Abstract
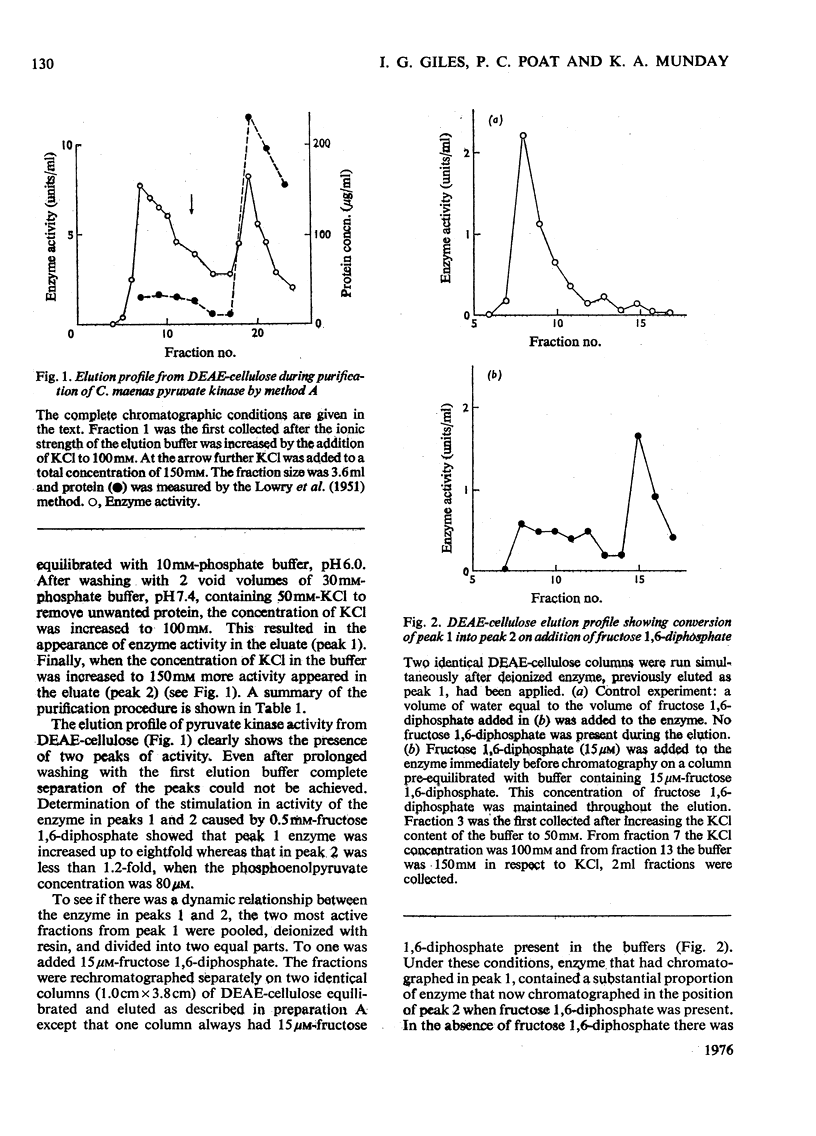
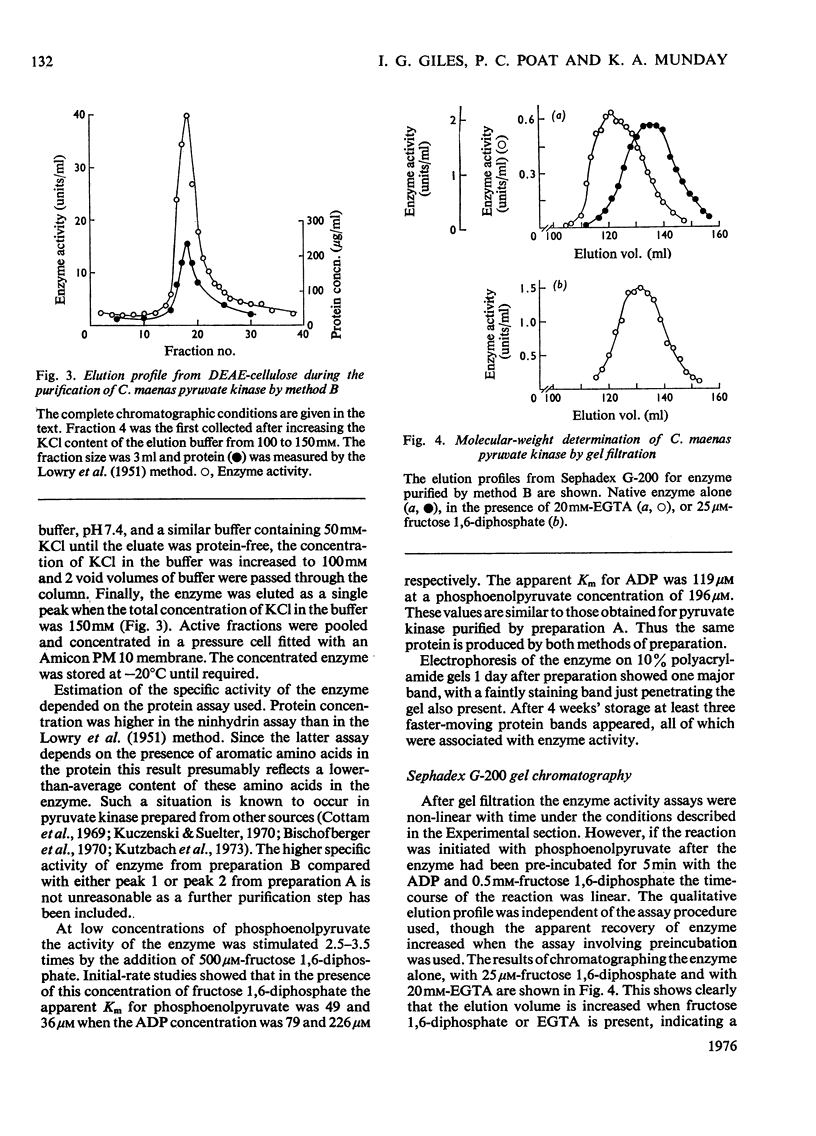
Pyruvatekinase from the hepatopancreas of the common shore crab, Carcinus maenas, was purified to a specific activity of 240 units/mg of protein in the assay conditions described. 2. In one method of purification the enzymic activity could be resolved into two fractions after chromatography on DEAE-cellulose. Fructose 1, 6-diphosphate was able to effect the conversion of one form (peak 1) into the second (peak 2). 3. In the presence of a saturating concentration of fructose 1, 6-diphosphate both forms of the enzyme were kinetically similar. 4. Polyacrylamide-gel electrophoresis of the enzyme 1 day after preparation showed a single protein band. On storage at least three protein bands became visible, all of which were associated with pyruvate kinase activity. 5. Chromatography of the enzyme on Sephadex G-200 indicated a mol.wt. of 247000, but in the presence of fructose 1, 6-diphosphate the elution volume of the enzyme increased corresponding to a mol.wt. of 193000. 6 Dissociation of the enzyme in sodium dodecyl sulphate and 2-mercaptoethanol followed by polyacrylamide-gel electrophoresis produced one major protein band with a mol.wt. of 55000.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins G. L. A simple digital-computer program for estimating the parameters of the hill equation. Eur J Biochem. 1973 Feb 15;33(1):175–180. doi: 10.1111/j.1432-1033.1973.tb02667.x. [DOI] [PubMed] [Google Scholar]
