 3-d-galactosyl disyringic acid, 3-E-galactosyl disyringic acid, Syringic acid-hexoside I, Syringic acid-hexoside II, Syringic acid-(dihydroxydimethoxybenzoic acid)-hexoside I, Syringic acid-(dihydroxydimethoxybenzoic acid)-hexoside II, Syringic acid-(dihydroxydimethoxybenzoic acid)-hexoside III, Syringic acid-(hydroxytrimethoxybenzoic acid)-hexoside, Syringic, Di-syringic acid hexoside, Di-syringic acid hexoside II, 3,4, 3,4-dihydroxy-5-methoxy-benzoicacid |
| Gallic Acid Derivatives | |
| Galloyl hexoside, Methyl-gallic acid, Digalloyl-hexoside I, Digalloyl-hexoside II, Digalloyl-dihexoside, Digalloyl-hexoside III, Trigalloyl-dihexoside, Trigalloyl-hexoside I, Trigalloyl-hexoside II, Galloyl-eudesmic acid-hexoside I, Galloyl-eudesmic acid-hexoside II | |
| Hydroxycinnamic Acids | |
| Chlorogenic acid (5-CQA), 3,5 or 4,5 di-CQA | |
| Ellagic Acid Derivatives | |
| Ellagic acid-hexoside I, Ellagic acid-hexoside II, Ellagic acid | |
| Terpenoids16,17 |
Steroids E-sitosterol, E-daucosterol, 7E-hydroxysitostero, B-sitosterol, stigmasterol Triterpenoids Lupeol, Erythrodiol, Erythrodiol-3-palmitate, Oplodiol Carotenoid Lutein, Lutein dipalmitate diesters, Lutein myristate palmitate, Lutein lauristate palmitate diesters, Lutein dimyristate, Lutein palmitate stearistate, Lutein Violaxanthin, Lutein neoxanthin violaxanthin, B-carotene, Zeaxanthin |
| Flavonoids16,18 | Quercetagetin, Quercetagetin-7-methyl ether, Quercetagetin-7-O-glucoside, Quercetagetin−7-O-(Ac-Pen-Hex)-3-O-hexoside I, Quercetagetin-3-O-hexoside, Quercetagitrin (Quercetagetin-7-O-β-glucopyranoside), Quercetagetin-7-O-dihexoside, Quercetagetin-7-O-(Ac-Pen-Hex)-3-O-hexoside II, Quercetagetin-7-O-(galloyl-hexoside), Quercetagetin-3-O-hexoside-7-O-(galloyl- hexoside), Quercetin 3-O-hexoside, Kaempferol 3-O-hexoside I, Kaempferol 3-O-hexoside II, Isorhamnetin 3-O-hexoside, 8-Hydroxyquercetagetin, Quercetin, Patulitrin, Kaempferol, Patuletin, Quercetagetin-5-methyl ether, Quercetagetin-5,7-dimethl ether, Quercetagetin 3-O-glucoside, Kaempferitrin |
| Amino Acid Derivatives18 | Phenylalanine, N-Malonylphenylalanine |
| Other Metabolites17,18 | Quinic acid, Quinic acid hexoside, Dihexoside, Shikimic acid hexoside, Theogallin, Tryptophan, Sinapoyl alcohol, 2Hβ,3-dihydro-euparin-14-O-β-D-glucosid, 6-ethoxy-2,4-dimethylquinoline, Uracil, 9Z,12Z,15Z-octadectrien-1-ol, n-hexadecane, n-tetratriacontane,3—galactosyl disyringic acid,3—galactosyl disyringic acid, Vitamin E, (3S,6R,7E)-hydroxy-4,7-megastigmadien-9-one, ethylene glycol linoleate |
Pharmacological research across various biological models reports multiple effects (Table 2, Tables A10–A11), with variations in methodologies, such as using different solvent extracts and administration dosages, highlighting the diversity of active metabolites in TE and their range of biological effects. An in vitro study using TE extracts with different solvents such as ethanol, methanol, and water demonstrated that ethanolic extracts had more significant antioxidant activity than aqueous ones, highlighting the importance of the solvent in biological activity.19 In an in vitro study conducted in Mexico, adult nematodes of the species Caenorhabditis elegans were exposed to different concentrations of aqueous extracts of TE and others. The results showed that both extracts blocked nematode motility and decreased egg-laying, indicating anthelmintic potential.20 Other studies report its larvicidal effectiveness,21 antioxidant and antimicrobial activity,22 cytotoxic activity against cancer cell lines,23 antimicrobial activity,22,24 and spasmolytic activity.25
Table 2.
Medicinal uses attributed to TE according to different categories of knowledge.
