Abstract
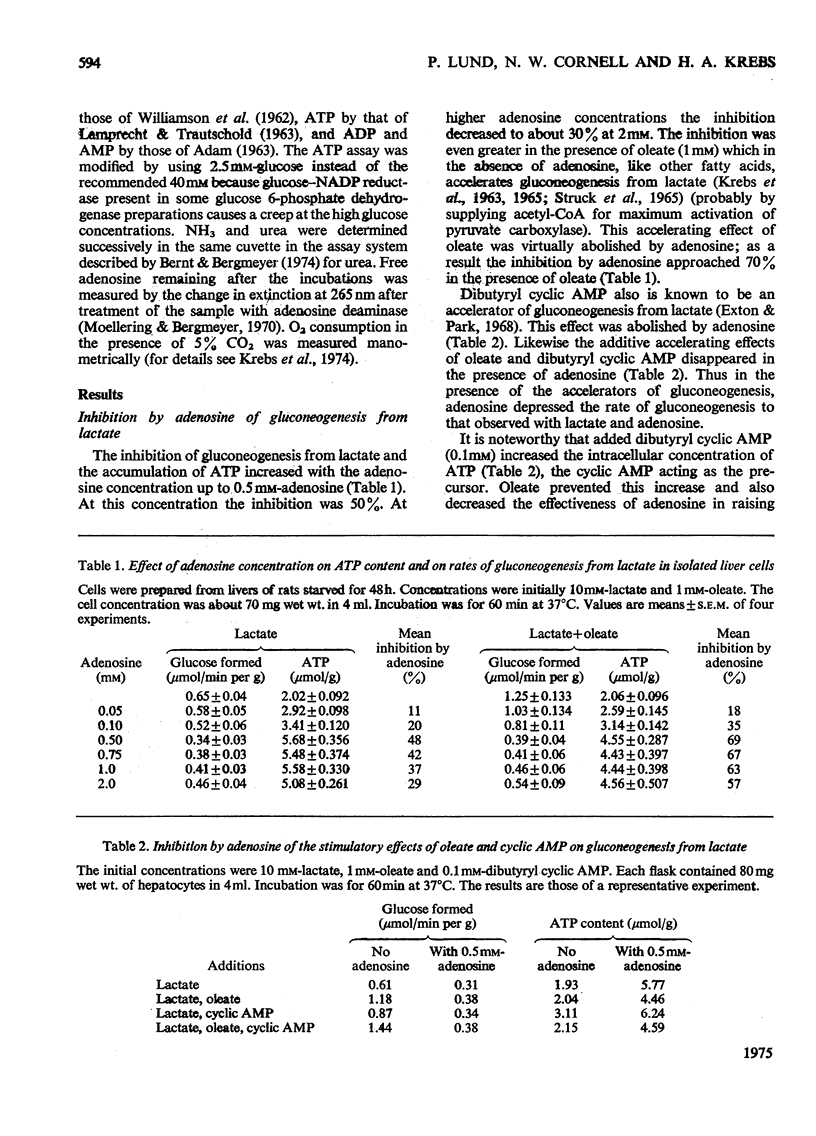
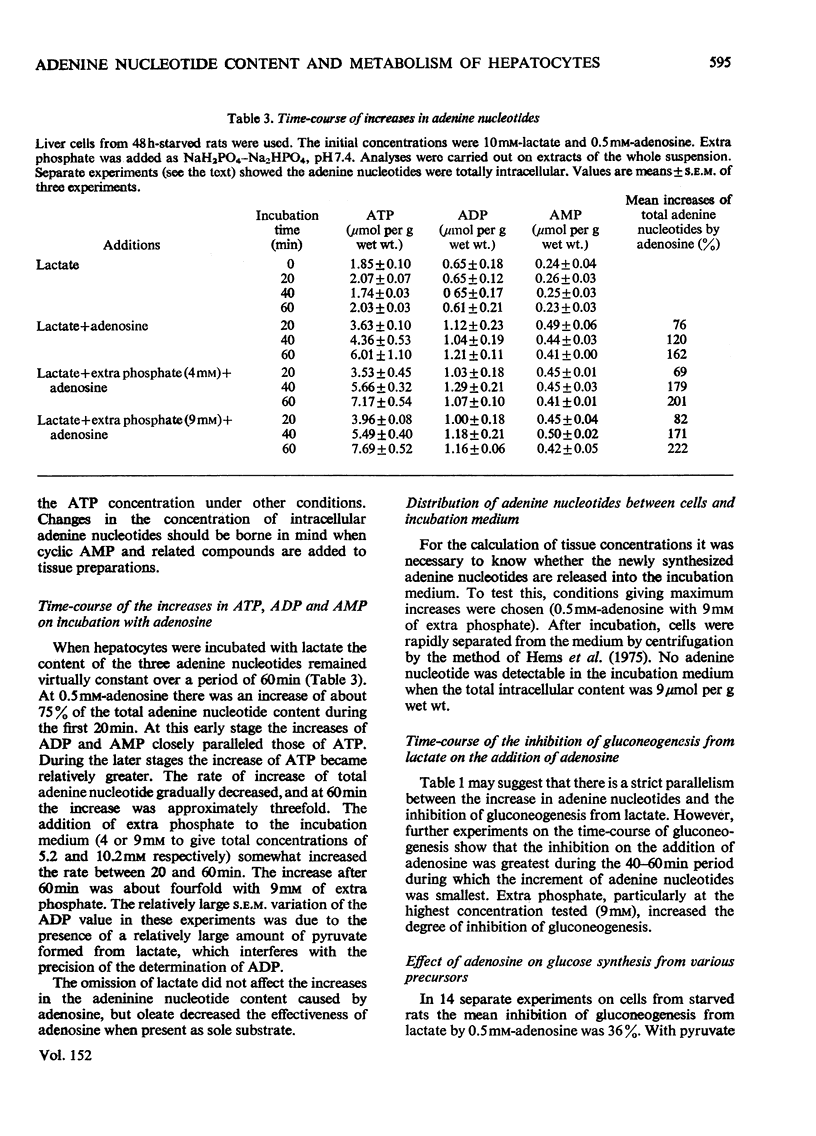
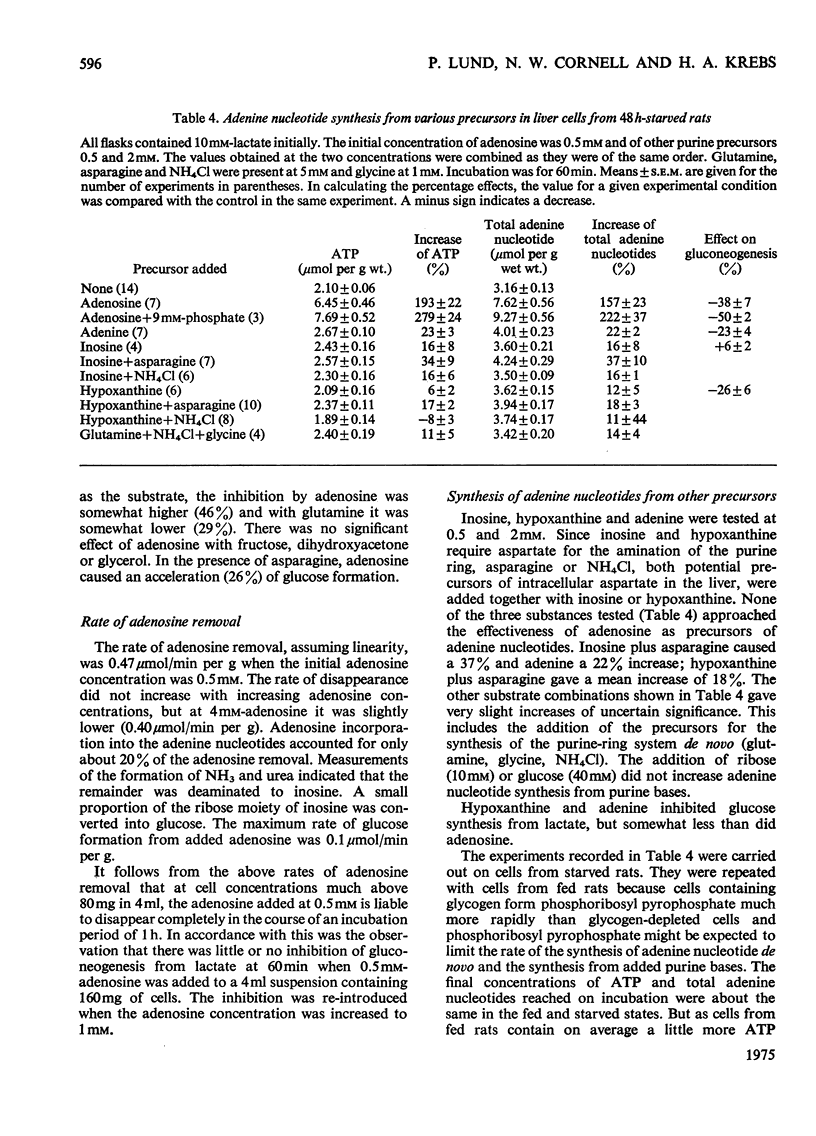
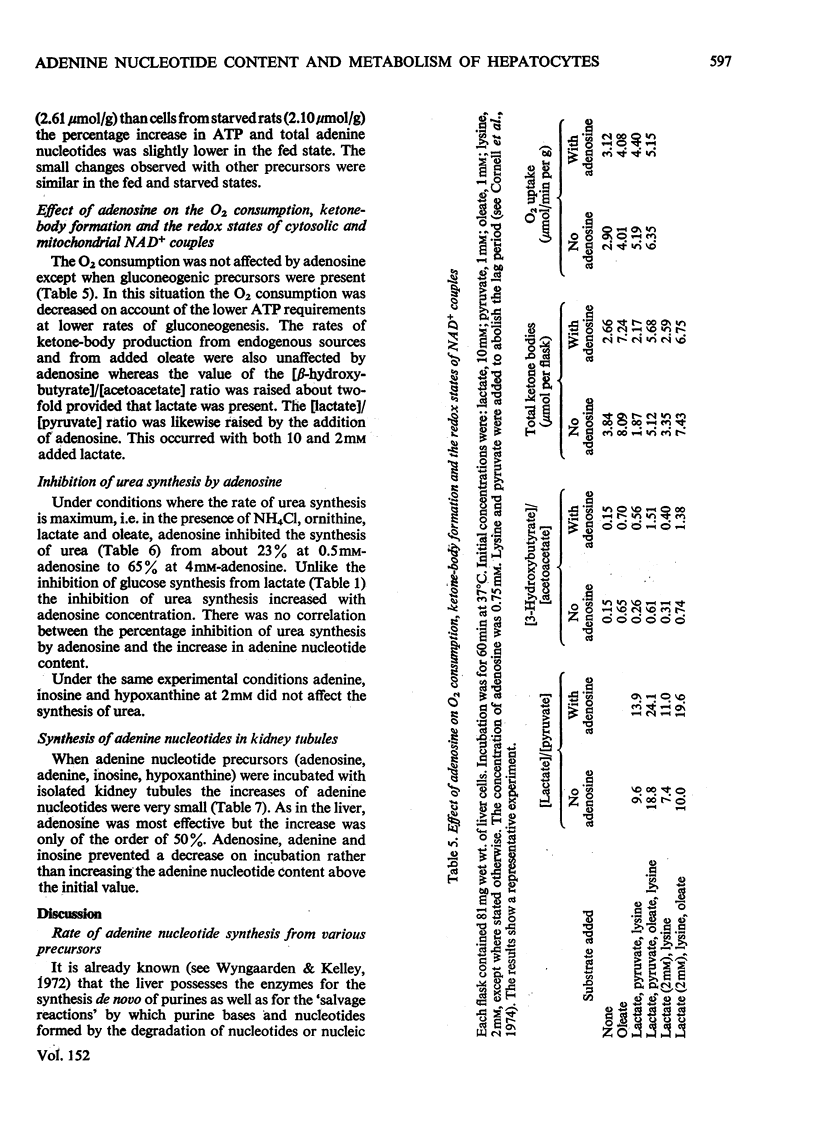
ADENOSINE (0.5 MM) added to hepatocyte suspensions increased the intracellular concentration of ATP and total adenine nucleotides within 60 min up to three-fold. 2. Adenosine at 0.5 mM inhibited gluconeogenesis from lactate by about 50%. At higher adenosine concentrations the inhibition was less. There was no strict parallelism between the time-course of the increase of the adenine nucleotide content and the time-course of the inhibition of gluconeogenesis from lactate. 3. Adenosine abolished the accelerating effects of oleate and dibutyryl cyclic AMP on gluconeogenesis from lactate. 4. Gluconeogenesis was no significant effect of adenosine with fructose, dihydroxyacetone or glycerol. With asparagine, adenosine caused anacceleration of glucose formation. 5. Adenosine incorporation into adenine nucleotides accounted for about 20% of the adenosine removal. 6. Inosine, hypoxanthine or adenine compared with adenosine gave relatively slight increases of adenine nucleotides. 