Drug delivery systems (DDSs) involved with nano/microparticles enjoy a superior advantage in accurately delivering drugs to the desired sites for higher therapeutic efficacies and lower side effects. Unfortunately, the conventional nano/microparticle-based DDSs, even with passive and active targeting properties, show a total targeting efficiency of less than 1%, attributed to the complicated microenvironment in vivo1. Therefore, it is urgent to delicately design the DDSs by using the factors that influence the fate of DDSs in vivo.
Many preclinical studies have shown that living cells, such as red blood cells (RBCs), platelets, macrophages, etc., can effectively load drugs and enhance delivery2, 3, 4. Two strategies have been widely utilized to load drugs onto living cells: (i) specifically connecting the drugs to certain sites on the cells through chemical coupling; (ii) non-specifically adhering the drugs to the cells through non-covalent interactions such as electrostatic forces, normally termed as “drug hitchhiking”. However, the chemical connection affects the inherent properties of cell carriers and induce adverse effects on the cells, shortening the circulation time5. On the contrary, physically loading onto the cell carriers always reduces the reticuloendothelial system (RES) drug uptake and prolongs the systemic circulation time6. Notably, the drug should be loaded on the cell surface and avoid being endocytosed by the cell carriers, suggesting that those cells with weaker phagocytic capacity can be selected for constructing living cell-based DDSs. Moreover, the properties of drug-containing particles, including particle size, shape, electrical properties, etc., can be optimized to reduce particle internalization.
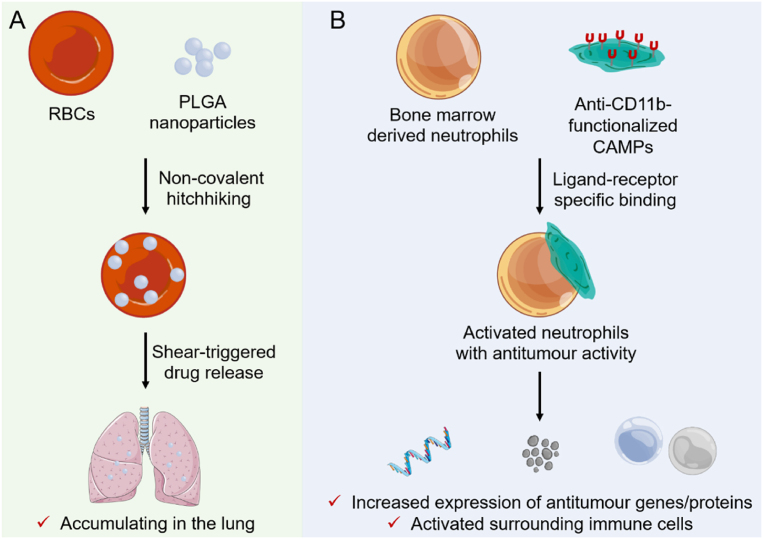
RBCs are the most extensively studied cell carriers due to little uptake and excellent circulation ability. However, drugs are ready to fall off during systemic circulation after binding to the surface of RBCs due to the weak force of physical coupling. To address the limitation, Zhao et al.7 anchored poly(lactic-co-glycolic acid) (PLGA) nanoparticles onto RBCs through multiple non-covalent forces, indicating efficient lung accumulation and therapeutic effects compared with the control group (Fig. 1A). However, they found that the leakage of drugs in the livers and other major organs is still profound.
Figure 1.
(A) Schematic illustration of RBC-anchored nanoparticles for enhanced lung targeting. The PLGA nanoparticles non-covalently hitchhiked onto the surface of injected RBCs will specifically accumulate in the lungs and colocalize with the metastatic cancer cells, and shear-induced detachment of nanoparticles in narrow lung capillaries further contributes to the nanoparticles depositing primarily in the lungs. (B) Graphical summary of the neutrophil–CAMP concept and key findings. Neutrophils are activated upon the attachment of anti-CD11b-functionalized CAMP, changing the gene expression and protein release from neutrophils, leading to alteration of the neutrophil phenotype and activation of surrounding immune cells, inducing a robust immune response and reducing the tumor burden in vivo.
