Abstract
Purpose
We aimed to identify the risk factors for severe neutropenia in the early phase of trifluridine-tipiracil (FTD/TPI) treatment, and their impact on overall survival (OS).
Methods
This single-center retrospective study included patients with unresectable metastatic colorectal cancer who were treated with FTD/TPI. The primary endpoint was OS, and the secondary endpoint was severe neutropenia during the first and second cycles of FTD/TPI. We assessed the association between outcomes and potential confounders using multivariate analysis.
Results
Of the 77 total patients, 33 developed severe neutropenia during the first and second treatment cycles. In Cox hazard analysis, the independent factors associated with OS were neutropenia ≥ grade 1 during cycles 1 and 2 (adjusted hazard ratio 0.43; 95% confidence interval (CI) 0.21–0.87), combined treatment with bevacizumab (0.47; 95% CI 0.27–0.83), number of metastatic organs ≥ 3 (2.15; 95% CI 1.22–3.82), and time since diagnosis of metastasis until commencement of FTD/TPI < 18 months (1.94; 95% CI 1.13–3.33). Severe neutropenia during cycles 1 and 2 was not associated with OS (0.75; 0.44–1.27). The risk of severe neutropenia adjusted for initial dose reduction was defined as renal impairment with creatinine clearance (Ccr) of < 60 ml/min (adjusted odds ratio, 4.67; 95% CI, 1.38–15.80) and absolute neutrophil count (per 1000/μl, 0.47; 0.27–0.81).
Conclusion
The neutropenia ≥ grade 1 during cycles 1 and 2 of FTD/TPI is a predictor of favorable outcomes; however, the effect of severe neutropenia on OS was not clear. Renal impairment was also associated with severe neutropenia.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00384-024-04798-2.
Keywords: Trifluridine-tipiracil, Severe neutropenia, Overall survival, Renal impairment
Introduction
Developments in targeted molecular and combination therapies have improved the prognosis of unresectable metastatic colorectal cancer. The more recent use of trifluridine-tipiracil (FTD/TPI) [1] and regorafenib [2] in later treatment stages has contributed to even further improvements in outcomes for this patient group. The SUNLIGHT study demonstrated that combination treatment with bevacizumab and FTD/TPI in later stages further improved treatment outcomes in these patients [3].
Several studies have reported that chemotherapy-induced neutropenia (CIN) in the early treatment phases represents a surrogate marker of higher plasma concentrations of FTD that contribute to better treatment outcomes [4–9]. However, the difficulty of this approach is that, in a situation where the only basis for setting the initial dose of FTD/TPI is body surface area (BSA), it is unclear how and on what basis to set the initial dose in order to maintain a high blood concentration of FTD/TPI. Moreover, it is also unclear whether inducing severe CIN actually contributes to better outcomes.
In this study, we aimed to identify the factors associated with severe CIN and analyze whether severe CIN induced in the early phases of FTD/TPI treatment affected long-term outcomes in patients with unresectable metastatic colorectal cancer.
Patients and methods
Study design
This retrospective study aimed to reveal the predictive factors of severe CIN > grade 3 during treatment cycles 1 and 2, and the general prognostic factors in patients with unresectable metastatic colorectal cancer being treated with FTD/TPI. It was approved by the institutional review board of Tokuyama Central Hospital (protocol number: K469-20,230,607) and was conducted in accordance with the ethical standards of the 2013 Declaration of Helsinki. The requirement for informed consent was waived due to the retrospective nature of the study. This study adhered to the STROBE statement [10].
Patients
Consecutive patients with unresectable metastatic colorectal cancer who were treated with FTD/TPI at our institution and started their treatments between May 2014 and April 2023 were included. Patients with locally advanced colorectal cancer without distant metastases were excluded. Patients who received FTD/TPI as first- or second-line treatment, under conditions that did not meet the starting criteria in Japan, such as myelosuppression (i.e., absolute neutrophil count < 1500/μl, platelet count < 75,000/μl, or hemoglobin level < 8.0 g/dl) or hepatic dysfunction (aspartate and alanine aminotransferase > 100 IU/l or total bilirubin > 1.5 mg/dl), except for cases of renal impairment (serum creatinine > 1.5 mg/dl) were also excluded, as were any patients who started FTD/TPI therapy at other institutions. FTD/TPI was administered as a third-line therapy in 35 patients, as a fourth-line therapy in 17 patients and as a more late-line therapy in 25 patients. FTD/TPI was administered at a starting dose of 70 mg/m2/day with modification by each physician, from day 1 to day 5 and day 8 to day 12 every 28 days. Bevacizumab was administered at a dose of 5 mg/kg intravenously on days 1 and 15. Patients visited the outpatient clinic once a week or every 2 weeks for the first cycle and once every 2 weeks after the second cycle to check for adverse events and laboratory abnormalities. The need for reducing the dose of FTD/TPI was determined by each physician based on clinical decision and the patient’s general condition.
