Abstract
Purpose
Previous studies have reported that infection-induced fever is associated with improved breast cancer prognosis, potentially through the modulation of cytokines. However, the key cytokines and the underlying mechanisms through which fever exerts its anti-tumor effects remain unclear.
Patients and Methods
A total of 794 breast cancer patients were recruited between 2008 and 2017, with follow-up extending until October 31st, 2023. Infection-induced fever was assessed using questionnaires, while a multiplex assay evaluated a panel of 27 cytokines. The mediation effects of various cytokines were analyzed through model-based causal mediation analysis. Additionally, we explored modifications to these mediation effect by examining interactions among the cytokines themselves as well as their interactions with infection-induced fever. Bioinformatic analyses were conducted to elucidate the biological pathways mediating infection-induced fever.
Results
The relationship between infection-induced fever and improved breast cancer prognosis was mediated by a decrease in interleukin-8 (IL-8) levels. Furthermore, our findings revealed that the downregulation of IL-8, which mediates the beneficial effects of fever, was antagonized by IL-2, IL12p70 and IL-7. By intersecting the biological pathways influenced by IL-8, alongside those affected by IL-2, IL12p70, or IL-7, we found that these latter cytokines antagonized the mediation effects of IL-8 via regulating critical pathways such as neutrophil degranulation, extracellular matrix organization and asparagine N-linked glycosylation.
Conclusion
Infection-induced fever may improve breast cancer prognosis through IL-8 downregulation and the mediation mechanisms may be involved in neutrophil degranulation, extracellular matrix organization and asparagine N-linked glycosylation. Such findings not only provide valuable insights into effectively managing febrile responses for breast cancer patients, but also underscore the therapeutic potential of cytokines in breast cancer patients.
Keywords: infection-induced fever, breast cancer, prognosis, cytokines, IL-8
Introduction
Cancer has emerged as a public health threat globally. It is estimated that there will be 2,001,140 new cancer cases and 611,720 cancer-related deaths in the United States in 2024.1 Breast cancer remains the leading cause of female cancers worldwide. Despite rapid advancements in therapeutic strategies, novel biomarkers and treatment regimens are still required to enhance treatment efficacy and overall prognosis for breast cancer patients.2
Since the emergence of coronavirus disease 2019 (COVID-19) as a global public health concern in 2019, fever has become one of the most prevalent clinical manifestations among vulnerable populations, such as breast cancer patients.3,4 The impact of fever on breast cancer prognosis has attracted considerable attention. Epidemiology studies have consistently demonstrated that infection-induced fever is associated with a reduced risk of breast cancer and improved breast cancer prognosis. Consistently observed phenomena include spontaneous regression of tumors following febrile responses induced by Coley’s toxin (heat-killed Streptococcus pyogenes and Serratia marcescens), vaccination therapy, or microorganism infection in clinical practice.5,6 This suggests an anti-tumor effect of fever attributed to immune system activation.6,7 Conversely, life-threatening complications arising from febrile infection and the following febrile neutropenia during cancer therapies may promote cancer progression and elevate patient mortality rates.8–10 Given these contradictory results, investigating the underlying mechanisms through which fever enhances breast cancer prognosis could illuminate strategies for effective fever management and prognostic improvement in this patient population.
Cytokine modulation represents a primary physiologic response to fever.11,12 Recent research has shown that cytokines play critical roles in oncogenesis exhibiting dual functions either suppressing or promoting tumor growth via regulating immune responses and cellular behavior.7,13–17 For instance, IL-8 has been widely recognized as a pro-tumor cytokine which operates through recruiting and activating myeloid cells, promoting angiogenesis and maintaining cancer stem cells.17,18 In contrast, IL-2, IL-7 and IL-12 have been reported to enhance anti-tumor immune responses mediated by T cells and natural killer (NK) cells.19 In the context of breast cancer, prior studies have indicated that the relationship between infection-induced fever and oncogenesis is influenced by interferon-γ (IFN-γ) polymorphism,20 while IL-6 polymorphism modify the association between fever and cancer prognosis.7 These findings highlight the critical role of cytokines in mediating the relationship between febrile responses and breast cancer progression. However, whether and which cytokines mediate the effects of infection-induced fever on breast cancer prognosis remain to be systematically evaluated. Given their pleiotropic nature, elucidating precise biological mechanisms linking infection-induced fever to breast cancer outcomes becomes complex even when specific mediators are identified. Leveraging the propensity of cytokines to interact with each other, we can uncover the mediation effects that can be modified and the biological pathways underlying these moderated mediation effects may elucidate the anti-tumor effects of infection-induced fever on breast cancer prognosis.
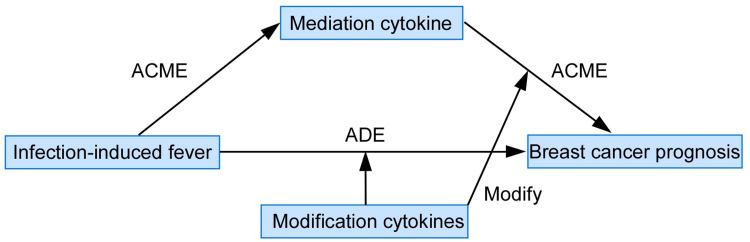
Utilizing data from Guangzhou Breast Cancer Study (GZBCS), we systematically investigated the mediation effects of 27 cytokines and identified that IL-8 downregulation mediated the anti-tumor effects of pre-diagnostic fever. Furthermore, our analysis revealed that the mediation effect of IL-8 was antagonized by IL-2, IL-7, and IL-12p70; additionally involving biological pathways related to neutrophil degranulation, extracellular matrix organization and asparagine N-linked glycosylation. These pathways likely represent key biological functions through which IL-8 mediates the associations between infection-induced fever and enhanced breast cancer prognosis (Figure 1). Based on these findings, combined levels of IL-8, IL-2, IL-7 and IL-12 may serve as a predictive biomarker for breast cancer outcomes in future studies. Correspondingly, recombinant IL-2, IL-7 or IL-12 and antagonists or monoclonal antibodies targeting IL-8 could potentially be utilized for patients either as monotherapy or in combination with other therapeutic modalities.19
Figure 1.
Model of moderated mediation effect evaluation elucidating the cytokine-mediated anti-tumor mechanisms of infection-induced fever on breast cancer prognosis.
Materials and Methods
Study Population and Serum Sample Collection
A total of 794 breast cancer patients who underwent serum cytokines testing were included in the current study. These patients were recruited from the First Affiliated Hospital of Sun Yat-sen University and Sun Yat-sen Memorial Hospital in Guangzhou, China, from 2008 to 2017. The inclusion and exclusion criteria were reported previously.21 Briefly, a total of 1775 patients with serum samples were included. We excluded patients for whom any of the following information was missing: clinical stage (N=243), estrogen receptor (ER) status (N=144), progesterone receptor (PR) status (N=147), human epidermal growth receptor 2 (HER2) status (N=207), and follow-up (N=108). The patients with poor serum quality were also excluded (N=204). All the serum samples were collected at the time of diagnosis before any treatment and stored at −80°C. Cytokines detection was performed on all the serum samples at the same time, which eliminates the potential batch effects. All the participants were informed with a written consent. This study was approved by the Ethics Committee of Sun Yat-Sen Memorial Hospital at Sun Yat-sen University.
Baseline Data Collection
Demographic information, including age at diagnosis and infection-induced fever history before breast cancer diagnosis were collected through a structural questionnaire by trained investigators as previously described.7 Medical records were used for collection of clinical and pathological characteristics like ER status, PR status, HER2 status, and clinical stage.
