Abstract
1. The apparent Km values for succinate uptake by whole cells of Escherichia coli K12 depend on pH in the range 6.5-7.4.2. Uptake of succinate in lightly buffered medium is accompanied by proton uptake. 3. The apparent Km values for succinate uptake and for succinate-induced proton uptake are similar. 4. Approximately two protons enter the cell with each succinate molecule. 5. The pattern of inhibition of succinate uptake is similar to that of succinate-induced proton uptake. 6. Uptake of fumarate and malate, which share the succinate-transport system, is also accompanied by the uptake of approximately two protons per molecule of fumarate or malate. 7. Uptake of aspartate by the dicarboxylic acid-transport system is accompanied by the uptake of approximatley two protons per molecule of asparatate. 8. It is concluded that uptake of dicarboxylic acids by the dicarboxylic acid-transport system is obligatorily coupled to proton uptake such that succinate, malate and fumarate are taken up in electroneutral form and asparate is taken up in cationic form. 9. These results are consistent with, though they do not definitely prove, the energization of succinate uptake of the deltapH.
Full text
PDF

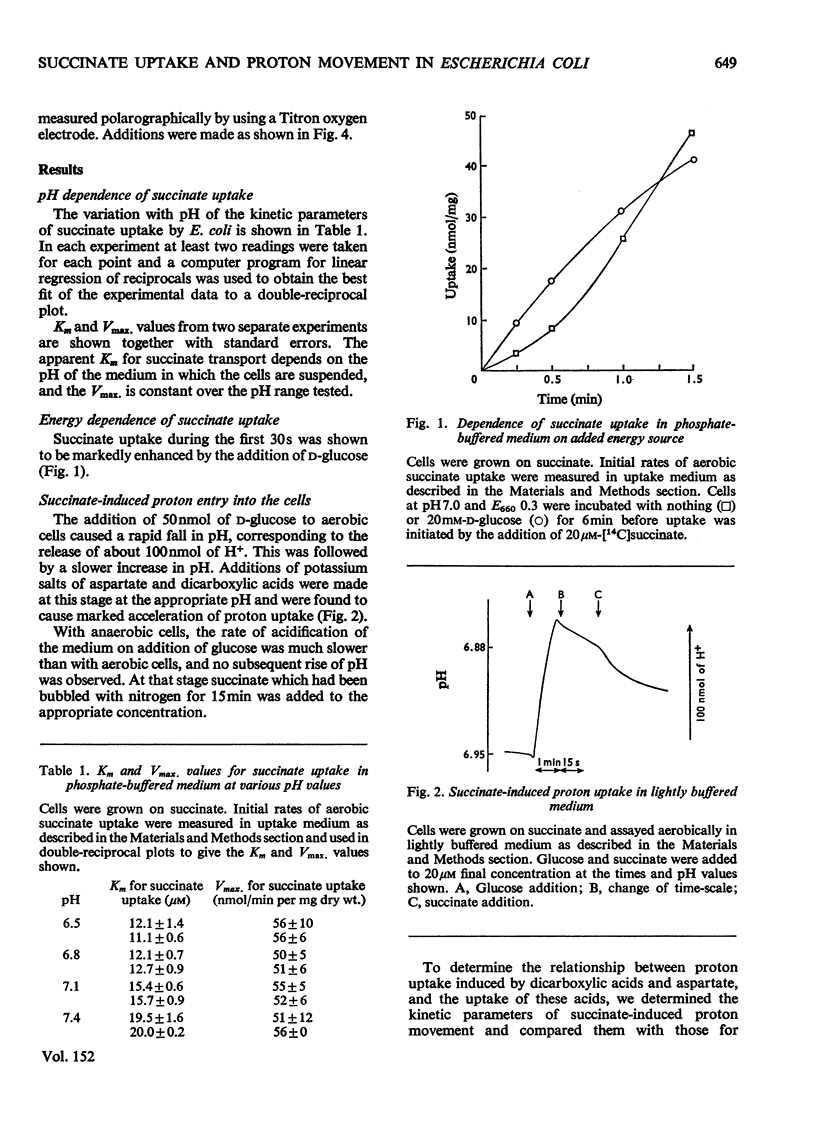
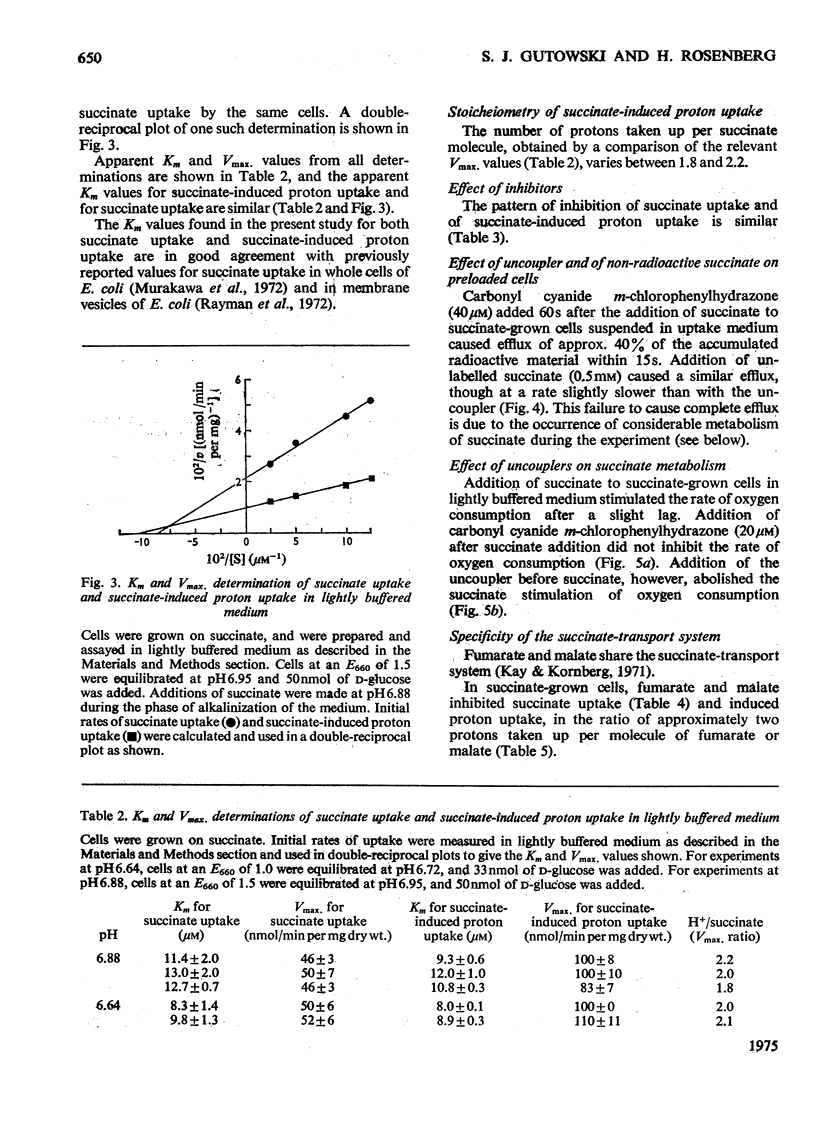
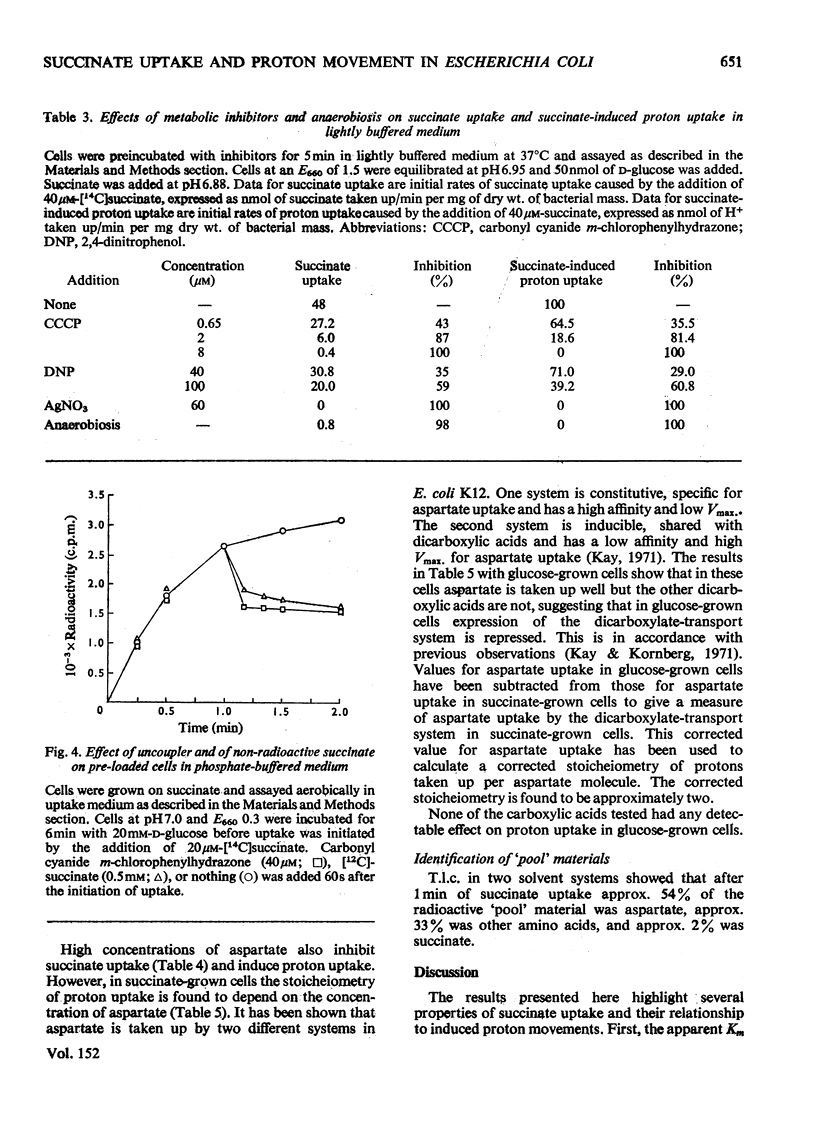
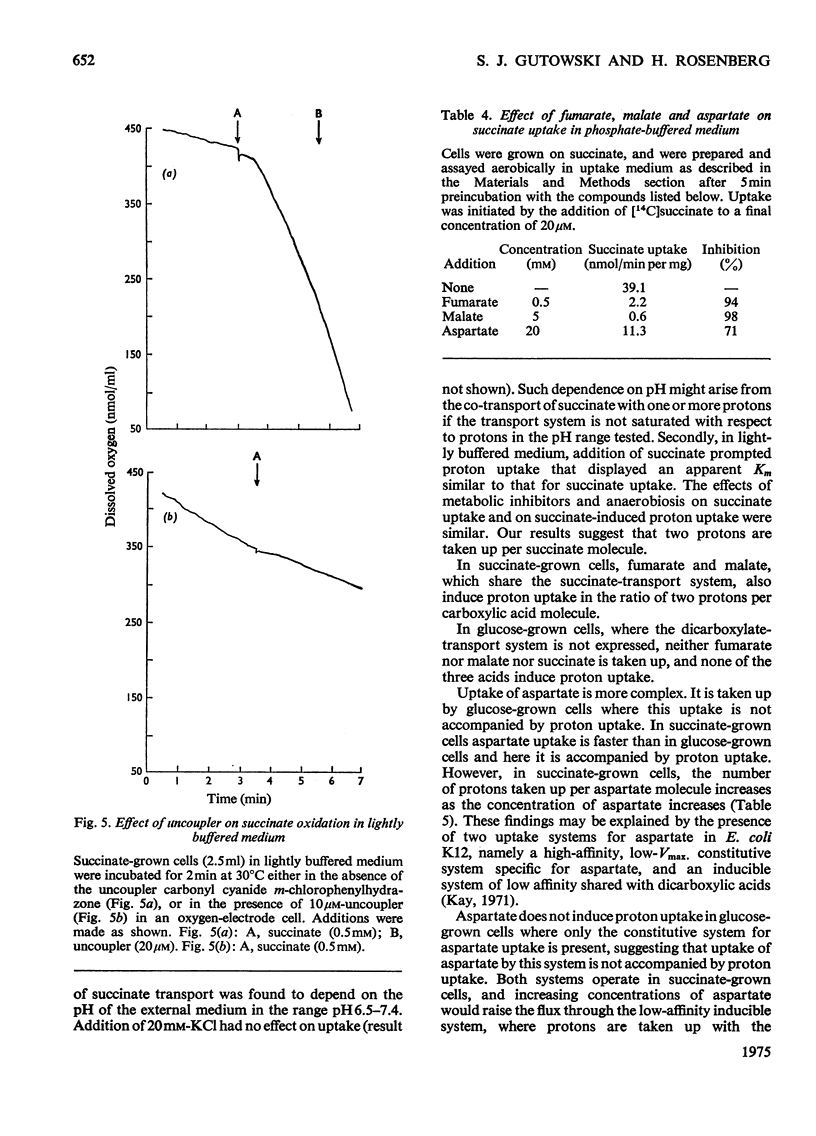

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K-12: the genetic and biochemical characterisations of a strain carrying a mutation in the uncB gene. Biochim Biophys Acta. 1973 Feb 22;292(2):366–375. doi: 10.1016/0005-2728(73)90043-1. [DOI] [PubMed] [Google Scholar]
- Harold F. M. Chemiosmotic interpretation of active transport in bacteria. Ann N Y Acad Sci. 1974 Feb 18;227:297–311. doi: 10.1111/j.1749-6632.1974.tb14395.x. [DOI] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Proton-coupled accumulation of galactoside in Streptococcus lactis 7962. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2866–2869. doi: 10.1073/pnas.70.10.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay W. W., Kornberg H. L. The uptake of C4-dicarboxylic acids by Escherichia coli. Eur J Biochem. 1971 Jan;18(2):274–281. doi: 10.1111/j.1432-1033.1971.tb01240.x. [DOI] [PubMed] [Google Scholar]
- Kay W. W. Two aspartate transport systems in Escherichia coli. J Biol Chem. 1971 Dec 10;246(23):7373–7382. [PubMed] [Google Scholar]