Procedures
Clinicopathological data were collected from the electronic medical records of each patient. These included age, sex, body surface area, body mass index, sidedness of the primary lesion, metastatic lesions, number of metastatic organs, complete blood cell counts, serum creatinine (calculated by the enzymatic method), serum transaminase, serum albumin on commencement of FTD/TPI, prior chemotherapy, relative dose intensity (RDI) of FTD/TPI, time from diagnosis of metastasis to induction of FTD/TPI, adverse events of FTD/TPI, RAS mutation status, previous gastrectomy history, and whether therapy was or was not combined with bevacizumab. All adverse events were graded according to the Common Terminology Criteria for Adverse Events version 5.0 [11], and neutropenia > grade 3 was defined as severe. Creatinine clearance (Ccr) was calculated using the Cockcroft-Gault method.
Outcomes
The primary endpoint was overall survival (OS), which was defined as the time elapsed from the commencement of FTD/TPI to death from any cause, or the last follow-up. The secondary endpoint was severe CIN in cycles 1 and 2 of FTD/TPI treatment. Progression-free survival (PFS) was defined as the time between the beginning of FTD/TPI and the date of confirmed radiological or clinical progression, or death from any cause in right-censoring event-free subjects at the time of the final follow-up.
Statistical analysis
Data are presented as medians and interquartile ranges (IQRs). Continuous variables were analyzed using the Wilcoxon rank-sum test, and categorical variables were analyzed using the chi-squared test or Fisher’s exact test, as appropriate. The Kaplan–Meier method was used to calculate PFS and OS, and differences were evaluated using the log-rank test.
We pre-specified the potential confounders for outcomes using clinical knowledge and a comprehensive review of the literature [12, 13]. The potential confounders chosen for severe CIN were age, sex, previous gastrectomy history, combined treatment with bevacizumab, number of treatment regimens used before FTD/TPI ≥ 3, absolute neutrophil count, serum albumin ≤ 3.9 g/dl, Ccr < 60 ml/min [14], and initial dose reduction for the recommended dose of FTD/TPI. Those for OS included age, sex, relative dose intensity, severe CIN or CIN ≥ grade 1 during cycles 1 and 2 [4–7], and during the whole treatment period [8], number of metastatic organs ≥ 3 [12, 13], time from diagnosis of metastasis to start of FTD/TPI < 18 months [12, 13], combination treatment with bevacizumab [3], sidedness of the primary lesion, and Ccr < 60 ml/min. Variables with a missingness rate > 10% were not included in our analysis.
Due to insufficient events (i.e., severe neutropenia and death), we examined the correlations between the study variables and the primary and secondary endpoints using multivariate data analysis adjusted for predetermined confounders. In the Cox regression model for OS, we partially adjusted the hazard ratio (HR): age was adjusted for sex, combination with bevacizumab, time since diagnosis of metastasis until commencement of FTD/TPI <18 months, and number of metastatic organs ≥3. Sex was adjusted for age, combination with bevacizumab, time since diagnosis of metastasis until commencement of FTD/TPI <18 months, and number of metastatic organs ≥3. Combination with bevacizumab was adjusted for age, sex, time since diagnosis of metastasis until commencement of FTD/TPI <18 months, and number of metastatic organs ≥3. Time since diagnosis of metastasis until commencement of FTD/TPI <18 months was adjusted for age, sex, combination with bevacizumab, and number of metastatic organs ≥3. Number of metastatic organs ≥3 was adjusted for age, sex, combination with bevacizumab, and time since diagnosis of metastasis until commencement of FTD/TPI <18 months. Furthermore, all other variables were adjusted for age, sex, combination with bevacizumab, time since diagnosis of metastasis until commencement of FTD/TPI <18 months, and number of metastatic organs ≥3. In the logistic regression model for severe CIN occurrence, the odds ratios (ORs) of all potential confounders were adjusted for the binary variable, whether initial dose reduction was performed or not.