- Bailey E., Stirpe F., Taylor C. B. Regulation of rat liver pyruvate kinase. The effect of preincubation, pH, copper ions, fructose 1,6-diphosphate and dietary changes on enzyme activity. Biochem J. 1968 Jul;108(3):427–436. doi: 10.1042/bj1080427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischofberger H., Hess B., Röschlau P. Molecular weight of yeast pyruvate kinase. Hoppe Seylers Z Physiol Chem. 1971 Aug;352(8):1139–1150. doi: 10.1515/bchm2.1971.352.2.1139. [DOI] [PubMed] [Google Scholar]
- Bischofberger H., Hess B., Röschlau P., Wieker H. J., Zimmermann-Telschow H. Amino-acid composition and subunit structure of yeast-pyruvate kinase. Hoppe Seylers Z Physiol Chem. 1970 Mar;351(3):401–408. doi: 10.1515/bchm2.1970.351.1.401. [DOI] [PubMed] [Google Scholar]
- Cottam G. L., Hollenberg P. F., Coon M. J. Subunit structure of rabbit muscle pyruvate kinase. J Biol Chem. 1969 Mar 25;244(6):1481–1486. [PubMed] [Google Scholar]
- Flanders L. E., Bamburg J. R., Sallach H. J. Pyruvate kinase isozymes in adult tissue and eggs of Rana pipiens. II. Physical and kinetic studies of purified skeletal and heart muscle pyruvate kinases. Biochim Biophys Acta. 1971 Sep 22;242(3):566–579. doi: 10.1016/0005-2744(71)90150-1. [DOI] [PubMed] [Google Scholar]
- Giles I. G., Poat P. C., Munday K. A. Regulation of pyruvate kinase from the hepatopancreas of the crab Carcinus maenas. Biochem Soc Trans. 1975;3(3):400–402. doi: 10.1042/bst0030400. [DOI] [PubMed] [Google Scholar]
- Hess B., Kutzbach C. Identification of two types of liver pyruvate kinase. Hoppe Seylers Z Physiol Chem. 1971 Mar;352(3):453–458. doi: 10.1515/bchm2.1971.352.1.453. [DOI] [PubMed] [Google Scholar]
- Kuczenski R. T., Suelter C. H. Yeast pyruvate kinase. Native and subunit molecular weight. Biochemistry. 1970 May 12;9(10):2043–2047. doi: 10.1021/bi00812a003. [DOI] [PubMed] [Google Scholar]