7. Urea synthesis from NH4Cl under optimum conditions i.e. in the presence of ornithine, lactate and oleate, was also inhibited by adenosine. The inhibition increased with the adenosine concentration and was 65% at 4 mM-adenosine. Again there was no correlation between the degree of inhibition of urea synthesis and the increase in the adenine nucleotide content. 8. The basal O2 consumption, the increased O2 consumption on the addition of oleate and the rate of formation of ketone bodies were not affected by the addition of adenosine. The [beta-hydroxybutyrate]/[acetoacetate] ratio was increased by adenosine, provided that lactate was present. 9. The increase of the adenine nucleotide content of the hepatocytes on the addition of adenosine may be explained on the assumption that adenosine kinase is not regulated by feedback but by substrate supply.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAPUTTO R. The enzymatic synthesis of adenylic acid; adenosinekinase. J Biol Chem. 1951 Apr;189(2):801–814. [PubMed] [Google Scholar]
- Chagoya de Sánchez V., Brunner A., Piña E. In vivo modification of the energy charge in the liver cell. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1441–1445. doi: 10.1016/s0006-291x(72)80138-4. [DOI] [PubMed] [Google Scholar]
- Cornell N. W., Lund P., Hems R., Krebs H. A. Acceleration of gluconeogenesis from lactate by lysine (Short Communication). Biochem J. 1973 Jun;134(2):671–672. doi: 