Variance inflation factors were calculated for every potential prognostic variable in each adjusted model. All statistical analyses were performed using R version 4.2.3 (R Foundation for Statistical Computing, Vienna, Austria) and its package rms 6.7-1.
Results
Patients
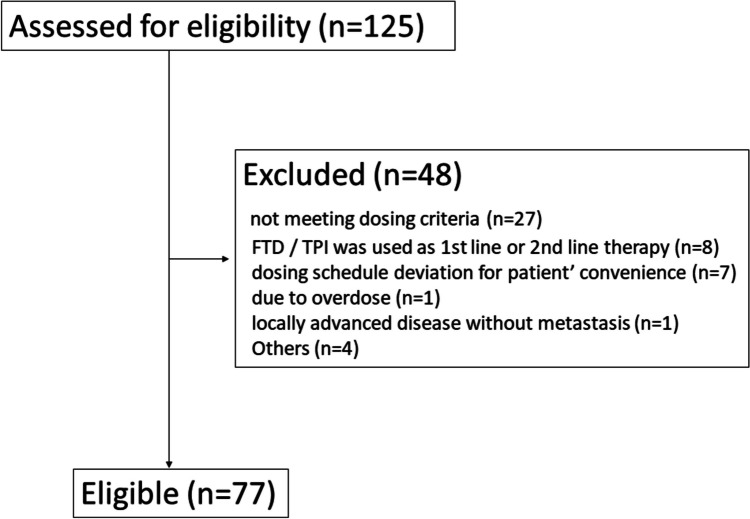
Among 125 eligible patients with unresectable metastatic tumors treated using FTD/TPI, 48 were excluded, leaving 77 (Fig. 1). Patient characteristics are shown in Table 1. In this study, deviation of serum creatinine level from the FTD/TPI starting criteria (i.e., ≥ 1.5 mg/dl) was not an exclusion criterion, but only one patient fell into this range, with a level of 1.85 mg/dl. Patients with Ccr < 60 ml/min and Ccr ≥ 60 ml/min were initiated at a full dose (70 mg/m2/day); this applied to 6 and 25 patients. A one-step lower dose (55–70 mg/m2/day) was administered in 13 and 29 patients, and at lower dosages (− 55 mg/m2/day) in 2 and 2 patients, respectively. In addition, dose reduction in cycle 2 was needed in 10 (47.6%) of 21 patients with Ccr < 60 ml/min and 13 (23.2%) of 56 patients with Ccr ≥ 60 ml/min.
Fig. 1.
Flow diagram of this study
Table 1.
Patient characteristics
| Characteristic | N = 77a |
|---|---|
| Age, year | 69 (61, 73) |
| Sex, male | 47 (61%) |
| BSA, m2 | 1.63 (1.49, 1.74) |
| ANC, /μl | 3494 (2771, 4576) |
| Albumin, g/dl | 3.80 (3.30, 4.10) |
| Creatinine, mg/dl | 0.77 (0.65, 0.88) |
| Creatinine clearance, ml/min | 73 (56, 91) |
| Creatinine clearance < 60 ml/min | 21 (27%) |
| Sidedness of primary lesion, right | 16 (21%) |
| RAS status | |
| Wild | 36 (47%) |
| Mutant | 30 (39%) |
| Missing | 11 (14%) |
| Liver metastasis | 50 (65%) |
| Number of metastatic organ sites | |
| 1 | 26 (34%) |
| 2 | 29 (38%) |
| 3 | 15 (19%) |
| 4 | 7 (9.1%) |
| Time since diagnosis metastasis until commencement of FTD/TPI, months | 20 (14, 31) |
| Prior regimens ≥ 3 | 42 (55%) |
| Number of regimens after FTD/TPI | |
| 0 | 31 (40%) |
| 1 | 21 (27%) |
| 2 | 18 (23%) |
| 3 | 3 (3.9%) |
| 4 | 3 (3.9%) |
| 5 | 1 (1.3%) |
| Initial dose reduction | 46 (60%) |
| RDI percentage in cycle 1 | 80 (57, 91) |
| With bevacizumab in cycle 1 | 29 (38%) |
|
With bevacizumab during the treatment period Previous gastrectomy |
35 (45%) 4 (5.2%) |
aMedian (IQR); n (%)
Incidence of severe adverse events during cycles 1 and 2
In this study, 41 patients (53.2%) developed severe adverse events, during cycles 1 and 2 (10 patients developed severe adverse events during both cycles 1 and 2). The details of severe adverse events during cycles 1 and 2 were 33 patients (42.9%) with CIN, 11 patients (14.3%) with anemia and thrombocytopenia, 5 patients (6.5%) with non-hematological toxicities: 2 with fatigue, 2 with anorexia, and 1 with nausea. In patients with Ccr < 60 ml/min and Ccr ≥ 60 ml/min, 12 (57.1%) and 21 (37.5%) patients developed severe CIN, and 2 (9.5%) and 3 (5.4%) patients developed severe non-hematological toxicities, respectively.