| Source | Medicinal uses |
|---|---|
| Ancient books |
Francisco Hernández, 183826 Treats cold diseases, relieves flatulence, aphrodisiac, treats cold stomach, induces urination and menstruation, induces sweating, treats intermittent fevers, alleviates cold-related conditions, cures cachexia, relieves liver ailments, relieves contracted limbs, cures hydropsy, induces vomiting in warm water. |
|
Brunfels [& Weiditz], 153027 Alleviates bladder pain and stranguria (taken with wine), reduces fever (administered in warm water), treats thigh pain (toasted with egg whites and vinegar, applied as a poultice), acts as a fumigant for infants, relieves nerve pain and reduces swelling in the feet (ground and mixed with oil), effective against foot pain (consumed as a potion), reduces body heat (externally applied, presumably the seeds), kills worms and helps in their elimination (seeds are drunk or eaten), promotes urination and helps expel bladder stones. | |
|
Gerard, 159728 It possesses poisonous and cooling qualities, it is concluded that these plants are most venomous and filled with poison, and thus should not be touched or smelled, much less used in food or medicine. | |
| Ethnobotany (Table A9) |
Neurological disorders - Stroke, cerebrovascular diseases. Respiratory and related ailments - Tuberculosis, cough, cold. Pain management - Ear pain, rheumatic pain, tooth pain, body pain, headache. Dermatological conditions - Blotch, skin disorders (including eczema, acne, scabies, dermatitis, abscess, boils), itchiness, rash, sores, warts, pimples, cuts. Gastrointestinal disorders - Blood dysentery, stomachache, diarrhea. Infectious diseases |
| Pharmacology in vivo (Table A10) |
Neurological and behavioral effects - Exhibits anxiolytic, sedative-like, and antidepressant effects. Anti-inflammatory and immune modulation - Reduces inflammatory responses in colitis and gastric conditions and modulates immune and inflammatory processes in cancer models. Renal function effects - Enhances urine output and electrolyte balance, impacting renal function and systemic fluid homeostasis. Cancer prevention - Offers protective mechanisms against cancer development through antioxidative, immunomodulatory, and anti-inflammatory effects. Infectious disease control - Demonstrates efficacy against parasitic infections such as malaria. Dermatological protection - Protects against UV-induced skin damage and photoaging. |
| Pharmacology in vitro (Table A11) |
Antiparasitic and antimicrobial effects Antioxidant and anti-inflammatory properties Anticancer and cytotoxic activities Spasmolytic effect Anti-plasmodial and schizonticidal activities |
| Clinical trials (lutein) (Table A12) |
Cognitive and neurological benefits - Improved working memory and cognitive functions. - Enhanced eye-hand coordination and smooth-pursuit eye movements. - Supplementation improved certain aspects of brain network connectivity. Visual health improvements - Increased macular pigment optical density, contrast sensitivity, and glare sensitivity. - Improved ocular blood flow and visual functions. Cardiovascular and metabolic health - Improved body composition and lipid profiles, including significant decreases in visceral fat, serum levels of total cholesterol, and LDL-cholesterol. Nutritional and systemic health - Increased concentrations of key nutrients like DHA and lutein in maternal blood and milk, indicating better nutritional status. - Significant increases in plasma and erythrocyte concentrations of lutein, demonstrating enhanced nutrient absorption and systemic distribution. Inflammatory and immune response - Supplementation led to improved inflammatory profiles, evidenced by increased levels of BDNF and decreased levels of pro-inflammatory cytokines, supporting cognitive and overall health improvements. |
These in vitro findings provide a basis for further exploration in in vivo models. For example, studies on albino mice suggest that a mixture of plant extracts offers protective effects against UV-induced photoaging,29 natriuretic and calcium-sparing effects,30 reduction in the severity of colitis and attenuation of inflammatory cytokine secretion, and improvement of endogenous antioxidant defense,31 suppression of parasitemia in malaria-infected mice,32 and protective effects against gastric cancer in Wistar rats by modulating oxidative stress, immune response, inflammation, and apoptosis,33 in addition to anxiolytic, sedative,34 and antidepressant effects.35
Methodological strengths include using control groups and dose-response studies, while limitations are related to small sample sizes and variability in or lack of detail about extract preparation (Tables A10–A11). These findings suggest its safety and therapeutic potential, although caution is advised for long-term use due to potential adverse effects, such as intestinal contractions, underscoring the need for careful consideration in future clinical trials of therapeutic applications.
Despite extensive pharmacological research, the lack of human studies on TE aside from lutein supplementation remains a significant gap. Lutein, often derived from TE but also found in many other species, has shown health benefits in various clinical trials (Table 2). There is a wide range of research indicating that lutein supplementation improves working memory performance, body composition, and lipid profile in cognitively healthy older adults and middle-aged obese individuals, reducing visceral fat and LDL cholesterol levels.36 In healthy women, lutein complex supplementation increased biomarkers of ocular blood flow and reduced diastolic blood pressure.37 In patients with high myopia and healthy adults, lutein significantly increased the optical density of macular pigment, suggesting benefits for eye health, such as improved contrast sensitivity, reduced visual fatigue,38 and increased nutrient levels in blood and breast milk.39
Moreover, the potential risks of self-medication with lutein supplements underscore the urgent need for further studies focusing on how it is used today as a supplement. Consumers might be led to believe that these supplements are panaceas for vision issues without understanding the potential risks or the lack of concrete evidence supporting these claims. By emphasizing the importance of transitioning from the laboratory to the patient's bedside, we can advocate for more transparent and evidence-based practices in the market and ensure that patients receive safe and effective treatments.
3.1.2. Ethnobotany – Modern uses
To fully understand the medicinal value of TE, it is crucial not only to consider its chemical profile and its pharmacological and clinical applications but also to examine how different cultures have utilized this plant throughout history; over the last 500 years, TE has spread globally, especially in temperate and warmer areas as documented in ethnobotanical studies (Table A9). Its cultivation is seasonal and prevalent in Asia, particularly India and Nepal, which contrasts with its limited ethnobotanical research in Mexico, the endemic region, where only three out of eighteen studies were conducted.
Rather, TE is primarily recognized for its ritual significance over its medicinal uses. In Mexico, these uses are part of local harvest festivals,40 and on the Día de los Muertos (Day of the Dead), symbolizing a connection between the living and deceased, aligned with its Mesoamerican heritage. Internationally, TE finds its place in diverse cultural festivities. It adorns celebrations during Diwali, Onam, and Ganesh Chaturthi in India, symbolizing prosperity and auspiciousness. In Nepal, during the Tihar Festival, and in China, for the Qingming Festival, TE is used in practices honouring the deceased and the divine. It is widely used in Thailand both as an ornament in temples and for diverse celebrations (M. Heinrich, pers. obser.).