Follow-Up
The follow-up of breast cancer patients was conducted by telephone calls or outpatient visits at a 3-month interval in the first year, 6-month interval in the second and third year and annually thereafter as previously described.7 The median follow-up time was 85.91 months. The primary and secondary endpoints of this study were overall survival (OS) and progression-free survival (PFS), calculated as the time from diagnosis to death and from diagnosis to the date of progression (recurrence or distant metastasis) or death, respectively. Survival statuses were censored at the latest follow-up date or October 31, 2023.
Infection-Induced Fever Assessment
As previously described,7 pre-diagnostic fever was assessed by asking the patients to recall the average times of fever per year over the past 10 years at the time of breast cancer diagnosis, including influenza, common cold, abscess, bronchitis, pneumonia, and herpes simplex (no, <1, 1–2, 3–4, or ≥5 times). Given that 27.4, 5.9, 1.5, and 0.6% patients experienced less than 1, 1–2, 3–4, and more than 5 times of fever over the past 10 years, we combined these levels, yielding a binary variable of pre-diagnostic fever (no and yes). During the follow-up, post-diagnostic fever was assessed by asking the patients to recall the fever frequency after breast cancer diagnosis using the same questionnaire (no, <1, 1–2, 3–4, or ≥5 times). Post-diagnostic fever was also a binary variable (no and yes) because of the low proportion of patients experienced 1–2, 3–4, and more than 5 times of fever after diagnosis (6.8, 9.8, 0.6, and 1.5%, respectively). Since the post-diagnostic fever variable was available in only 62.2% participants, the post-diagnostic fever variable was only used in the sensitivity analysis.
Serological Cytokine Assay
The Bio-Plex Pro Human Cytokine 27-plex assay (Bio-Rad, M500KCAF0Y) was used to examine 27 cytokines from serum samples of 794 breast cancer patients according to the protocols of the manufacturer. Briefly, fluorescently dyed magnetic microspheres (beads) were first added to the 96-well microplate, followed by diluted samples, standards, blank and controls. After washing, the detection antibodies were added to capture the cytokines that w covalently coupled to the beads. Next, after a series of washes, a Streptavidin-PE detection antibody is added to form a sandwich complex. Lastly, assay buffer was added and the plate was read using the Luminex 200 platform (Luminex Corporation, Austin, TX, USA). The standard curves were constructed and the assay data was analyzed using Bio-Plex Manager 6.0 (Bio-Rad Laboratories). For cytokine values below the assay’s lower detection limit, values equal to half of the lower limit of detection were used. After log-transformation and standardization, the cytokine values were used for analyses.
Statistical Analysis
To identify the potential cytokines altered by infection-induced fever, unpaired Wilcoxon test was performed to compare the cytokine levels between patients with or without pre-diagnostic fever history. To exclude the potential reverse causation, we performed sensitivity analyses by evaluating the associations between cytokine levels and post-diagnostic fever.
Cox regression analysis was performed to assess the associations between pre-diagnostic fever and breast cancer prognosis and the associations between cytokines levels and breast cancer prognosis, respectively. The hazard ratio (HR) and 95% confidence interval (CI) were derived. The adjusted variables were detailed in the figure legends.
The model-based causal mediation analysis was performed with mediation R package to identify the cytokines with mediation effect.22 Briefly, we specified two statistical models, the mediator model for the conditional distribution of the mediator (cytokines) given the exposure (pre-diagnostic fever) and the outcome model for the conditional distribution of the outcome (breast cancer prognosis) given exposure (pre-diagnostic fever) and mediator (cytokines). These models are fitted separately and then their fitted objects comprise the main inputs to the mediation model, which estimates the Average Causal Mediation Effect (ACME) and Average Direct Effect (ADE). ACME and ADE quantified the effects of exposure (pre-diagnostic fever) on outcome (breast cancer prognosis) through mediator (cytokines) and the effects of exposure (pre-diagnostic fever) on outcome (breast cancer prognosis) without mediator (cytokines), respectively. To further quantify the magnitude of mediation, the percentage of the association mediated by cytokines was calculated by ACME/[ADE+ACME]. The model-based causal mediation analysis was performed on all the 27 cytokines.
The interactions between the cytokines with each other as well as the interactions between cytokines and pre-diagnostic fever were investigated, to explore the moderated mediation effect. Tests for multiplicative interactions were conducted using −2 log likelihood ratio test, which compared models with and without the interaction terms. By intersecting the biological pathways induced by the mediators and the modifiers, we might better reveal the exact mechanisms mediating the association of infection-induced fever and better breast cancer prognosis.
All statistical analysis was conducted by R software (version 4.1.2) and significance was assigned when p < 0.05.
Sensitivity Analysis
To mitigate the potential reverse causation, we conducted sensitivity analyses by assessing the relationship between baseline cytokines and post-diagnostic fever. We also conducted another sensitivity analyses by exploring the associations between pre-diagnostic fever frequency and baseline cytokines.
Bioinformatic Analysis
The gene expression profiles and clinical information of breast cancer patients from the TCGA database were downloaded using TCGABiolink R package. We applied single sample gene set enrichment analysis (ssGSEA) method to assess the enrichment of cytokines signaling as the surrogate of cytokines exposure. ssGSEA is a pathway enrichment method that can quantify the activation of given gene sets.23,24 The signatures used to estimate the cytokines signaling were derived from PathCards database (https://pathcards.genecards.org/).25
We applied Spearman correlation analysis to identify the genes that might be regulated by different cytokines exposure. Multiple hypothesis correction was performed and false discovery rate (FDR) less than 0.05 was significant. The genes co-regulated by the mediators and the cytokines modifying the mediation effects were identified. Reactome pathway enrichment analysis was performed to identify the biological pathways.
Results
Characteristics at Baseline and Their Associations with Breast Cancer Prognosis
As demonstrated in Table 1, over half of breast cancer patients (62.5%, n=496) at diagnosis were 50 ± 9 years old. Besides, most of the patients were ER-positive (75.6%, n=600), PR-positive (66.1%, n=525), HER2-negative (58.1%, n=461), lymph node-negative (55.9%, n=442) and diagnosed with early clinical stage (stage I/II: 78.9%, n=626) (Table 1). During the follow-up time (85.91 months), 148 patients experienced cancer progression and 84 died in total (Table 1). Age over 60 (HR=2.07, 95% CI: 1.02–4.19 for OS), lymph node-positive (HR=2.52, 95% CI: 1.60–3.96 for OS; HR=2.01, 95% CI=1.45–2.81 for PFS), clinical stage III (HR=6.22, 95% CI: 3.13–12.36 for OS; HR=5.69, 95% CI=3.38–9.56 for PFS) and clinical stage IV (HR=15.41, 95% CI: 6.93–34.29 for OS; HR=13.82, 95% CI=7.36–25.95 for PFS) were associated with worse breast cancer prognosis, while ER-positive and PR-positive decreased risk of progression (ER-positive: HR=0.49, 95% CI: 0.32–0.77 for OS and HR=0.55, 95% CI: 0.40–0.78 for PFS; PR-positive: HR=0.48, 95% CI: 0.31–0.73 for OS and HR=0.53, 95% CI: 0.39–0.74 for PFS) (Table 1).
Table 1.