- Komor E., Tanner W. The hexose-proton symport system of Chlorella vulgaris. Specificity, stoichiometry and energetics of sugar-induced proton uptake. Eur J Biochem. 1974 May 2;44(1):219–223. doi: 10.1111/j.1432-1033.1974.tb03476.x. [DOI] [PubMed] [Google Scholar]
- Lawford H. G., Haddock B. A. Respiration-driven proton translocation in Escherichia coli. Biochem J. 1973 Sep;136(1):217–220. doi: 10.1042/bj1360217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo T. C., Rayman M. K., Sanwal B. D. Transport of succinate in Escherichia coli. I. Biochemical and genetic studies of transport in whole cells. J Biol Chem. 1972 Oct 10;247(19):6323–6331. [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G., COHN M. Sur la biosynthèse de la beta-galactosidase (lactase) chez Escherichia coli; la spécificité de l'induction. Biochim Biophys Acta. 1951 Nov;7(4):585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Translocations through natural membranes. Adv Enzymol Relat Areas Mol Biol. 1967;29:33–87. doi: 10.1002/9780470122747.ch2. [DOI] [PubMed] [Google Scholar]
- Myers W. F., Huang K. Y. Separation of intermediates of the citric acid cycle and related compounds by thin-layer chromatography. Anal Biochem. 1966 Nov;17(2):210–213. doi: 10.1016/0003-2697(66)90199-0. [DOI] [PubMed] [Google Scholar]