TE has been reported to help manage a wide range of health problems (Table 2, Table A9), For example, in Mauritius, ethnobotanical studies were conducted using face-to-face interviews with semi-structured questionnaires. Here, TE is used to prepare an infusion of the flower in water, consumed once a day for a month to treat cerebrovascular diseases and strokes.41 In Nepal, information was collected in the Ramechhap District through semi-structured interviews with local inhabitants, including traditional healers. The plant treats fever, rheumatic pain, and skin problems using the juice extracted from the plant.42 In India, in the districts of Shimoga and Kathua, ethnobotanical research was conducted through semi-structured interviews and casual conversations. The use of TE leaves was mentioned for treating wounds in livestock, ear pain, dysentery, and tuberculosis. The preparation includes applying a paste of leaves directly to the wounds or ingesting leaf extract.43,44 In Mexico, in the Purépecha plateau, semi-structured questionnaires were used to gather data from key informants. Here, the plant is used for dermatological conditions such as erysipelas, wounds, and rashes by applying an infusion of flowers and leaves directly to the skin.45
TE is not just as a medicinal plant but also as a cultural symbol. Recognizing and preserving ethnobotanical knowledge about TE could offer novel perspectives on sustainable practices and lead to the discovery of new medical uses, highlighting the potential for intercultural exchange of plant knowledge. Although beneficial, this also requires caution; as TE moves from local to global contexts, decontextualization is a significant development. This aspect is crucial to consider, especially to ensure that such exchanges do not dilute the original context but rather enhance the understanding and appreciation of “traditional” medicine globally.
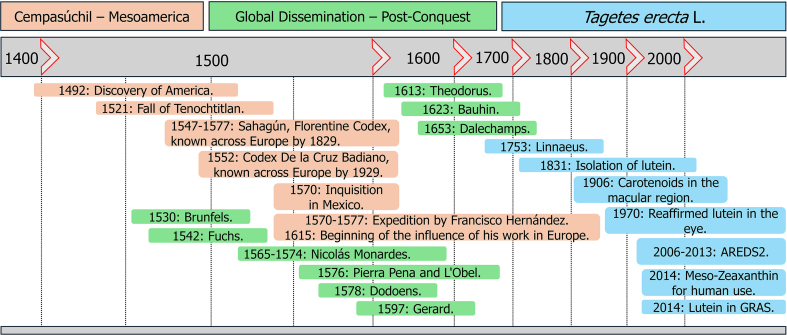
3.2. Cempasúchil in Mesoamerica (Fig. 1)
Fig. 1.
Timeline of TE Key historical, medical, and botanical events. This timeline delineates the significant historical, botanical, and medical milestones in the evolution of TE, from its origins as the pre-Columbian cempasúchil to its contemporary scientific identification.
3.2.1. Mesoamerican medicine
Now we turn to Mesoamerica, a region encompassing present-day Mexico and parts of Central America, dominated by advanced civilizations such as the Aztecs and Mayans. In this area, human conglomeration and rich biodiversity allowed the development of a sophisticated medical practice. This practice included using various medicinal plants, including TE, which played a principal role in complex rituals and as part of knowledge passed down through generations. However, the absence of written records from pre-conquest times challenges understanding the full scope of ancient Mesoamerican medical traditions. However, an analysis of post-conquest documents and ethnographic studies reveals a system where the divine played a pivotal role in the conceptualization of illness. For instance, plants such as Quahyyauhtli and Iztauhyatl, identified as Tagetes lucida Cav., Artemisia ludoviciana subsp. mexicana (Willd. ex Spreng.) D.D.Keck, respectively, are associated with diseases believed to be caused by the god Tláloc and other deities within the rain-agriculture-fertility complex and were used as remedies to combat these ailments. Similarly, TE also had a significant ritual meaning and was used in medicinal and ceremonial practices related to water phenomena.
Another significant element in Mesoamerican culture is the naming of plants in Nahuatl, the predominant language. This naming convention carries ritual, medical, and cultural repercussions that substantiate the modern uses attributed to a plant. Cempasúchil is the most common Nahuatl term attributed to TE, which possesses a variety of names in different indigenous languages (Table A4), demonstrating great cultural diversity. The word comprises two terms: CEMPOHUAL-LI, meaning twenty, and XOCHI-TL, meaning flower.46 The number twenty holds profound ritual significance in Maya culture in the calendar (based on a month with twenty days); the number also reflected a cosmological and ritual view, revered as the moon's cycle encompassing a visible period, a symbol of completion, wholeness, and the fullness of time, signifying the completion of life (death).47 Currently, in Mexico, cempasúchil is central to the Day of the Dead, reflecting the continuity of cultural heritage.
3.2.2. Codex De la Cruz Badiano
The Codex Cruz-Badiano represents a text written or dictated in Nahuatl and translated into Latin, describing Mesoamerican medical tradition based on the perspectives of an Aztec healer working within a Catholic convent. European medicine demonstrates the use of medicinal plants among indigenous peoples from the author's perspective. It remained largely unnoticed during the European colonial period and early independence until its rediscovery in 1929. This Codex is the first American written reference to mention what we now know as the genus Tagetes. Throughout history, various authors have referred to members of this genus as either TE, T. mexicana Ortega or T. lucida Cav., using multiple Nahuatl names and with different and sometimes contrasting identifications (Table A5).
The debate regarding the presence of TE in the Codex Cruz-Badiano is complex, stemming from the absence of explicit textual references to this species. Folios 30v and 54v48 are of particular interest for their potential identification with TE based on graphic and clinical contexts. However, the task of identification is challenged due to linguistic ambiguities, insufficiently detailed illustrations, and the phenotypic variability of the plants.48
The Codex's context is unmistakably religious, possibly influenced by Fray Jacobo de Grado, who commissioned the translation into Latin and likely oversaw the compilation49 suggesting his involvement might have influenced the selection of content to emphasize the religious and evangelical aspects and its structure. Given its composition at the Colegio de Tlatelolco, a religiously embedded institution focusing on educating indigenous elites in Christian doctrine and European arts and sciences,49 presumably, these influences shaped its contents.
The absence of a taxon like TE with significant ritual, cultural, and social importance in Mesoamerica and contemporary Mexico from the Codex and another relevant reference of the era calls for a revaluation of its role in “traditional” Mexican medicine and its applications in pre- and post-Hispanic rituals and medicine.