Clinicopathologic Characteristics and the Association with Breast Cancer Prognosis
| Characteristics | Total (%) | OS | PFS | ||
|---|---|---|---|---|---|
| Events | HR (95% CI) | Events | HR (95% CI) | ||
| Age at diagnosis | |||||
| ≤40 | 185 (23.3) | 13 | 1.00 (reference) | 34 | 1.00 (reference) |
| 41–59 | 496 (62.5) | 52 | 1.35 (0.73, 2.47) | 84 | 0.81 (0.55, 1.21) |
| ≥60 | 113 (14.2) | 19 | 2.07 (1.02, 4.19) | 30 | 1.22 (0.75, 1.99) |
| ER status | |||||
| Negative | 194 (24.4) | 32 | 1.00 (reference) | 52 | 1.00 (reference) |
| Positive | 600 (75.6) | 52 | 0.49 (0.32, 0.77) | 96 | 0.55 (0.40, 0.78) |
| PR status | |||||
| Negative | 269 (33.9) | 41 | 1.00 (reference) | 68 | 1.00 (reference) |
| Positive | 525 (66.1) | 43 | 0.48 (0.31, 0.73) | 80 | 0.53 (0.39, 0.74) |
| HER2 status | |||||
| Negative | 461 (58.1) | 45 | 1.00 (reference) | 84 | 1.00 (reference) |
| Positive | 333 (41.9) | 39 | 1.52 (0.99, 2.34) | 64 | 1.31 (0.94, 1.81) |
| Lymph node status | |||||
| Negative | 442 (55.9) | 29 | 1.00 (reference) | 60 | 1.00 (reference) |
| Positive | 348 (44.1) | 53 | 2.52 (1.60, 3.96) | 86 | 2.01 (1.45, 2.81) |
| Clinical stage | |||||
| I | 234 (29.5) | 11 | 1.00 (reference) | 20 | 1.00 (reference) |
| II | 392 (49.4) | 27 | 1.53 (0.76, 3.08) | 58 | 1.84 (1.11, 3.06) |
| III | 132 (16.6) | 32 | 6.22 (3.13, 12.36) | 50 | 5.69 (3.38, 9.56) |
| IV | 36 (4.5) | 14 | 15.41 (6.93, 34.29) | 20 | 13.82 (7.36, 25.95) |
| Pre-diagnostic fever | |||||
| No | 446 (56.2) | 50 | 1.00 (reference) | 87 | 1.00 (reference) |
| Yes | 281 (35.4) | 24 | 0.60 (0.37, 0.97) | 45 | 0.66 (0.46, 0.95) |
| Not available | 67 (8.4) | 10 | 1.13 (0.57, 2.24) | 16 | 1.13 (0.66, 1.92) |
Notes: Bold character indicates statistically significant result. HR > 1 indicates that the factor is associated with worse prognosis, while HR values < 1 indicates that the factor is associated with better prognosis.
Abbreviations: OS, overall survival; PFS, progression-free survival; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; HR, hazard ratios; CI, confidence interval.
Consistent with our previous research,20 patients with pre-diagnostic fever history exhibited a remarkably decreased risk of progression when compared with those who had no fever history before (HR=0.60, 95% CI: 0.37–0.97 for OS; HR= 0.66, 95% CI: 0.46–0.95 for PFS; Table 1), after adjusting for potential prognostic factors of breast cancer.
Association Between Pre-Diagnostic Fever and Cytokines
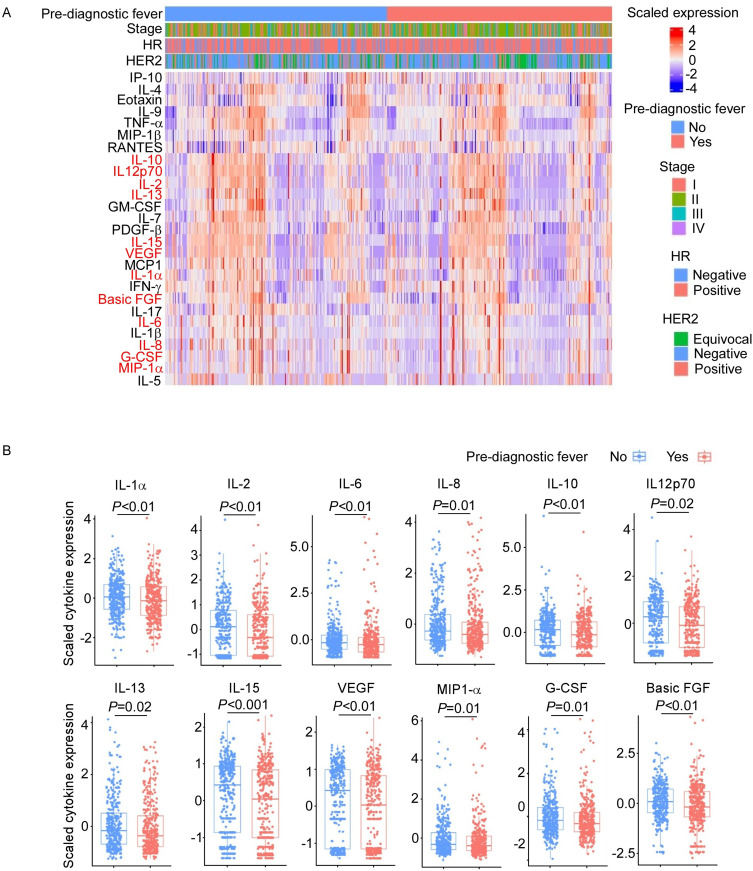
To explore the cytokines that might be altered by infection-induced fever, we examined the values of 27 cytokines at the time of diagnosis. As shown in Figure 2, several cytokines were significantly lower in patients with pre-diagnostic fever history, including IL-1α, IL-2, IL-6, IL-8, IL-10, IL12p70, IL-13, IL-15, VEGF, MIP1-α, G-CSF and basic FGF. None of the cytokines exhibited elevated levels in patients with pre-diagnostic fever history.
Figure 2.
The downregulated cytokine expression in breast cancer patients with or without pre-diagnostic fever history. (A) Heatmap showing cytokine expression in breast cancer patients. Red font shows cytokines that are significantly downregulated in breast cancer patients with fever history. (B) The significantly downregulated genes in patients with pre-diagnostic fever history.
To mitigate the potential reverse causation, we conducted sensitivity analyses by assessing the relationship between baseline cytokines and post-diagnostic fever. As shown in Supplementary Table 1, the concentrations of IL-1α, IL-2, IL-6, IL-8, IL12p70, IL-15, VEGF, MIP-1α and G-CSF did not show significant associations with post-diagnostic fever. This indicates that these cytokines are likely regulated by pre-diagnostic fever rather than serving as markers for susceptibility to infection-induced febrile responses. We also conducted another sensitivity analyses by exploring the associations between pre-diagnostic fever frequency and baseline cytokines. As shown in Supplementary Table 2, we found that the higher frequency of fever was associated with lower IL-6, IL-1α, IL-2, IL-1β, Basic FGF, VEGF, PDGF-β, IL-13, MCP1, IL-8, MIP-1α, IL-10, G-CSF, IL-15, IL-7 and IL12p70, further supporting that the changes in these cytokines were likely caused by pre-diagnostic fever.
Taken together, our results suggested that IL-1α, IL-2, IL-6, IL-8, IL12p70, IL-15, VEGF, MIP-1α and G-CSF might be altered by the infection-induced fever.
Associations Between Cytokines and Breast Cancer Prognosis
As shown in Table 2, univariate analyses revealed a significant association between IL-8 levels and shorter OS (HR=2.06, 95% CI: 1.32–3.21) as well as PFS (HR=1.84, 95% CI: 1.32–2.57), while lower level of MIP-1α was only significantly associated with shorter PFS (HR=1.44, 95% CI: 1.04–2.00). After adjusting for potential confounding factors, the result of multivariate Cox regression analyses further verified that IL-8 was associated with worse breast cancer prognosis (HR=1.83, 95% CI: 1.17–2.87 for OS; HR=1.70, 95% CI: 1.22–2.38 for PFS).
Table 2.