10.1042/bj1340671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell N. W., Lund P., Krebs H. A. The effect of lysine on gluconeogenesis from lactate in rat hepatocytes. Biochem J. 1974 Aug;142(2):327–337. doi: 10.1042/bj1420327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton J. H., Park C. R. Control of gluconeogenesis in liver. II. Effects of glucagon, catecholamines, and adenosine 3',5'-monophosphate on gluconeogenesis in the perfused rat liver. J Biol Chem. 1968 Aug 25;243(16):4189–4196. [PubMed] [Google Scholar]
- HOHORST H. J., KREUTZ F. H., BUECHER T. [On the metabolite content and the metabolite concentration in the liver of the rat]. Biochem Z. 1959;332:18–46. [PubMed] [Google Scholar]
- Hems R., Lund P., Krebs H. A. Rapid separation of isolated hepatocytes or similar tissue fragments for analysis of cell constituents. Biochem J. 1975 Jul;150(1):47–50. doi: 10.1042/bj1500047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB M. I., BERNE R. M. Metabolism of purine derivatives by the isolated cat heart. Am J Physiol. 1960 Feb;198:322–326. doi: 10.1152/ajplegacy.1960.198.2.322. [DOI] [PubMed] [Google Scholar]
- KREBS H. A., HEMS R., GASCOYNE T. RENAL GLUCONEOGENESIS. IV. GLUCONEOGENESIS FROM SUBSTRATE COMBINATIONS. Acta Biol Med Ger. 1963;11:607–615. [PubMed] [Google Scholar]