3.2.3. Florentine Codex
The Florentine Codex, “Historia general de las cosas de la Nueva España” by the Franciscan monks Bernardino De Sahagún (1499–1590), offers a detailed exploration of medicinal plants in Book XI (Natural history), Chapter VII (Concerning other herbs) and paragraph VII (Medicinal herbs). Notably, it lacks any mention of TE. However, in the same chapter, Paragraph VIII (Of wild flowers and herbs) describes various types of flower heads, including cempasúchil, accompanied by an illustration (Fig. 2). It displays a “group” of plants with similar characteristics, emphasizing the existence of two varieties: ‘female and male cempasúchil.50 This description does not attribute any particular medicinal or ritual use to the plant. Instead, it categorizes it as an ornamental flower (Table A6).
Fig. 2.
Historical illustrations and references of TE and related species. In 1542, Leonhart Fuchs's De historia stirpium commentarii insignes introduced the term "Tagetes" as Tagetes Indica, representing most likely Tagetes lucida Cav.51 Subsequently, in 1563, Pietro Mattioli's Comentarii denuo aucti in libros sex Pedacii Dioscoridis Anazarbei de materia medica52 mentioned the "Caryophyllus Indicus Major" Nicolas Monardes, as a reference, has not mentioned TE and Bernardino de Sahagún’s Codex Florentinus (1570) had no influence in Europe until 1829. In his 1597 The herball or General historie of plantes, John Gerard28 classified TE as the French or African Marigold. The pivotal moment came with Francisco Hernández's acknowledgment of the plant's Mexican origins in 1651 through his work, Rerum medicarum Novae Hispaniae thesaurus. Carl Linnaeus's Species plantarum (1753) definitively included the plant with its formal binomial nomenclature, Tagetes erecta L.
Sahagún approach to documenting medicinal knowledge diverges notably from the rest of his work. The sections dedicated to illnesses and medicinal plants underwent more alterations and revisions than the rest of the Florentine Codex.40 This allows us to glean additional insights from incidental references in other sections, enabling us to expand and rectify instances where magical and religious medicinal content was either suppressed or omitted. Overall, it enriches our understanding of the ritual and therapeutic context of cempasúchil during that period.
The Florentine Codex, particularly in Book II (Festivals to the gods and ceremonies), provides a nuanced description of the cempasúchil flower head, presenting its characteristics from three different perspectives: As an object held by participants during ceremonies, as garlands for adorning the neck or head, and as a “symbolic” stone used in throwing rituals (Table A7). These descriptions enable us to comprehend the profound ritual significance of this flower at the time, taking into account the strong relationship between the divine, magic, diseases, gods, plants, and treatments.40 The apparent effort to conceal the ritual connection with the gods and their ceremonies emerged from the spiritual conquest.
3.2.4. Francisco Hernández
Francisco Hernández (1514–1587) and his indigenous collaborators compiled the uses and applications of American medicinal plants. They presented cempasúchil not only in terms of its physical characteristics, name, classification, distribution, and cultivation but also its therapeutic utility. By describing its properties and uses, he established a link between Indigenous medicinal practices and European medical theories, adding details such as the flower's “temperament,” taste, and odor (Table 2, Table A8).
Hernández's text, further edited and disseminated in Europe, played a pivotal role in disseminating information on cempasúchil with an extensive description referencing the various terminologies used for cempasúchil, illustrating the species' multifaceted identity in different cultural contexts. Hernández's observations predate the widespread recognition of the plant's presence in European horticulture. He noted its proliferation, especially in warmer Spanish regions, and its appreciation by other foreign nations. This early acknowledgment of cempasúchil's adaptability and appeal highlights the plant's smooth integration into the botanical landscape of the Old World (Table A8).
His pragmatic project enlists the medicinal properties of cempasúchil. Among the pathologies mentioned (Table 2), we find hydropsy, a disease linked to magical-religious concepts. This condition, known as a disease of water, along with ascites (fluid accumulation in the abdomen), was symbolically linked to the rain god Tláloc and other rain and water deities. The ascitic body was perceived as a vessel filled with water, serving a specific function for Tláloc. This fact highlights both the symbolic and medical descriptions of ascites, considered a sacred condition and a divine manifestation in pre-Hispanic cultures.53 A fundamental belief of the Aztecs was that certain diseases were associated with specific gods.54 This context underscores the significant medical importance of cempasúchil in the management of certain divine ailments during the pre-Conquest period through rituals.
3.3. Global dissemination post-conquest (Fig. 1)
It is challenging to establish a specific route for the diffusion of a species, as various paths existed during the 16th and 17th centuries, illustrating the complex and far-reaching impact of trade networks. The Treasure Fleets (Flota de Indias) and Manila Galleons connected the Americas and Asia to Spain, while European overland routes and Mediterranean ports further distributed these goods. The Canary Islands and Cape Verde played critical roles in the transatlantic journey, linking Europe, Africa, and the Americas. Unofficial routes emerged due to piracy, expanding the reach of American goods into other parts of Europe.55
The Port of Seville played a crucial role in the transatlantic trade, acting as a primary gateway for American goods into Europe. Based on Crailsheim,55 these commercial networks in the city allowed Nicolas Monardes (1493–1588), a Sevillian physician, to leverage access to exotic American plants. His studies of these plants contributed to the spread of American botanical knowledge in Europe. Monardes' work exemplifies the broader impact of Seville's trade with America, influencing economic and political realms and shaping European scientific and medicinal understanding.
“Historia medicinal de las cosas que se traen de nuestras Indias Occidentales” (1565, 1569, 1574) by Monardes mentions plants such as Tobacco (Nicotiana tabacum L.), Sassafras [Sassafras albidum (Nutt.) Nees], Sunflower (Helianthus annuus L.), Guaiacum or Lignum vitae (Guaiacum officinale L.), and Balsam of Peru [Myroxylon balsamum (L.) Harms].56 Notably, he does not mention TE, possibly because this species was already widely distributed in Europe then and did not represent any commercial or medically recognized advantage compared to other species. Also, in Europe, it was considered an ornamental rather than a medicinal species. During the Renaissance and early modern period in Europe, American plants spread rapidly, acquiring names, denominations of origin, and distinct uses. (for a more detailed historical perspective on TE in Europe from the 16th century to Linnaeus, see Section A1). Importantly, these data demonstrate the ambiguity of what was used during the 16th and 17th centuries in European medicine, with other yellow-coloured Asteraceae and TE not necessarily being differentiated and thus used interchangeably.