Survival analysis
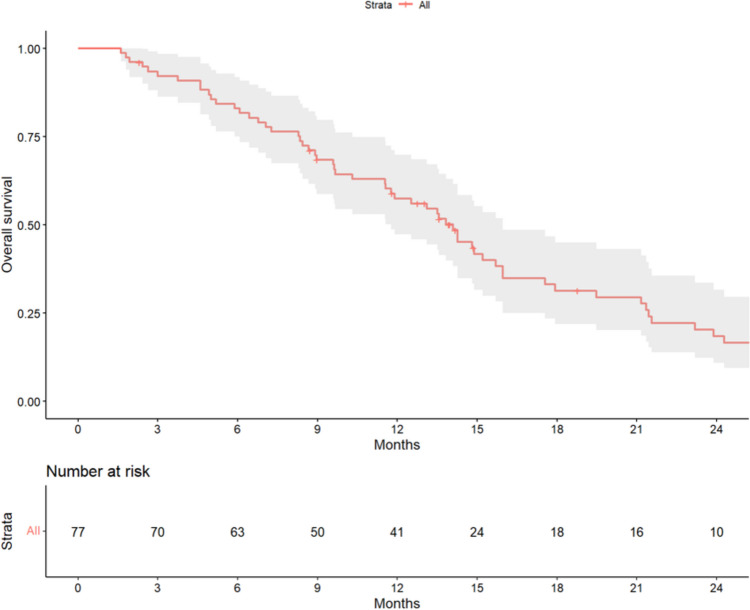
On the data cutoff date of October 30, 2023, 75 progression events had been recorded, with a median PFS of 4.6 months (95% CI, 3.7–6.0). A total of 61 death events had also been recorded, putting the median OS at 14.1 months (95% CI, 11.8–16.0; Fig. 2).
Fig. 2.
Overall survival of all 77 patients. The shaded area indicates the upper and lower boundaries of the 95% confidence interval
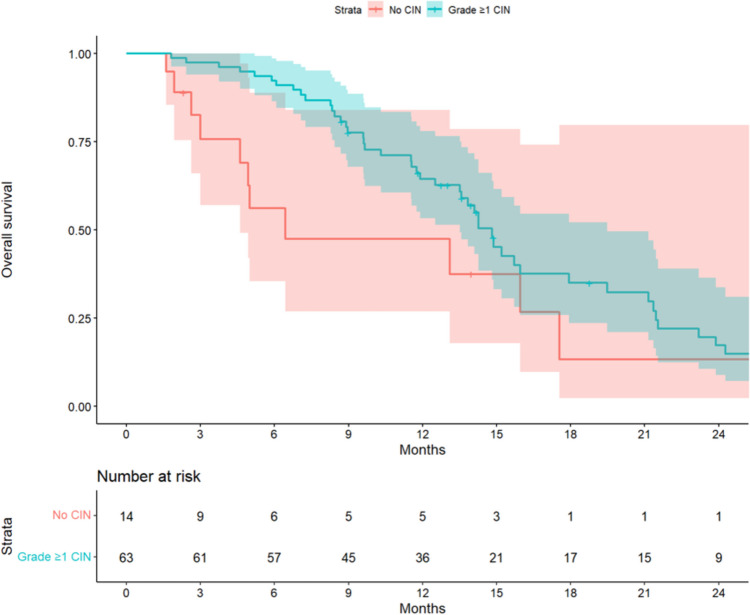
The associations between the prognostic variables and OS are shown in Table 2. Combination therapy with bevacizumab (adjusted HR, 0.47; 95% CI, 0.27–0.83) was associated with better outcomes, whereas the number of metastatic organs ≥ 3 (adjusted HR, 2.15; 95% CI, 1.22–3.82) and the time since diagnosis of metastasis until the commencement of FTD/TPI < 18 months (adjusted HR, 1.94; 95% CI, 1.13–3.33) were associated with poorer outcomes. While CIN of any grade during cycles 1 and 2 (adjusted HR, 0.43; 95% CI, 0.21–0.87) was associated with favorable outcomes, severe CIN during this period (adjusted HR, 0.75; 95% CI, 0.44–1.27) and over the entire treatment period (adjusted HR, 0.60; 95% CI, 0.34–1.07) were not associated with OS in this cohort. Renal impairment with Ccr < 60 ml/min (adjusted HR, 0.75; 95% CI, 0.38–1.50) was also not associated with survival. None of the variance inflation factors was > 2. The Kaplan–Meier curves for OS stratified by the presence or absence of CIN of any grade during cycles 1 and 2, adjusted for age, sex, combination with bevacizumab, time since diagnosis of metastasis until commencement of FTD/TPI < 18 months, and number of metastatic organs ≥ 3, are shown in Fig. 3 (median OS, 14.8 months [95% CI, 13.5–19.5) vs 6.4 months [95% CI, 4.6–not available]). Moreover, those stratified by the presence or absence of severe CIN during cycles 1 and 2, adjusted for the same factors, are shown in Supplementary Fig. 1 (median OS, 14.1 months [95% CI, 11.5–23.9) vs 14.3 months [95% CI, 11.9–21.2]). The Kaplan–Meier curves for OS in patients treated with and without