Antigen-modified particles bind specifically to surface antibodies inherent to some living cell carriers, preventing cell damage during the nanoparticle loading, changing the nanoparticle fate in vivo, and improving targeting efficiency8. Recently, Kumbhojkar et al.8 constructed neutrophils bearing cyto-adhesive micropatches (CAMPs) for immunotherapy of cancer in a drug-free manner. They demonstrated that the discoidal CAMPs physically adhered to the surfaces of neutrophils and polarized the neutrophils into antitumor phenotype, exerting an effective antitumor effect by themselves as well as by activating the antitumor phenotype of surrounding immune cells (Fig. 1B). These findings provide a promising strategy of utilizing cell bearing particles for prevention and treatment of serious diseases. To extend CAMPs-laden cell therapy, they loaded 6.04-μm anti-CD11b Fab-modified polylactic-co-glycolic acid (PLGA) particles onto neutrophils. As expected, the PLGA particles1 adhered onto neutrophils rather than internalized into cells.
Neutrophils change to antitumor (N1) phenotype and produce multiple antitumor effects after activation9,10. CAMP-activated neutrophils released primary granules of myeloperoxidase in vitro cell model and maintained them for a long time in mice. Furthermore, CAMP-laden neutrophils did not produce obvious side effects in tumor-bearing mice, confirmed by the vital organs’ IL-6 levels and hematoxylin and eosin staining. Attachment of CAMP showed almost no effects on the circulation dynamics of neutrophils in tumor-bearing mice while neutrophils-bearing CAMPs accumulated in the tumor significantly. Then, the potential antitumor effects of CAMP-loaded neutrophils were systematically explored. The N1 phenotype of neutrophils was activated by the adhesion of CAMPs and released several pro-inflammatory cytokines, enhancing systemic antitumor immune response. Moreover, the CAMP-laden neutrophils activated the antitumor phenotype of surrounding immune cells, including CD8+ T cells, natural killer (NK) cells, dendritic cells (DCs) and macrophages, significantly promoting the systemic antitumor immune response.
To demonstrate the effectiveness of neutrophil-based cancer immunotherapy, Kumbhojkar et al.8 evaluated the antitumor efficacy of neutrophil-CAMPs in tumor-bearing mice. Compared with saline-treated groups, intravenous administration of CAMP-adhered neutrophils reduced tumor growth rate and increased median survival in tumor-bearing mice. At the same time, the therapeutic benefit was not found in the mice treated by neutrophils alone. Due to the specific accumulation of CAMP-attached neutrophils and subsequent recruitment of DCs to the tumor-draining lymph nodes, they thought CAMP-attached neutrophils could work collaboratively with clinically used checkpoint blockades, intensifying the early antitumor immune response. Therefore, combining therapies of CAMP-attached neutrophils and clinically relevant checkpoint blockades, such as anti-cytotoxic T-lymphocyte associated protein 4 (anti-CTLA4) checkpoint inhibitor and anti-programmed cell death protein 1 (anti-PD1) checkpoint inhibitor, were performed to study the cancer immunotherapy. As expected, the two combined therapy regimens showed synergistic effects on antitumor activities, as evidenced by slowed tumor growth and improved survival.
In summary, this study designed a novel cell carrier different from the traditional live cell-based DDSs. The chosen neutrophils are both carriers and therapeutic entities, which avoid leakage during delivery, reduce the corresponding side effects, and improve the antitumor treatment effect. In this system, the micropatches adhered to the neutrophils changed the fate of the microparticles in the systemic circulation by the homing characteristics of the neutrophils. This study is interesting; however, increasing experiments are required to clarify several directions.
-
(1)
Even if the system is not loaded with drugs, the human body has complex physiological conditions, and nanoparticles may fall off under blood flow environments with high shear forces. Will this affect the body's environment and produce physiological effects that we do not expect?
-
(2)
The authors verified the efficient homing characteristics of the system. The accumulation of CAMP-attached neutrophils in the tumor site reached 3.5% injected dose (ID) per gram 24 h after injection. The authors selected ordinary neutrophils as a control. However, the tumor accumulation of simple disk-attached nanoparticles is unclear.