Medical uses have been attributed across history in Europe (Table 2). However, since the works of Gerard (1597), a different view of the taxon emerged, describing it as poisonous. “The plant is considered to be of a poisonous and venomous quality and is advised not to be touched, smelled, or used in food or medicine, suggesting that the use of Tagetes, especially the common sort with single flower heads, can lead to adverse reactions like swelling and in some cases, death in animals.28” Various authors began to emphasize the unpleasant scent of the taxon and its potential toxicity when used as medicine.57,58 In Europe, it has also become of interest as an ornamental flower and for other aesthetic purposes.59 This appears to be one of the first steps for TE to lose its medicinal status in Europe and to be simplified as an ornamental flower associated with strong toxicity.
3.4. Tagetes erecta L. (lutein): Modern impact and global acceptance (Fig. 1)
A comparison between the historical uses of TE and its clinical applications reveals major differences (Table 2). For example, lutein, a metabolite extracted from TE, has become a focal point for treating ocular diseases. However, in its historical and ethnobotanical tradition, no such uses for eye ailments could be found. This discrepancy allows for a discussion on how the isolation of lutein alters the perception of the plant compared to the full spectrum of metabolites present in the whole plant.
Lutein, extracted from TE, is increasingly recognized for its therapeutic potential in preventing and treating eye disease. This focus marks a shift from considering the whole plant's range of metabolites to isolating a single, active metabolite. The landmark Age-Related Eye Disease Study 2 (AREDS2) was the second phase of a comprehensive, multicentre, double-masked clinical trial conducted by the National Eye Institute, a division of the National Institutes of Health (NIH) in the United States (U.S.). This study, conducted between 2006 and 2013, highlighted its role in preventing aging-related macular degeneration (AMD) and cataracts. This trialled the adoption of lutein, zeaxanthin, and omega-3 fatty acids in nutritional supplements in place of beta-carotene. This groundbreaking research elevated lutein status and secondarily altered TE's significance within the biomedical industry in modern pharmacology and clinical practice.60
The AREDS2 findings prompted a re-evaluation of TE's global standing as a lutein source. affecting its demand and regulatory perception. Consumer awareness has boosted demand for lutein, reflecting in health and wellness trends and leading to TE fluctuating inclusion in pharmacopeias, included in the 1846 edition of the Mexican Pharmacopoeia,61 and excluded in the 2013 and 2021 editions, which mirrors changes in scientific evidence, medical practices, and international regulatory standards.
The regulations initially recognized the use of lutein for chicken feed, stablishing not only the safety and efficacy of lutein in enhancing poultry health and productivity but also its role in a culturally significant practice: The pigmentation of chicken skin to a yellow hue. However, with evolving research, a significant regulatory milestone was reached in 2014 when Meso-Zeaxanthin, a derivative of lutein from TE, was acknowledged for human use.62 Also, The National Centre for Advancing Translational Sciences, U.S. (NCATS) approved TE in 2014, and the clinical acknowledgment of various lutein forms highlights the plant's increasing acceptance and versatility in product development. Additionally, the 2014 classification of lutein as Generally Recognized as Safe (GRAS) in the U.S. and the patenting of its extracts for enhancing visual quality and mitigating the effects of intense light exposure reflects a growing recognition of its potential.
These dynamics highlight a commitment to ensuring lutein's safety, quality, and efficacy in drug manufacturing, dispensing, and preventive therapeutic applications. This evolving scientific and regulatory landscape mirrors the broader dynamics of medicinal plants within the global economy and underscores TE's economic impact and versatility. The story of TE, thus, transcends its scientific and medical evolution, reflecting the economic forces shaping its utilization and perception.
With plant-based/botanical extracts in the global market, including lutein from TE, the industry is burgeoning in 2021, valued at USD 2.2 billion.63 Lutein is largely consumed as a food colorant, and its sales amount to USD 150 million in the U.S. alone. In the E.U., lutein extracted from higher plants using hexane or other accepted solvents is listed as E161b when used as a feed additive.63 The forecasted growth is due to increasing demand for natural and organic products in the health, cosmetics, and food industries. North America and Asia Pacific are leading this expansion, with the lutein market expected to grow at a Compound Annual Growth Rate (CAGR) of 5.4 % from 2021 to 2026, driven by consumer preference for organic products and its application as a feed additive in poultry and aquaculture for its antioxidant properties.
4. Limitations of this study
Potential biases in the selected databases, documents, evidence interpretation, and language constraints may influence the outcomes of our analysis. Future research could expand on this methodology by having a broader range of languages and databases and exploring further applications of TE in different cultural contexts. The coverage of the literature cannot be comprehensive either since more detailed archival research needs to be initiated.
5. Conclusion
Our investigation re-evaluates the traditional and contemporary roles of TE, highlighting the necessity to distinguish historical uses from contemporary medicinal applications. As demonstrated, TE's prominence in ancient rituals is evident, yet its medicinal uses have gradually diminished over the centuries. This phenomenon is not unique to TE but can be observed in various medicinal plants, where historical and cultural significances do not always align with contemporary medicinal utilisations. For instance, despite the effectiveness of specific metabolites like lutein derived from TE, it does not necessarily affirm the whole plant's therapeutic benefits.
Our research emphasizes the inseparable ties between medicine and culture, articulating that medical applications are deeply entrenched in the language and traditions that shape them. This perspective is crucial when demonstrating that our multidisciplinary understanding of a plant's medicinal potential is continually shaped by cultural, biomedical, linguistic, and historical contexts.