bevacizumab stratified by the presence or absence of severe CIN during cycles 1 and 2 are shown in Supplementary Fig. 2 (survival of patients treated with bevacizumab: median OS, 14.9 months [95% CI, 9.7–not available) vs 17.9 months [95% CI, 15.7–not available], p = 0.57) and Supplementary Fig. 3 (survival of patients treated without bevacizumab: median OS, 13.2 months [95% CI, 9.6–not available) vs 9.5 months [95% CI, 6.4–14.8], p = 0.076).
Table 2.
Multivariable regression models for potential prognostic variables associated with overall survival
| Variable | Adjusted hazard ratio | p-value |
|---|---|---|
| Age, per 10 years | 0.92 (0.71–1.21) | 0.5726 |
| Sex | 0.9327 | |
| Female | 1 (ref) | |
| Male | 0.97 (0.53–1.80) | |
| Primary lesion sidedness | 0.6229 | |
| Left | 1 (ref) | |
| Right | 1.17 (0.63–2.17) | |
| Bevacizumab | 0.0092 | |
| Without | 1 (ref) | |
| With | 0.47 (0.27–0.83) | |
| Number of metastatic organs ≥ 3 | 2.15 (1.22–3.82) | 0.0086 |
| Time since diagnosis of metastasis until commencement of FTD/TPI < 18 months | 1.94 (1.13–3.33) | 0.0171 |
| CIN ≥ grade 1 during cycles 1 and 2 | 0.0191 | |
| None | 1 (ref) | |
| Occurred | 0.43 (0.21–0.87) | |
| Severe CIN during cycles 1 and 2 | 0.2767 | |
| None | 1 (ref) | |
| Occurred | 0.75 (0.44–1.27) | |
| Severe CIN during the entire treatment period | 0.0815 | |
| None | 1 (ref) | |
| Occurred | 0.60 (0.34–1.07) | |
| RDI in all cycle, per 10% | 1.16 (0.98–1.38) | 0.0929 |
| Creatinine clearance | 0.4192 | |
| ≥ 60 ml/min | 1 (ref) | |
| < 60 ml/min | 0.75 (0.38–1.50) |
Data are hazard ratio with 95% CI in parentheses
Age, sex, combination with bevacizumab, time since diagnosis of metastasis until commencement of FTD-TPI ≤ 18 months, and number of metastatic organs ≥ 3 were adjusted each other; and all other variables were adjusted for age, sex, combination with bevacizumab, time since diagnosis of metastasis until commencement of FTD-TPI ≤ 18 months, and number of metastatic organs ≥ 3
FTD/TPI, trifluridine tipiracil; CIN, chemotherapy-induced neutropenia; RDI, relative dose intensity
Fig. 3.