Univariate and Multivariate Cox Regression for Scaled Cytokine Expression with Breast Cancer OS and PFS
| Cytokines | OS | PFS | ||
|---|---|---|---|---|
| HR (95% CI)a | HR (95% CI)b | HR (95% CI) a | HR (95% CI)b | |
| MIP-1β | 0.87 (0.57, 1.34) | 0.89 (0.57, 1.37) | 0.95 (0.69, 1.31) | 0.99 (0.72, 1.37) |
| IL-6 | 1.21 (0.79, 1.86) | 1.20 (0.78, 1.86) | 1.14 (0.82, 1.58) | 1.12 (0.81, 1.54) |
| IFN-γ | 0.94 (0.61, 1.44) | 0.92 (0.60, 1.41) | 0.93 (0.68, 1.29) | 0.92 (0.67, 1.27) |
| IL-1α | 1.42 (0.92, 2.19) | 1.37 (0.89, 2.12) | 1.22 (0.88, 1.68) | 1.21 (0.87, 1.67) |
| IL-5 | 0.81 (0.53, 1.26) | 0.83 (0.54, 1.30) | 0.83 (0.60, 1.15) | 0.88 (0.64, 1.23) |
| GM-CSF | 1.03 (0.67, 1.58) | 1.12 (0.73, 1.73) | 0.94 (0.68, 1.30) | 1.02 (0.73, 1.41) |
| TNF-α | 0.97 (0.63, 1.49) | 0.96 (0.62, 1.48) | 1.34 (0.97, 1.86) | 1.36 (0.98, 1.89) |
| RANTES | 0.95 (0.62, 1.46) | 0.91 (0.59, 1.40) | 0.87 (0.63, 1.21) | 0.86 (0.62, 1.20) |
| IL-2 | 0.98 (0.64, 1.51) | 1.03 (0.67, 1.59) | 0.83 (0.60, 1.15) | 0.88 (0.64, 1.22) |
| IL-1β | 1.29 (0.84, 1.99) | 1.31 (0.85, 2.02) | 1.24 (0.90, 1.72) | 1.22 (0.88, 1.69) |
| Eotaxin | 1.11 (0.72, 1.70) | 1.05 (0.68, 1.62) | 0.92 (0.67, 1.28) | 0.84 (0.60, 1.16) |
| Basic FGF | 1.03 (0.67, 1.58) | 1.12 (0.72, 1.73) | 0.95 (0.69, 1.32) | 0.99 (0.71, 1.37) |
| VEGF | 1.05 (0.68, 1.61) | 1.19 (0.77, 1.84) | 0.89 (0.65, 1.24) | 0.97 (0.70, 1.34) |
| PDGF-β | 0.95 (0.62, 1.46) | 1.01 (0.66, 1.56) | 0.96 (0.70, 1.33) | 1.04 (0.75, 1.44) |
| IP-10 | 1.51 (0.98, 2.32) | 1.28 (0.83, 1.98) | 1.23 (0.89, 1.69) | 1.05 (0.76, 1.46) |
| IL-13 | 1.03 (0.67, 1.59) | 1.00 (0.65, 1.55) | 1.07 (0.77, 1.48) | 1.08 (0.78, 1.50) |
| IL-4 | 1.16 (0.75, 1.78) | 1.11 (0.72, 1.71) | 1.12 (0.81, 1.55) | 1.10 (0.80, 1.52) |
| MCP1 | 1.14 (0.74, 1.75) | 1.08 (0.70, 1.67) | 1.01 (0.73, 1.39) | 0.98 (0.71, 1.36) |
| IL-8 | 2.06 (1.32, 3.21) | 1.83 (1.17, 2.87) | 1.84 (1.32, 2.57) | 1.70 (1.22, 2.38) |
| MIP-1α | 1.47 (0.95, 2.26) | 1.29 (0.83, 1.99) | 1.44 (1.04, 2.00) | 1.32 (0.95, 1.83) |
| IL-10 | 1.24 (0.81, 1.92) | 1.43 (0.92, 2.21) | 1.15 (0.83, 1.59) | 1.30 (0.94, 1.80) |
| G-CSF | 1.38 (0.90, 2.12) | 1.04 (0.67, 1.61) | 1.31 (0.95, 1.81) | 1.05 (0.75, 1.46) |
| IL-15 | 1.13 (0.73, 1.74) | 1.31 (0.85, 2.03) | 0.88 (0.63, 1.21) | 0.97 (0.70, 1.35) |
| IL-7 | 0.99 (0.64, 1.52) | 0.94 (0.61, 1.45) | 1.02 (0.73, 1.40) | 0.97 (0.70, 1.34) |
| IL12p70 | 0.84 (0.55, 1.30) | 0.90 (0.59, 1.39) | 0.82 (0.59, 1.14) | 0.91 (0.66, 1.26) |
| IL-17 | 0.99 (0.64, 1.52) | 0.93 (0.61, 1.43) | 0.92 (0.67, 1.28) | 0.87 (0.63, 1.20) |
| IL-9 | 0.81 (0.53, 1.24) | 0.88 (0.57, 1.36) | 0.97 (0.70, 1.34) | 1.03 (0.75, 1.43) |
Notes: Bold character indicates statistically significant result. aUnivariate Cox Regression; bMultivariate Cox Regression.
Mediation Effects of IL-8 on the Association Between Pre-Diagnostic Fever and Breast Cancer Prognosis
We next performed mediation analysis on all the 27 cytokines to identify the potential ones that might mediate the effect of pre-diagnostic fever on breast cancer prognosis. As shown in Table 3, Supplementary Table 3 and Supplementary Table 4, the ACME of IL-8 was statistically significant for PFS (p=0.036) and was marginally significant for OS (p=0.052). The ADE of IL-8 was not significant for PFS (p=0.67) and OS (p=0.71). This result suggested that IL-8 decrease might mainly mediate the anti-tumor effect of fever on breast cancer prognosis. The proportions of mediation by IL-8 in the anti-tumor effect of fever were found to be 45.82% for OS and 39.76% for PFS, respectively (Table 3).
Table 3.
Mediation Analysis of IL-8 Expression Between Pre-Diagnostic Fever and Breast Cancer Prognosisa
| Cytokines | ACMEb | ADEc | Percentage (%)d | ||
|---|---|---|---|---|---|
| β | p | β | p | ||
| OS | |||||
| IL-8 | 30.90 | 0.052 | 36.53 | 0.71 | 45.82 |
| PFS | |||||
| IL-8 | 21.27 | 0.036 | 32.23 | 0.67 | 39.76 |
Notes: Bold character indicates statistically significant result. aThe analysis was adjusted for HR, HER2, and clinical stage. bACME, average causal mediation effect. cADE, average direct effect. dPercentage describes the proportion of the effect of fever on the prognosis mediated by IL8; Percentage=ACME/[ADE + ACME].
Modification of IL12p70, IL-2 and IL-7 on the Mediation Effects
Although we found that IL-8 reduction mediated the anti-tumor effect of pre-diagnostic fever on breast cancer prognosis, the underlying mechanism was still unclear because of the pleiotropy of cytokines. By exploring the mediation effect of IL-8 that can be modified, we can better uncover the mediation mechanisms of IL-8. As shown in Table 4, IL-8 was significantly associated with breast cancer progression only among the patients with low levels of IL12p70 (HR=1.42, 95% CI: 1.13–1.77), IL-2 (HR=1.62, 95% CI: 1.17–2.24) or IL-7 (HR=1.32, 95% CI: 1.05–1.65). In contrast, the association of IL-8 with breast cancer progression was not statistically significant in patients with high levels of IL12p70, IL-2 or IL-7 (all p values>0.05). The interactions between IL-8 and IL12p70, IL-2, or IL-7 were significant, with pinteraction of 0.014, 0.033, 0.048, respectively.
Table 4.