- Kutzbach C., Bischofberger H., Hess B., Zimmermann-Telschow H. Pyruvate kinase from pig liver. Hoppe Seylers Z Physiol Chem. 1973 Oct-Nov;354(10-11):1473–1489. doi: 10.1515/bchm2.1973.354.2.1473. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Llorente P., Marco R., Sols A. Regulation of liver pyruvate kinase and the phosphoenolpyruvate crossroads. Eur J Biochem. 1970 Mar 1;13(1):45–54. doi: 10.1111/j.1432-1033.1970.tb00897.x. [DOI] [PubMed] [Google Scholar]
- MATHEWS C. K., BROWN F., COHEN S. S. VIRUS-INDUCED ACQUISITION OF METABOLIC FUNCTION. VII. BIOSYNTHESIS DE NOVO OF DEOXYCYTIDYLATE HYDROXYMETHYLASE. J Biol Chem. 1964 Sep;239:2957–2963. [PubMed] [Google Scholar]
- Melchior J. B. The role of metal ions in the pyruvic kinase reaction. Biochemistry. 1965 Aug;4(8):1518–1525. doi: 10.1021/bi00884a009. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Pogson C. I. Adipose-tissue pyruvate kinase. Properties and interconversion of two active forms. Biochem J. 1968 Nov;110(1):67–77. doi: 10.1042/bj1100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle J. R. The molecular weight of the undegraded polypeptide chain of yeast hexokinase. Biochem Biophys Res Commun. 1970 Apr 8;39(1):46–52. doi: 10.1016/0006-291x(70)90755-2. [DOI] [PubMed] [Google Scholar]
- SWINGLE S. M., TISELIUS A. Tricalcium phosphate as an adsorbent in the chromatography of proteins. Biochem J. 1951 Feb;48(2):171–174. doi: 10.1042/bj0480171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Harano Y., Sue F., Morimura H. Crystallization, characterization and metabolic regulation of two types of pyruvate kinase isolated from rat tissues. J Biochem. 1967 Jul;62(1):71–91. doi: 10.1093/oxfordjournals.jbchem.a128639. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