Overall survival for patients stratified by the presence or absence of CIN of any grade during cycles 1 and 2, adjusted for age, sex, combination with bevacizumab, time since diagnosis of metastasis until commencement of FTD/TPI < 18 months, and number of metastatic organs ≥ 3. The shaded area indicates the upper and lower boundaries of the 95% confidence interval
Risk factors for severe neutropenia during cycles 1 and 2
Multivariate analysis adjusted for initial dose reduction revealed renal impairment with Ccr < 60 ml/min (adjusted OR, 4.67; 95% CI, 1.38–15.80) and absolute neutrophil count (per 1000/μl; adjusted OR, 0.47; 95% CI, 0.27–0.81) for the risk of severe CIN (Table 3).
Table 3.
Multivariable regression models for potential variables associated with severe CIN during cycles 1 and 2
| Variable | Adjusted odds ratio | p-value |
|---|---|---|
| Initial dose reduction | 0.30 (0.11–0.82) | 0.0186 |
| Age, per 10 years | 1.22 (0.76–1.95) | 0.4090 |
| Sex | 0.3410 | |
| Female | 1 (ref) | |
| Male | 0.61 (0.22–1.70) | |
| Previous gastrectomy | 0.1370 | |
| Without | 1 (ref) | |
| With | 5.00 (0.60–41.70) | |
| Bevacizumab | 0.9900 | |
| Without | 1 (ref) | |
| With | 1.01 (0.35–2.93) | |
| Time since diagnosis of metastasis until commencement of FTD/TPI, per month | 1.01 (0.99–1.04) | 0.2200 |
| Number of regimen before FTD/TPI | 0.1020 | |
| < 3 | 1 (ref) | |
| ≥ 3 | 2.45 (0.84–7.17) | |
| ANC, per 1000/μl | 0.47(0.27–0.81) | 0.0066 |
| Serum albumin | 0.2210 | |
| > 3.9 g/dl | 1 (ref) | |
| ≤ 3.9 g/dl | 2.01 (0.66–6.16) | |
| Creatinine clearance | 0.0130 | |
| ≥ 60 ml/min | 1 (ref) | |
| < 60 ml/min | 4.67 (1.38–15.80) |
Data are odds ratio with 95% CI in parentheses
All variables ware adjusted for initial dose reduction
CIN, chemotherapy-induced neutropenia; FTD/TPI, trifluridine tipiracil; ANC, absolute neutrophil counts
Discussion
There were three important findings from this retrospective study. First, renal impairment was a significant risk factor for severe CIN in patients treated with FTD/TPI. Second, development of CIN of any grade [9], concomitant bevacizumab administration [3], a number of metastatic organs ≥ 3, and time since diagnosis of metastasis until commencement of FTD/TPI < 18 months had prognostic impact on these patients, as has been previously reported [13]. Finally, severe CIN during cycles 1and 2 had no impact on OS.
The SUNLIGHT trial revealed the favorable impact of combination treatment with bevacizumab on survival [3]. Severe CIN was more frequent in the combination group than in the FTD/TPI alone group in the SUNLIGHT study; however, the difference was not significant in our cohort. Whether the myelosuppressive effects of FTD/TPI are further accelerated by bevacizumab, especially in patients with impaired renal function, is unknown. Further the number of metastatic sites of < 3 and time since initial diagnosis of metastasis to the commencement of FTD/TPI treatment of ≥ 18 months were good prognostic factors revealed by Tabernero et al. in the RECOURSE trial cohort [13]. Cox proportional hazard analysis using these three established prognostic factors, together with age and sex as covariates, revealed that severe CIN during cycles 1 and 2 was not a prognostic factor. By contrast, the development of CIN of any grade was identified as a favorable prognostic factor. For regiments containing oral anticancer agents, treatment intensity is dependent on patients’ adherence and the occurrence of mild CIN may reflect true relative dose intensity.