Modification Analysis of Cytokines Between Pre-Diagnostic Fever/IL-8 and Prognosis
| Cytokines | Pre-Diagnostic Fever | IL-8 | ||
|---|---|---|---|---|
| HR (95% CI) | P for Interaction | HR (95% CI) | P for Interaction | |
| IL12p70 | 0.026 | 0.014 | ||
| <median | 1.22 (0.72, 2.05) | 1.42 (1.13, 1.77) | ||
| >median | 0.55 (0.32, 0.93) | 0.94 (0.74, 1.19) | ||
| IL-2 | 0.042 | 0.033 | ||
| <median | 1.11 (0.66, 1.88) | 1.62 (1.17, 2.24) | ||
| >median | 0.53 (0.31, 0.91) | 1.03 (0.83, 1.27) | ||
| IL-7 | 0.062 | 0.048 | ||
| <median | 1.13 (0.66, 1.93) | 1.32 (1.05, 1.65) | ||
| >median | 0.60 (0.36, 1.01) | 0.93 (0.73, 1.19) | ||
Notes: Bold character indicates statistically significant result.
We also observed that IL12p70, IL-2 and IL-7 modified the association between pre-diagnostic fever and overall survival with pinteraction of 0.026, 0.042, 0.062, respectively. In consistence, pre-diagnostic fever was associated with favorable survival only among the patients with high IL12p70 (HR=0.55, 95% CI: 0.32–0.93), IL-2 (HR=0.53, 95% CI: 0.31–0.91) or IL-7 (HR=0.60, 95% CI: 0.36–1.01) levels.
Taken together, such results suggested that IL12p70, IL-2 and IL-7 might modify the mediation effects of IL-8 on the association between infection-induced fever and breast cancer prognosis. The biological functions of IL-8 which can be modified by IL12p70, IL-2, or IL-7 might be the critical mechanisms mediating the effects of infection-induced fever on breast cancer prognosis.
Identification of the Biological Functions of IL-8 Underlying the Mediation Effect
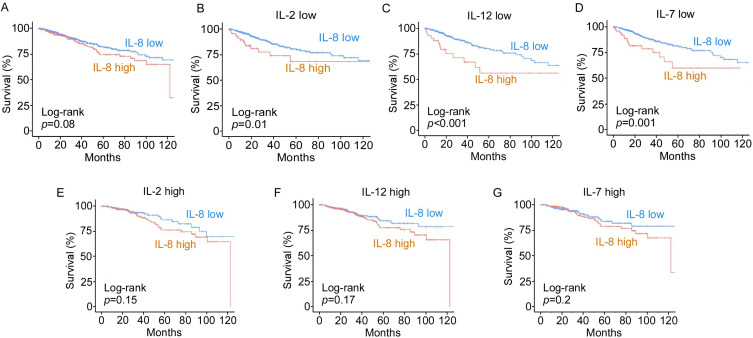
We next analyzed the transcriptomic data from the TCGA database to investigate biological functions of different cytokines on breast tumors. The enrichment of cytokine signaling was assessed as the surrogate of cytokines exposure. Consistent with our result from GZBCS mentioned earlier in this paper, breast cancer patients with low IL-8 signaling had better prognosis with marginal significance (p=0.08, Figure 3A). Moreover, the association between IL-8 signaling and breast cancer prognosis was more pronounced among the patients with low levels of IL-2 signaling (p=0.01, Figure 3B), IL-12 signaling (p<0.001, Figure 3C) or IL-7 signaling (p=0.001, Figure 3D). In contrast, the association between IL-8 and breast cancer prognosis did not reach statistical significance among patients with high levels of IL-2 signaling (p=0.15, Figure 3E), IL-12 signaling (p=0.17, Figure 3F) or IL-7 signaling (p=0.2, Figure 3G).
Figure 3.
The prognostic implications of IL-8 downregulation are modified by IL-2, IL-7 and IL-12 in the TCGA-BRCA dataset. (A) A Kaplan-Meier curve showing survival associated with IL-8 level in breast cancer patients. (B–G) Kaplan-Meier curves showing survival associated with IL-8 levels in breast cancer patients stratified by low or high levels of IL-2, IL-12 or IL-7, respectively.
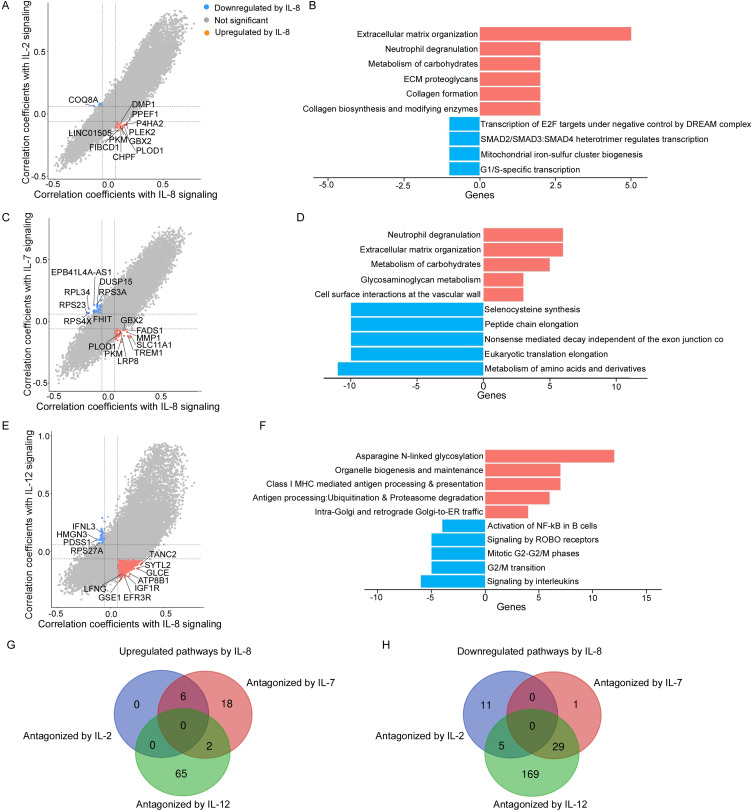
The biological functions of IL-8 exposure were revealed by the genes significantly correlated with IL-8 signaling. Among these genes, those regulated by IL-2, IL-7 or IL12p70 in opposite directions might elucidate how the mediation effect is modified and the enriched pathways should mediate the anti-tumor effects of infection-induced fever on breast cancer prognosis.26 We identified 24 genes that were upregulated by IL-8 and downregulated by IL-2. These genes were found to be enriched in 6 pathways, including extracellular matrix organization, neutrophil degranulation and ECM proteoglycans. We also found 8 genes downregulated by IL-8 while upregulated by IL-2 and they were related to 16 pathways including transcription of E2F targets under negative control by p107 (RBL1) and p130 (RBL2) in complex with HDAC1, SMAD2/SMAD3:SMAD4 heterotrimer regulates transcription, and mitochondrial iron-sulfur cluster biogenesis (Figure 4A and B; Supplementary Table 5).
Figure 4.
Bioinformatics analysis of the biological interaction between IL-8 and IL-2, IL-8 and IL-7 or IL-8 and IL-12. (A, C and E) Genes upregulated by IL-8 while downregulated by IL-2, IL-7 or IL-12 and genes downregulated by IL-8 while upregulated by IL-2, IL-7 or IL-12, respectively. (B, D and F) REACTOME pathways analysis of the antagonistic effects of IL-2, IL-7 or IL-12 on IL-8. (G and H) Venn diagram showing the overlapping upregulated (G) and downregulated (H) pathways by IL-8 while antagonized by IL-2, IL-7 and IL-12, respectively.