Live cell-based therapy is a promising means of targeted delivery/treatment. For the clinical translation, several points should be considered. For example:
-
(1)
The purification process is complicated in the preparation process, which may affect the activity of neutrophils in clinical application. Cell-based therapeutics can also be learned from CAR-T therapy if the homology of the cell source and patient is required.
-
(2)
Considering that adequate particles are critical to activating neutrophils and producing efficient therapeutic efficacy in vivo, controlling the number of patches on each cell during the preparation process is essential.
Author contributions
Xiaotong Li and Jianhua He drafted the manuscript. Wei He proposed the topic and corrected the manuscript. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Nos. 82073782 and 82241002), the Shanghai Science and Technology Committee (No. 19430741500, China), and the Key Laboratory of Modern Chinese Medicine Preparation of Ministry of Education of Jiangxi University of Traditional Chinese Medicine (zdsys-202103, China).
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
References
- 1.Li X.T., Zou J.H., He Z.S., Sun Y.H., Song X.R., He W. The interaction between particles and vascular endothelium in blood flow. Adv Drug Deliver Rev. 2024;207 doi: 10.1016/j.addr.2024.115216. [DOI] [PubMed] [Google Scholar]
- 2.Huang Y.K., Gao X.L., Chen J. Leukocyte-derived biomimetic nanoparticulate drug delivery systems for cancer therapy. Acta Pharm Sin B. 2018;8:4–13. doi: 10.1016/j.apsb.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tao Y., Lan X.M., Zhang Y., Fu C.X., Liu L., Cao F., et al. Biomimetic nanomedicines for precise atherosclerosis theranostics. Acta Pharm Sin B. 2023;13:4442–4460. doi: 10.1016/j.apsb.2022.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang S.L., Wang R.F., Meng N.N., Lu L.W., Wang J., Zhou J.F., et al. Engineered platelets-based drug delivery platform for targeted thrombolysis. Acta Pharm Sin B. 2022;12:2000–2013. doi: 10.1016/j.apsb.2022.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muzykantov V.R., Murciano J.C., Taylor R.P., Atochina E.N., Herraez A. Regulation of the complement-mediated elimination of red blood cells modified with biotin and streptavidin. Anal Biochem. 1996;241:109–119. doi: 10.1006/abio.1996.0384. [DOI] [PubMed] [Google Scholar]
- 6.Zhang S.Q., Fu Q., Zhang Y.J., Pan J.X., Zhang L., Zhang Z.R., et al. Surface loading of nanoparticles on engineered or natural erythrocytes for prolonged circulation time: strategies and applications. Acta Pharm Sin B. 2021;42:1040–1054. doi: 10.1038/s41401-020-00606-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Z.M., Ukidve A., Krishnan V., Fehnel A., Pan D.C., Gao Y.S., et al. Systemic tumour suppression via the preferential accumulation of erythrocyte-anchored chemokine-encapsulating nanoparticles in lung metastases. Nat Biomed Eng. 2021;5:441–454. doi: 10.1038/s41551-020-00644-2. [DOI] [PubMed] [Google Scholar]
- 8.Kumbhojkar N., Prakash S., Fukuta T., Adu-Berchie K., Kapate N., An R., et al. Neutrophils bearing adhesive polymer micropatches as a drug-free cancer immunotherapy. Nat Biomed Eng. 2024:1–14. doi: 10.1038/s41551-024-01180-z. [DOI] [PubMed] [Google Scholar]
- 9.Chan Y.T., Tan H.Y., Lu Y., Zhang C., Cheng C.S., Wu J., et al. Pancreatic melatonin enhances anti-tumor immunity in pancreatic adenocarcinoma through regulating tumor-associated neutrophils infiltration and NETosis. Acta Pharm Sin B. 2023;13:1554–1567. doi: 10.1016/j.apsb.2023.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yee P.P., Wei Y.J., Kim S.Y., Lu T., Chih S.Y., Lawson C., et al. Neutrophil-induced ferroptosis promotes tumor necrosis in glioblastoma progression. Nat Commun. 2020;11:5424. doi: 10.1038/s41467-020-19193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]