- Niven D. F., Hamilton W. A. Mechanisms of energy coupling to the transport of amino acids by Staphylococcus aureus. Eur J Biochem. 1974 May 15;44(2):517–522. doi: 10.1111/j.1432-1033.1974.tb03510.x. [DOI] [PubMed] [Google Scholar]
- Rayman M. K., Lo T. C., Sanwal B. D. Transport of succinate in Escherichia coli. II. Characteristics of uptake and energy coupling with transport in membrane preparations. J Biol Chem. 1972 Oct 10;247(19):6332–6339. [PubMed] [Google Scholar]
- Robin A., Kepes A. The mechanism of maintenance of electroneutrality during the transport of gluconate by E. coli. FEBS Lett. 1973 Oct 15;36(2):133–136. doi: 10.1016/0014-5793(73)80354-0. [DOI] [PubMed] [Google Scholar]
- Rosenberg H., Cox G. B., Butlin J. D., Gutowski S. J. Metabolite transport in mutants of Escherichia coli K12 defective in electron transport and coupled phosphorylation. Biochem J. 1975 Feb;146(2):417–423. doi: 10.1042/bj1460417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West I. C., Mitchell P. Stoicheiometry of lactose-H+ symport across the plasma membrane of Escherichia coli. Biochem J. 1973 Mar;132(3):587–592. doi: 10.1042/bj1320587. [DOI] [PMC free article] [PubMed] [Google Scholar]