Similarly, we found 51 genes upregulated by IL-8 while downregulated by IL-7, which were enriched in 26 pathways including neutrophil degranulation, extracellular matrix organization and metabolism of carbohydrates. Besides, 46 genes downregulated by IL-8 while upregulated by IL-7 were revealed, and 30 related downregulated pathways were enriched including selenocysteine synthesis, peptide chain elongation and nonsense mediated decay independent of the exon junction complex (Figure 4C and D; Supplementary Table 6).
We also found that 1015 genes were upregulated by IL-8 while downregulated by IL-12p70. And these genes were enriched in 67 pathways including asparagine N-linked glycosylation, organelle biogenesis and maintenance and class I MHC mediated antigen processing and presentation. Similarly, 35 genes were downregulated by IL-8 while upregulated by IL-12p70 and they were enriched in 203 downregulated pathways including activation of NF-kappaB in B cells, signaling by ROBO receptors and mitotic G2-G2/M phases (Figure 4E and F; Supplementary Table 7).
We did not find any pathway that was upregulated by IL-8 and co-inhibited by IL-2, IL-12p70 and IL-7 (Figure 4G). The pathways that were downregulated by IL-8 and co-stimulated by IL-2, IL-12p70 and IL-7 were not found neither (Figure 4H).
Taking together, the key pathways that were upregulated by IL-8 while antagonized by IL-2, IL-7 or IL-12p70 were revealed, including neutrophil degranulation, extracellular matrix organization and asparagine N-linked glycosylation. These pathways have been reported to correlate with poor cancer prognosis,27–30 which provide a possible biological explanation for our results.
Discussion
The current study suggests that pre-diagnostic fever is associated with improved breast cancer prognosis through the downregulation of IL-8 levels. Furthermore, we found that IL-2, IL-12p70 and IL-7 antagonized the mediation effect of IL-8. The biological pathways underlying cytokine interaction include neutrophil degranulation, extracellular matrix organization and asparagine N-linked glycosylation, which may represent critical mechanisms by which infection-induced fever exerts anti-tumor effects on breast cancer prognosis.
Previous investigations have reported a beneficial role for infection-induced fever in improving cancer patient prognosis,7,31,32 with cytokines potentially playing significant roles.7 It has been hypothesized that infection-induced fever may improve breast cancer prognosis by inducing the production and release of pro-inflammatory cytokines to activate innate and adaptive immunity.33 However, our study did not find evidence supporting cytokine upregulation or immune activation in patients with a history of pre-diagnostic fever. This discrepancy may be attributed to prior studies measuring single nucleotide polymorphism of cytokines rather than actual cytokine levels.7,20 In contrast, we observed significantly lower levels of several inflammatory cytokines in patients with fever history, including IL-234,35 and IL-6.36 These findings suggest that evaluating actual cytokine concentrations can provide new insights into the biological mechanisms mediating the anti-tumor effects associated with infection-induced fever.
By systematically assessing the mediation effect among 27 cytokines, we determined that infection-induced fever might enhance breast cancer prognosis through decreased levels of IL-8. This result is robust; sensitivity analyses indicated no association between baseline IL-8 levels and post-diagnostic fevers—thus excluding potential reverse causation where low IL-8 could increase susceptibility to febrile responses. Additionally, exploratory analyses showed that higher pre-diagnostic fever frequency was associated with lower IL-8 level, indicating that IL-8 changes was possibly changed by fever. We also demonstrated an association between reduced IL-8 signaling and improved breast cancer outcomes within TCGA database analyses. Such results supported our hypothesis that infection-induced fever improves the breast cancer prognosis via downregulating IL-8 levels.
Cytokines have been closely associated with cancer prognosis due to their extensive communication with the host immune system and tumor microenvironment.19 The elevated level of IL-8 has been notably recognized as an unfavorable prognostic indicator in various cancers, which critically induces neutrophil migration,37 degranulation38 and neutrophil extracellular traps (NETs) formation.18 Specifically within breast cancer contexts, it has been shown that increased expression of IL-8 promotes disease progression by facilitating enhanced invasion capabilities among malignant cells alongside stem cell formation while also contributing to angiogenesis and distant metastasis development.39,40 These results are consistent with our study. Furthermore, in clinical practice, other medical treatments may also influence breast cancer outcomes through modulating cytokines, such as anesthetics used during cancer surgery.41–43 Together, these studies emphasize the importance of cytokine-targeted therapies in improving cancer outcome.
The pleiotropic nature of IL-839,44 complicates elucidation regarding precise mechanisms through which it mediates anti-tumor effects stemming from infection-related febrile responses. To further address this issue, we explored the moderated mediation effect of IL-8. The biological functions of IL-8 that are modified by these cytokines should be the pivotal mechanisms underlying the anti-tumor effect of infection-induced fever. The rational was that certain biological mechanisms of IL-8 mediated the effects of infection-induced fever on breast cancer prognosis, and the cytokines modifying the mediation effects should influenced these mechanisms as well. Therefore, the biological pathways co-regulated by IL-8 and the cytokines modifying the mediation effects should be the pivotal mechanisms underlying the anti-tumor effects of infection-induced fever.
Our findings indicate that IL-2, IL-12p70 and IL-7 antagonize the mediation effects of IL-8, with biological pathways such as neutrophil degranulation, extracellular matrix organization and asparagine N-linked glycosylation being implicated. Consistent with prior studies, neutrophil degranulation emerges as a principal biological function of IL-8 that contributes to poor cancer prognosis.27 Notably, several granule proteins are released into the extracellular milieu during neutrophil degranulation, which accelerates tumor progression by promoting angiogenesis or creating an immunosuppressive niche.27 These results support our strategy of investigating modifications in mediation effects to elucidate underlying mechanisms.
In addition to neutrophil degranulation, our data suggest that infection-induced fever may enhance breast cancer prognosis by downregulating IL-8 levels and mitigating its detrimental impacts on extracellular matrix organization and asparagine N-linked glycosylation. Specifically, IL-8 stimulates the secretion of matrix metalloproteinases (MMPs) from neutrophils, subsequently leading to extracellular matrix remodeling and tumor progression.28 Asparagine N-linked glycosylation has also been reported to facilitate tumor progression through induction of proliferation, invasion and metastasis.29,30 However, the relationship between IL-8 and asparagine N-linked glycosylation remains unexplored; thus our findings provide preliminary evidence warranting further investigation into the adverse effects of IL-8 on this process.