Returning to our starting question, what makes a medicine ‘traditional” and is it TE a traditional medicine, it is apparent most of the current uses are not linked to local and traditional practices, even though clearly, we now make use of this element of Mexican biodiversity on a global level and in multiple ways. Very little of what has been described here is traditional. Instead, we see a series of appropriations or exploitations, innovations, and further developments of this quintessential Mexican indigenous species. Based on the current international regulations, including the Nagoya Protocol (2014)64 and the Convention on Biological Diversity (Rio Convention 1992),65 the region of origin is the core basis for defining access and benefits, and the use as such is of secondary importance. Consequently, if such a development would happen today, the provider countries would have significant rights over their resources.
The plant has been reinterpreted through various lenses throughout its transition, from a ritual and symbolic element of Mesoamerican culture to a scientific subject, medicine, and ornamental element. It provides an illuminating example of the multifaceted nature and complexity of medicinal plants when viewed from the perspective of pharmaco-botanical history and the importance of incorporating this perspective for a profound understanding of a taxon. It casts into doubt the way the term traditional is used in the fields of medicine, ethnopharmacology, pharmacy, and economic botany.
Despite the extensive historical, medicinal, botanical, phytochemical, pharmacological, clinical, and economic context surrounding TE, each year, at the beginning of November, during the Día de Muertos celebrations (November 1st and 2nd), Mexico becomes adorned with the vibrant color and fragrant aroma of this flower. TE holds a profound symbolic significance and is deeply rooted in Mesoamerican culture. To this day, it represents a form of “resistance,” “syncretism,” “integration,” and “globalization” among countries and cultures. TE is a multifaceted flower that has been cast into numerous roles and, for any Mexican, remains indelibly the cempasúchil, or “the flower of the dead.” This enduring cultural relevance underscores its importance beyond its diverse utilitarian applications, embedding it deeply in the cultural and emotional fabric of Mexican society.
Funding
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors. MH is a Yushan Fellow at China Medical University, Taiwan.
Conflict of interest
None of the authors has any conflict of interest.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2024.08.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Howes M.J.R., Quave C.L., Collemare J., et al. Molecules from nature: reconciling biodiversity conservation and global healthcare imperatives for sustainable use of medicinal plants and fungi. Plants, People, Planet. 2020;2(5):463–481. [Google Scholar]
- 2.Taylor J., Rabe T., McGaw L., Jäger A., Van Staden J. Towards the scientific validation of traditional medicinal plants. Plant Growth Regul. 2001;34:23–37. [Google Scholar]
- 3.Menendez-Baceta G., Aceituno-Mata L., Reyes-García V., Tardío J., Salpeteur M., Pardo-de-Santayana M. The importance of cultural factors in the distribution of medicinal plant knowledge: a case study in four Basque regions. J Ethnopharmacol. 2015;161:116–127. doi: 10.1016/j.jep.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Mazzocchi F. Western science and traditional knowledge: despite their variations, different forms of knowledge can learn from each other. EMBO Rep. 2006;7(5):463–466. doi: 10.1038/sj.embor.7400693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jütte R., Heinrich M., Helmstädter A., et al. Herbal medicinal products – evidence and tradition from a historical perspective. J Ethnopharmacol. 2017;207:220–225. doi: 10.1016/j.jep.2017.06.047. [DOI] [PubMed] [Google Scholar]
- 6.Heinrich M. Quality and safety of herbal medical products: regulation and the need for quality assurance along the value chains. Br J Clin Pharmacol. Jul 2015;80(1):62–66. doi: 10.1111/bcp.12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mann F. Chinese traditional medicine: a practitioner's view. China Q. 1965;23(23):28–36. doi: 10.1017/S0305741000009875. [DOI] [Google Scholar]
- 8.Croizier R.C. Vol. 1. Harvard University Press; 1968. pp. 101–180. (Traditional Medicine in Modern China: Science, Nationalism, and the Tensions of Cultural Change). [Google Scholar]
- 9.Heinrich M., Kum K.Y., Yao R. Routledge; 2022. Decontextualised Chinese Medicines: Their Uses as Health Foods and Medicines in the ‘global North’. Routledge Handbook Of Chinese Medicine; pp. 721–741. [Google Scholar]
- 10.Gelfand M. Vol. 1. Harvill Press Ltd.; 1964. p. 191. (Witch Doctor: Traditional Medicine Man of Rhodesia). [Google Scholar]
- 11.Heinrich M. 1994. Ethnobotany of Mexican Compositae: An Analysis of Historical and Modern Sources; pp. 475–503. [Google Scholar]
- 12.Villaseñor J.L. Checklist of the native vascular plants of Mexico. Rev Mex Biodivers. 2016;87(3):559–902. [Google Scholar]