- KREBS H. A., SPEAKE R. N., HEMS R. ACCELERATION OF RENAL GLUCONEOGENESIS BY KETONE BODIES AND FATTY ACIDS. Biochem J. 1965 Mar;94:712–720. doi: 10.1042/bj0940712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. S., Feinberg H. Incorporation of adenosine-8-14/C and inosine-8-14C into rabbit heart adenine nucleotides. Am J Physiol. 1971 May;220(5):1242–1248. doi: 10.1152/ajplegacy.1971.220.5.1242. [DOI] [PubMed] [Google Scholar]
- Maguire M. H., Lukas M. C., Rettie J. F. Adenine nucleotide salvage synthesis in the rat heart; pathways of adnosine salvage. Biochim Biophys Acta. 1972 Mar 14;262(2):108–115. doi: 10.1016/0005-2787(72)90223-7. [DOI] [PubMed] [Google Scholar]
- Struck E., Ashmore J., Wieland O. Stimulierung der Gluconeogenese durch langkettige Fettsäuren und Glucagon. Biochem Z. 1965 Nov 5;343(1):107–110. [PubMed] [Google Scholar]
- TSUBOI K. K., BUCKLEY N. M. METABOLISM OF PERFUSED C14-LABELED NUCLEOSIDES AND BASES BY THE ISOLATED HEART. Circ Res. 1965 Apr;16:343–352. doi: 10.1161/01.res.16.4.343. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkening J., Nowack J., Decker K. The dependence of glucose formation from lactate on the adenosine triphosphate content in the isolated perfused rat liver. Biochim Biophys Acta. 1975 Jun 12;392(2):299–309. doi: 10.1016/0304-4165(75)90011-2. [DOI] [PubMed] [Google Scholar]