Based on our study, serum levels of IL-8, IL-2, IL-7 and IL-12 could serve as a combined biomarker with high prognostic value, particularly in breast cancer patients with a pre-diagnostic infection-induced fever history. For instance, a high level of IL-8 accompanied by concurrent low levels of IL-2, IL-7 and IL-12 might predict a worse breast cancer outcome. Correspondingly, these patients are more likely to benefit from cytokine-based anti-cancer therapies, including the administration of recombinant IL-2, IL-7 or IL-12, and IL-8-targeted antagonists or monoclonal antibodies. However, in pre-clinical and clinical trials, cytokine-based monotherapies have demonstrated a relatively low response rate, a short half-life and a high incidence of severe adverse events, which restrict their wide application. In contrast, combining therapies with chemotherapy, radiotherapy, immune checkpoint inhibitors (ICIs) and chimeric antigen receptor (CAR) therapies offer unique advantages over monotherapies in terms of safety, tolerance and efficacy, making them a promising therapeutic modality for breast cancer treatment.19 In particular, it is promising to combine ICIs with IL-8-targeted therapy for more optimal activation of cytotoxic T and NK cells while inhibition of immunosuppressive myeloid cells, which helps overcome ICIs resistance and exploit the immune system.45,46
Some limitations should be acknowledged. First of all, infection-induced fever history was self-reported by breast cancer patients via questionnaire, which inevitably caused a recall bias. However, patients recruited in this prospective cohort study were unaware of the hypothesis, thus the results might get biased equally towards good or bad prognosis. Further studies should use alternative data collection methods like medical records to explore the association between infection-induced fever and breast cancer prognosis. Secondly, the factors that might influence cytokine levels were not fully considered in the current study, which might cause confounding bias and made the association between infection-induced fever and cytokines less convincing. However, these potential confounding factors might not substantially influence the cytokine levels and would cause non-differential bias, reducing the confounding roles to some extent. Furthermore, we have conducted sensitivity analyses to support that the cytokines changes were possibly caused by fever. Further studies considering more confounding factors and in vitro or in vivo studies were needed to support that infection-induced fever caused these cytokines changes. Thirdly, the sample size of the current study was not big enough, which limited the conduction of several stratification analyses. For example, the mediation effect of IL-8 on the association of fever frequency and breast cancer prognosis was not investigated. Also, the single cohort and single cancer design of the current study limited generalization of the results. Further studies with larger sample size, multi-center and multi-cancer design should be conducted to explore the relationships between fever, cytokines and cancer prognosis. Fourthly, the present study lacked measurements of cytokines levels throughout follow-up periods of breast cancer patients. And we might overlook the potential impact of cytokines that were not evaluated currently due to the limitation of the cytokine assay. The dynamic values and enlarged detection range of cytokines could provide a novel insight for exploring the mediation role of cytokines on the association between infection-induced fever and breast cancer progression. Last but not least, the bioinformatics analysis based on the mRNA sequencing data from TCGA database might be influenced by the discrepancies between mRNA and protein levels due to post-transcriptional and translational regulation.47 Therefore, our findings regarding the biological pathways require further validation at the protein level to enhance clinical reliability.
Conclusion
In conclusion, our study suggests that infection-induced fever improved breast cancer prognosis through decreasing IL-8 levels. We further demonstrate that the moderated mediation effect of IL-8 downregulation can be antagonized by IL-2, IL12p70 and IL-7, emphasizing the significance of cytokine interactions in tumor progression. Besides, we propose the critical biological pathways underlying cytokine modulation mechanism, including neutrophil degranulation, extracellular matrix organization and asparagine N-linked glycosylation. These results inspire us to develop a combined prognostic biomarker, as well as to develop novel cytokine-based therapies for breast cancer patients.
Funding Statement
This study was funded by China Postdoctoral Science Foundation (2024M763778) and GuangDong Basic and Applied Basic Research Foundation (2023A1515110943). The funders played no role in study design, data collection, analysis and interpretation of data, or the writing of this manuscript.
Abbreviations
ACME, average causal mediation effect; CAR, chimeric antigen receptor; CI, confidence interval; COVID-19, coronavirus disease 2019; ECM, extracellular matrix; ER, estrogen receptor; FDR, false discovery rate; FGF, fibroblast growth factor; G-CSF, Granulocyte Colony-Stimulating Factor; GZBCS, Guangzhou Breast Cancer Study; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; ICIs, immune checkpoint inhibitors; IL, interleukin; MIP1-α, macrophage Inflammatory Protein 1-alpha; MMP, metalloproteinase; NETs, neutrophil extracellular traps; OS, overall survival; PFS, progression-free survival; PR, progesterone receptor; TCGA, the Cancer Genome Atlas; VEGF, vascular Endothelial Growth Factor.
Data Sharing Statement
The dataset and code used and analyzed during the current study is available from the corresponding author Zhuozhi Liang (liangzhzh6@mail2.sysu.edu.cn) on reasonable request.
Ethics Approval and Consent to Participate
This study complies with the Declaration of Helsinki. This study was approved by the Ethics Committee of Sun Yat-Sen Memorial Hospital (No. SYSKY-2024-621-01).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
- 1.Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. doi: 10.3322/caac.21820 [DOI] [PubMed] [Google Scholar]
- 2.Sonkin D, Thomas A, Teicher BA. Cancer treatments: past, present, and future. Cancer Genet. 2024;286–287:18–24. doi: 10.1016/j.cancergen.2024.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Islam MA, Kundu S, Alam SS, Hossan T, Kamal MA, Hassan R. Prevalence and characteristics of fever in adult and paediatric patients with coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis of 17515 patients. PLoS One. 2021;16(4):e0249788. doi: 10.1371/journal.pone.0249788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooksley T, Font C, Scotte F, et al. Emerging challenges in the evaluation of fever in cancer patients at risk of febrile neutropenia in the era of COVID-19: a MASCC position paper. Support Care Cancer. 2021;29(2):1129–1138. doi: 10.1007/s00520-020-05906-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kucerova P, Cervinkova M. Spontaneous regression of tumour and the role of microbial infection--possibilities for cancer treatment. Anticancer Drugs. 2016;27(4):269–277. doi: 10.1097/CAD.0000000000000337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hobohm U. Fever and cancer in perspective. Cancer Immunol Immunother. 2001;50(8):391–396. doi: 10.1007/s002620100216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye H, Tang LY, Liang ZZ, et al. Effects of Infection-Induced Fever and the Interaction with IL6 rs1800796 Polymorphism on the Prognosis of Breast Cancer. Cancer Epidemiol Biomarkers Prev. 2022;31(11):2030–2037. doi: 10.1158/1055-9965.EPI-22-0498 [DOI] [PubMed] [Google Scholar]
- 8.Pizzo PA. Management of Patients With Fever and Neutropenia Through the Arc of Time: a Narrative Review. Ann Intern Med. 2019;170(6):389–397. doi: 10.7326/M18-3192 [DOI] [PubMed] [Google Scholar]