- 13.Myers N., Mittermeier R.A., Mittermeier C.G., Da Fonseca G.A., Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403(6772):853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 14.Shin T., Fujikawa K., Moe A.Z., Uchiyama H. Traditional knowledge of wild edible plants with special emphasis on medicinal uses in Southern Shan State, Myanmar. J Ethnobiol Ethnomed. 2018;14(1):1–13. doi: 10.1186/s13002-018-0248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sujatha C., Nify B., Pratheesh V. Isolation, stabilization and characterization of xanthophyll from marigold flower-Tagetes erecta-L. Mod Appl Sci. 2009;3(2):19–28. [Google Scholar]
- 16.Xu L.-W., Wang G.-Y., Shi Y.-P. Chemical constituents from Tagetes erecta flowers. Chem Nat Compd. 2011;47:281–283. [Google Scholar]
- 17.Xu L-w, Juan C., Qi H-y, Shi Y-p. Phytochemicals and their biological activities of plants in Tagetes L. Chin Herb Med. 2012;4(2):103–117. [Google Scholar]
- 18.Burlec A.F., Pecio Ł., Kozachok S., et al. Phytochemical profile, antioxidant activity, and cytotoxicity assessment of Tagetes erecta L. flowers. Molecules. 2021;26(5):1201. doi: 10.3390/molecules26051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong Y., Liu X., He W.H., Xu H.G., Yuan F., Gao Y.X. Investigation into the antioxidant activity and chemical composition of alcoholic extracts from defatted marigold (Tagetes erecta L.) residue. Fitoterapia. Apr 2012;83(3):481–489. doi: 10.1016/j.fitote.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 20.Piña-Vázquez D.M., Mayoral-Peña Z., Gómez-Sánchez M., Salazar-Olivo L.A., Arellano-Carbajal F. Anthelmintic effect of Psidium guajava and Tagetes erecta on wild-type and Levamisole-resistant Caenorhabditis elegans strains. J Ethnopharmacol. 2017;202:92–96. doi: 10.1016/j.jep.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Nikkon F., Habib M.R., Saud Z.A., Karim M.R. Tagetes erecta Linn. and its mosquitocidal potency against Culex quinquefasciatus. Asian Pac J Trop Biomed. 2011;1(3):186–188. doi: 10.1016/S2221-1691(11)60024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burlec A.F., Cioancă O., Mircea C., et al. Antioxidant and antimicrobial properties of Chrysanthemum and Tagetes selective extracts. FARMACIA. 2019;67(3):405–410. [Google Scholar]
- 23.Oliveira PFd, Alves J.M., Damasceno J.L., et al. Cytotoxicity screening of essential oils in cancer cell lines. Rev Bras Farmacogn. 2015;25:183–188. [Google Scholar]
- 24.Gupta P., Vasudeva N. In vitro antiplasmodial and antimicrobial potential of Tagetes erecta roots. Pharm Biol. Nov 2010;48(11):1218–1223. doi: 10.3109/13880201003695142. [DOI] [PubMed] [Google Scholar]
- 25.Ventura-Martínez R., Angeles-López G.E., Rodríguez R., González-Trujano M.E., Déciga-Campos M. Spasmolytic effect of aqueous extract of Tagetes erecta L. flowers is mediated through calcium channel blockade on the Guinea-pig ileum. Biomed Pharmacother. Jul 2018;103:1552–1556. doi: 10.1016/j.biopha.2018.04.166. [DOI] [PubMed] [Google Scholar]
- 26.Hernández F. Quatros libros de la naturaleza y virtudes medicinales de las plantas y animales de la Nueva España. vol 1. en casa de la Viuda de Diego Lopez Daualos; 1838:81.
- 27.Brunfels O, Weiditz H. Herbarum Vivae Eiconeb. vol 1. Apud Joannem Schottum; 1530:251.
- 28.Gerard J, Bollifant E, Norton J, Norton B, Rogers W, Dodoens R. The Herball, or, Generall Historie of Plantes. vol 1. Edm. Bollifant for Bonham Norton and Iohn Norton; 1597:609-612.
- 29.Auh J.-H., Madhavan J. Protective effect of a mixture of marigold and rosemary extracts on UV-induced photoaging in mice. Biomed Pharmacother. 2021;135 doi: 10.1016/j.biopha.2020.111178. [DOI] [PubMed] [Google Scholar]
- 30.Zanovello M., Mariano L.N.B., Cechinel-Zanchett C.C., et al. Tagetes erecta L. flowers, a medicinal plant traditionally used to promote diuresis, induced diuretic and natriuretic effects in normotensive and hypertensive rats. J Ethnopharmacol. 2021;279 doi: 10.1016/j.jep.2021.114393. [DOI] [PubMed] [Google Scholar]
- 31.Meurer M., de Oliveira B.M., Cury B.J., et al. Extract of Tagetes erecta L., a medicinal plant rich in lutein, promotes gastric healing and reduces ulcer recurrence in rodents. J Ethnopharmacol. 2022;293 doi: 10.1016/j.jep.2022.115258. [DOI] [PubMed] [Google Scholar]
- 32.Chaniad P., Techarang T., Phuwajaroanpong A., Na-Ek P., Viriyavejakul P., Punsawad C. Preclinical evaluation of antimalarial activity of CPF-1 formulation as an alternative choice for the treatment of malaria. Evid Based Complement Alternat Med. 2023;23(1):144. doi: 10.1155/2021/1270902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cui G., Wei F., Wei M., Xie L., Lin Z., Feng X. Modulatory effect of Tagetes erecta flowers essential oils via Nrf2/HO-1/NF-κB/p65 axis mediated suppression of N-methyl-Nʹnitro-N-nitroguanidine (MNNG) induced gastric cancer in rats. Mol Cell Biochem. 2021;476:1541–1554. doi: 10.1007/s11010-020-04005-0. [DOI] [PubMed] [Google Scholar]
- 34.Pérez-Ortega G., Angeles-López G.E., Argueta-Villamar A., González-Trujano M.E. Preclinical evidence of the anxiolytic and sedative-like activities of Tagetes erecta L. reinforces its ethnobotanical approach. Biomed Pharmacother. 2017;93:383–390. doi: 10.1016/j.biopha.2017.06.064. [DOI] [PubMed] [Google Scholar]
- 35.Khulbe A., Pandey S., Sah S.P. Antidepressant-like action of the hydromethanolic flower extract of Tagetes erecta L. in mice and its possible mechanism of action. Indian J Pharmacol. 2013;45(4):386. doi: 10.4103/0253-7613.115026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hajizadeh-Sharafabad F., Tarighat-Esfanjani A., Ghoreishi Z., Sarreshtedari M. Lutein supplementation combined with a low-calorie diet in middle-aged obese individuals: effects on anthropometric indices, body composition and metabolic parameters. Br J Nutr. 2021;126(7):1028–1039. doi: 10.1017/S0007114520004997. [DOI] [PubMed] [Google Scholar]