- 9.Yan T, Yin W, Zhou L, et al. Postoperative fever: the potential relationship with prognosis in node negative breast cancer patients. PLoS One. 2010;5(12):e15903. doi: 10.1371/journal.pone.0015903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaya T, Dilek A, Ozaras R, Balcik OS, Leblebicioglu H. COVID 19 and febrile neutropenia: case report and systematic review. Travel Med Infect Dis. 2022;47:102305. doi: 10.1016/j.tmaid.2022.102305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keitelman IA, Sabbione F, Shiromizu CM, et al. Short-Term Fever-Range Hyperthermia Accelerates NETosis and Reduces Pro-inflammatory Cytokine Secretion by Human Neutrophils. Front Immunol. 2019;10:2374. doi: 10.3389/fimmu.2019.02374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mota CMD, Madden CJ. Neural circuits mediating circulating interleukin-1beta-evoked fever in the absence of prostaglandin E2 production. Brain Behav Immun. 2022;103:109–121. doi: 10.1016/j.bbi.2022.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chao Y, Wu CY, Kuo CY, et al. Cytokines are associated with postembolization fever and survival in hepatocellular carcinoma patients receiving transcatheter arterial chemoembolization. Hepatol Int. 2013;7(3):883–892. doi: 10.1007/s12072-012-9409-9 [DOI] [PubMed] [Google Scholar]
- 14.Rook GA, Dalgleish A. Infection, immunoregulation, and cancer. Immunol Rev. 2011;240(1):141–159. doi: 10.1111/j.1600-065X.2010.00987.x [DOI] [PubMed] [Google Scholar]
- 15.Yi M, Li T, Niu M, et al. Targeting cytokine and chemokine signaling pathways for cancer therapy. Signal Transduct Target Ther. 2024;9(1):176. doi: 10.1038/s41392-024-01868-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Habanjar O, Bingula R, Decombat C, Diab-Assaf M, Caldefie-Chezet F, Delort L. Crosstalk of Inflammatory Cytokines within the Breast Tumor Microenvironment. Int J Mol Sci. 2023;24(4). doi: 10.3390/ijms24044002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah D, Soper B, Shopland L. Cytokine release syndrome and cancer immunotherapies - historical challenges and promising futures. Front Immunol. 2023;14:1190379. doi: 10.3389/fimmu.2023.1190379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teijeira A, Garasa S, Ochoa MC, et al. IL8, Neutrophils, and NETs in a Collusion against Cancer Immunity and Immunotherapy. Clin Cancer Res. 2021;27(9):2383–2393. doi: 10.1158/1078-0432.CCR-20-1319 [DOI] [PubMed] [Google Scholar]
- 19.Propper DJ, Balkwill FR. Harnessing cytokines and chemokines for cancer therapy. Nat Rev Clin Oncol. 2022;19(4):237–253. doi: 10.1038/s41571-021-00588-9 [DOI] [PubMed] [Google Scholar]
- 20.Su Y, Tang LY, Chen LJ, et al. Joint effects of febrile acute infection and an interferon-gamma polymorphism on breast cancer risk. PLoS One. 2012;7(5):e37275. doi: 10.1371/journal.pone.0037275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye HM, Lu MJ, Liu Q, Lin Y, Tang LY, Ren ZF. Beneficial Effect of Toxoplasma gondii Infection on the Prognosis of Breast Cancer Was Modified by Cytokines. Clin Epidemiol. 2023;15:469–481. doi: 10.2147/CLEP.S408182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tofighi D, MacKinnon DP. RMediation: an R package for mediation analysis confidence intervals. Behav Res Methods. 2011;43(3):692–700. doi: 10.3758/s13428-011-0076-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinf. 2013;14(1):7. doi: 10.1186/1471-2105-14-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bagaev A, Kotlov N, Nomie K, et al. Conserved pan-cancer microenvironment subtypes predict response to immunotherapy. Cancer Cell. 2021;39(6):845–865e847. doi: 10.1016/j.ccell.2021.04.014 [DOI] [PubMed] [Google Scholar]
- 25.Belinky F, Nativ N, Stelzer G, et al. PathCards: multi-source consolidation of human biological pathways. Database. 2015;2015:bav006. doi: 10.1093/database/bav006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang ZZ, Zhang YX, Zhu RM, et al. Identification of epigenetic modifications mediating the antagonistic effect of selenium against cadmium-induced breast carcinogenesis. Environ Sci Pollut Res Int. 2022;29(15):22056–22068. doi: 10.1007/s11356-021-17355-z [DOI] [PubMed] [Google Scholar]
- 27.Mollinedo F. Neutrophil Degranulation, Plasticity, and Cancer Metastasis. Trends Immunol. 2019;40(3):228–242. doi: 10.1016/j.it.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 28.Verma D, Zanetti C, Godavarthy PS, et al. Bone marrow niche-derived extracellular matrix-degrading enzymes influence the progression of B-cell acute lymphoblastic leukemia. Leukemia. 2020;34(6):1540–1552. doi: 10.1038/s41375-019-0674-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radovani B, Gudelj I. N-Glycosylation and Inflammation; the Not-So-Sweet Relation. Front Immunol. 2022;13:893365. doi: 10.3389/fimmu.2022.893365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ugonotti J, Chatterjee S, Thaysen-Andersen M. Structural and functional diversity of neutrophil glycosylation in innate immunity and related disorders. Mol Aspects Med. 2021;79:100882. doi: 10.1016/j.mam.2020.100882 [DOI] [PubMed] [Google Scholar]
- 31.Jeys LM, Grimer RJ, Carter SR, Tillman RM, Abudu A. Post operative infection and increased survival in osteosarcoma patients: are they associated? Ann Surg Oncol. 2007;14(10):2887–2895. doi: 10.1245/s10434-007-9483-8 [DOI] [PubMed] [Google Scholar]
- 32.De Bonis P, Albanese A, Lofrese G, et al. Postoperative infection may influence survival in patients with glioblastoma: simply a myth? Neurosurgery. 2011;69(4):864–868. doi: 10.1227/NEU.0b013e318222adfa [DOI] [PubMed] [Google Scholar]
- 33.Tong X, Zhan T, Dong X, Xu D. Fever of unknown origin associated with immune checkpoint inhibitors. Front Immunol. 2024;15:1364128. doi: 10.3389/fimmu.2024.1364128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muhammad S, Fan T, Hai Y, Gao Y, He J. Reigniting hope in cancer treatment: the promise and pitfalls of IL-2 and IL-2R targeting strategies. Mol Cancer. 2023;22(1):121. doi: 10.1186/s12943-023-01826-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bae J, Liu L, Moore C, et al. IL-2 delivery by engineered mesenchymal stem cells re-invigorates CD8(+) T cells to overcome immunotherapy resistance in cancer. Nat Cell Biol. 2022;24(12):1754–1765. doi: 10.1038/s41556-022-01024-5 [DOI] [PubMed] [Google Scholar]
- 36.Orange ST, Leslie J, Ross M, Mann DA, Wackerhage H. The exercise IL-6 enigma in cancer. Trends Endocrinol Metab. 2023;34(11):749–763. doi: 10.1016/j.tem.2023.08.001 [DOI] [PubMed] [Google Scholar]
- 37.Schalper KA, Carleton M, Zhou M, et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med. 2020;26(5):688–692. doi: 10.1038/s41591-020-0856-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84(4):1045–1049. doi: 10.1172/JCI114265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh JK, Simoes BM, Howell SJ, Farnie G, Clarke RB. Recent advances reveal IL-8 signaling as a potential key to targeting breast cancer stem cells. Breast Cancer Res. 2013;15(4):210. doi: 10.1186/bcr3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen WT, Ebelt ND, Stracker TH, Xhemalce B, Van Den Berg CL, Miller KM. ATM regulation of IL-8 links oxidative stress to cancer cell migration and invasion. Elife. 2015;4. doi: 10.7554/eLife.07270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu H, Dilger JP, Lin J. Effects of local anesthetics on cancer cells. Pharmacol Ther. 2020;212:107558. doi: 10.1016/j.pharmthera.2020.107558 [DOI] [PubMed] [Google Scholar]
- 42.Li R, Liu H, Dilger JP, Lin J. Effect of Propofol on breast Cancer cell, the immune system, and patient outcome. BMC Anesthesiol. 2018;18(1):77. doi: 10.1186/s12871-018-0543-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li R, Mukherjee MB, Jin Z, et al. The Potential Effect of General Anesthetics in Cancer Surgery: meta-Analysis of Postoperative Metastasis and Inflammatory Cytokines. Cancers. 2023;15(10):2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vilotic A, Nacka-Aleksic M, Pirkovic A, Bojic-Trbojevic Z, Dekanski D, Jovanovic Krivokuca M. IL-6 and IL-8: an Overview of Their Roles in Healthy and Pathological Pregnancies. Int J Mol Sci. 2022;23(23). doi: 10.3390/ijms232314574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu H, Dong A, Rasteh AM, Wang P, Weng J. Identification of the novel exhausted T cell CD8 + markers in breast cancer. Sci Rep. 2024;14(1):19142. doi: 10.1038/s41598-024-70184-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong RS, Ong RJ, Lim JS. Immune checkpoint inhibitors in breast cancer: development, mechanisms of resistance and potential management strategies. Cancer Drug Resist. 2023;6(4):768–787. doi: 10.20517/cdr.2023.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu H, Guo Z, Wang P. Genetic expression in cancer research: challenges and complexity. Gene Rep. 2024;37:102042. doi: 10.1016/j.genrep.2024.102042 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset and code used and analyzed during the current study is available from the corresponding author Zhuozhi Liang (liangzhzh6@mail2.sysu.edu.cn) on reasonable request.