- 37.Harris A., Siesky B., Huang A., et al. Lutein complex supplementation increases ocular blood flow biomarkers in healthy subjects. Int J Vitam Nutr Res. 2019;89(1-2):5–12. doi: 10.1024/0300-9831/a000576. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida K., Sakai O., Honda T., et al. Effects of astaxanthin, lutein, and zeaxanthin on eye-hand coordination and smooth-pursuit eye movement after visual display terminal operation in healthy subjects: a randomized, double-blind placebo-controlled intergroup trial. Nutrients. 2023;15(6):1459. doi: 10.3390/nu15061459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schaefer E., Demmelmair H., Horak J., et al. Multiple micronutrients, lutein, and docosahexaenoic acid supplementation during lactation: a randomized controlled trial. Nutrients. 2020;12(12):3849. doi: 10.3390/nu12123849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Montellano B.O. Medicina, salud y nutrición aztecas. Siglo XXI. 1993;29(94):235. [Google Scholar]
- 41.Mahomoodally M.F., Mooroteea K. A comparative ethno-religious study of traditionally used medicinal plants employed in the management of cardiovascular diseases. J Herb Med. 2021;25 [Google Scholar]
- 42.Pradhan S.P., Chaudhary R.P., Sigdel S., Pandey B.P. Ethnobotanical knowledge of Khandadevi and Gokulganga rural municipality of Ramechhap district of Nepal. Ethnobot Res Appl. 2020;20:1–32. [Google Scholar]
- 43.Rajakumar N., Shivanna M. Ethno-medicinal application of plants in the eastern region of Shimoga district, Karnataka, India. J Ethnopharmacol. 2009;126(1):64–73. doi: 10.1016/j.jep.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 44.Rao P., Hasan S., Bhellum B., Manhas R. Ethnomedicinal plants of Kathua district, J&K, India. J Ethnopharmacol. 2015;171:12–27. doi: 10.1016/j.jep.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 45.Esquivel-García R., Pérez-Calix E., Ochoa-Zarzosa A., García-Pérez M.-E. Ethnomedicinal plants used for the treatment of dermatological affections on the Purépecha Plateau, Michoacán, Mexico. Acta Bot Mex. 2018;125:2448–7589. doi: 10.21829/abm125.2018.1339. [DOI] [Google Scholar]
- 46.Karttunen F.E. Vol. 30. University of Oklahoma Press; 1992. p. 329. (An Analytical Dictionary of Nahuatl). [Google Scholar]
- 47.Rice P.M. Maya calendar origins: monuments, mythistory, and the materialization of time. University of Texas Press. 2007;43(44):174. 1. [Google Scholar]
- 48.Tucker A.O., Janick J. 59. Springer; 2020. Flora of the Codex Cruz-Badianus. 92, 103, 243. [Google Scholar]
- 49.Viesca C. Y Martín de la Cruz, autor del Códice de la Cruz Badiano, era un médico tlatelolca de carne y hueso. Estud Cult Náhuatl. 1995;25:479–498. [Google Scholar]
- 50.De Sahagún B.B., Carlos María de, Guerra Mier Noriega Y., Jstd Historia general de las cosas de Nueva España. Impr. del ciudadano A. Valdés. 1829;1–3(59) 63, 64, 127, 136, 139, 140, 148, 149. [Google Scholar]
- 51.Fuchs L, Füllmaurer H, Füllmaurer H, et al. De historia stirpium commentarii insignes. vol 1. In officina Isingriniana; 1542:47-49.
- 52.Mattioli PA. Commentarii denuo aucti in libros sex Pedacii Dioscoridis Anazarbei de medica materia. vol 1. Ex Officina Valgrisiana; 1565:1303.
- 53.Viesca-Treviño C., Macuil-García C., Monzón-Barranco A., Rosas-Peña J. Tláloc y la ascitis. Rev Med IMSS (Inst Mex Seguro Soc) 2009;47(3):251–258. [PubMed] [Google Scholar]
- 54.De Montellano B.O. Las hierbas de Tláloc. Estud Cult Náhuatl. 1980;14:287–314. [Google Scholar]
- 55.Crailsheim E. Seville and Manila: illegal trade, corruption, and the phenomenon of trust in the Spanish Empire. Int J Marit Hist. 2017;29(1):175–181. [Google Scholar]
- 56.Monardes N. Historia medicinal de las cosas, que se traen de nuestras Indias occidentales que sirven en medicina. vol 1,2,3. En casa de Fernando Diaz; 1580:345.
- 57.Dalechamps J, Desmoulins J. Histoire generale des plantes. vol 1. 1653:727.
- 58.Ray J, Camel GJ, Tournefort JPd. Historia Plantarum vol 1. 1686:342-343.
- 59.Theodorus I, Bauhin C, Braun N. Neuw Vollkommentlich Kreuterbuch. vol 1. 1613:43-45.
- 60.Chew E.Y., SanGiovanni J.P., Ferris F.L., et al. Lutein/zeaxanthin for the treatment of age-related cataract: AREDS2 randomized trial report no. 4. JAMA Ophthalmol. 2013;131(7):843–850. doi: 10.1001/jamaophthalmol.2013.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farmacopea mexicana (Imprenta a cargo de Manuel N. de la Vega) 26 (1846).
- 62.David B., Wolfender J.-L., Dias D.A. The pharmaceutical industry and natural products: historical status and new trends. Phytochem Rev. 2015;14:299–315. [Google Scholar]
- 63.Fernández-Sevilla J.M., Acién Fernández F., Molina Grima E. Biotechnological production of lutein and its applications. Appl Microbiol Biotechnol. 2010;86:27–40. doi: 10.1007/s00253-009-2420-y. [DOI] [PubMed] [Google Scholar]
- 64.Buck M., Hamilton C. The Nagoya Protocol on access to genetic resources and the fair and equitable sharing of benefits arising from their utilization to the Convention on Biological Diversity. Rev Eur Community Int Environ Law. 2011;20(1):47–61. [Google Scholar]
- 65.Convention on Biological Diversity: Text and Annexes (United Nations Environment Programme) vols. 1–5, 12-13 (2011).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.