In this study, we analyzed the risk factors for severe CIN in cycle 1 and found that moderate-to-severe renal impairment could affect the occurrence of severe CIN. This is consistent with previous research [14]. Since TPI, which is formulated to inhibit the metabolism of FTD in the body, is excreted through the kidneys, renal impairment is believed to delay TPI elimination and enhance the effect of FTD. Saif et al. reported that the FTD area under the curve (AUC) did not differ between patients with impaired vs normal renal function; however, in their pharmacokinetic study, the TPI AUC increased with the severity of renal impairment [15]. In addition, severe CIN was observed in 90.9% of patients with mild renal impairment (i.e., Ccr < 60 ml/min) in their study [15]. Initially, we hypothesized that a previous gastrectomy, which is involved in drug absorption, or serum albumin levels, which may be related to free FTD/TPI concentrations in the blood, might be associated with the development of severe CIN. However, their involvement in severe CIN was not seen. By contrast, a lower absolute neutrophil count was associated with the development of severe CIN. We considered the possibility of bone marrow exhaustion due to the longer treatment period preceding FTD/TPI; however, the length of the preceding treatment period (i.e., time since diagnosis of metastasis until commencement of FTD/TPI) was not associated with the incidence of severe neutropenia. Therefore, it is not clear whether a lower absolute neutrophil count prior to the treatment is due to each patient’s natural bone marrow function or continued treatment over time. The initial dose of FTD/TPI determined using BSA alone may not be sufficient, and dose reduction may be needed for patients with more than moderately impaired renal function (i.e., Ccr < 60 ml/min) and low absolute neutrophil counts.
Because the incidence of severe CIN depends on the dose of FTD/TPI and patient renal function, as has been previously reported [14, 15] and described here, dose design using severe CIN as a surrogate marker is particularly challenging for general clinicians. Moreover, it may even put patients at risk if not done correctly by a highly experienced oncologist. It is important to set a safe dosage that does not induce severe CIN (> grade 3), with objective reference to the result that patients with ≥ grade 1 CIN have better outcomes than those without, as was shown here and in a study by Yoshino et al. [9].
This study had some key limitations worth noting. First, this was a single-center, retrospective study of a small number of patients. We were therefore unable to adjust for all potential confounders in the multivariate model because of the small number of events. Although initial dose reduction was performed in many patients and the RDI was not sufficient, the median OS for the entire cohort was 14.1 months, which is comparable to what has been previously reported [3, 8, 14].
Renal impairment with Ccr < 60 ml/min was associated with the occurrence of severe CIN during cycles 1 and 2 in patients with unresectable metastatic colorectal cancer who were treated using FTD/TPI. The onset of CIN, even if mild, in the early phase of FTD/TPI treatment was a predictor of favorable outcome; however, severe CIN could not contribute to better outcome.
Supplementary Information
Below is the link to the electronic supplementary material.
(PNG 38.8 KB)
(PNG 57.7 KB)
(PNG 61.1 KB)
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Author contribution
Yoshiro Omori and Satoshi Matsukuma conceived the idea and planned the analysis. Mikiko Kawa, Kazuki Ishimitsu, Toru Kawaoka, Kazuhisa Tokuno and Yuji Fujita collected the data and contributed to the interpretation of the results. Yoshiro Omori and Satoshi Matsukuma carried out the analysis and drafted the manuscript. Norio Akiyama, Shinya Sato and Shigeru Yamamoto supervised this work.All authors discussed the results and contributed to the final manuscript.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N et al (2015) Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 372(20):1909–1919. 10.1056/NEJMoa1414325 [DOI] [PubMed] [Google Scholar]
- 2.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M et al (2013) Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381(9863):303–312. 10.1016/S0140-6736(12)61900-X [DOI] [PubMed] [Google Scholar]
- 3.Prager GW, Taieb J, Fakih M, Ciardiello F, Van Cutsem E, Elez E et al (2023) Trifluridine-tipiracil and bevacizumab in refractory metastatic colorectal cancer. N Engl J Med 388(18):1657–1667. 10.1056/NEJMoa2214963 [DOI] [PubMed] [Google Scholar]
- 4.Hamauchi S, Yamazaki K, Masuishi T, Kito Y, Komori A, Tsushima T et al (2017) Neutropenia as a predictive factor in metastatic colorectal cancer treated with TAS-102. Clin Colorectal Cancer 16(1):51–57. 10.1016/j.clcc.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 5.Kamiimabeppu D, Osumi H, Shinozaki E, Ooki A, Wakatsuki T, Yoshino K et al (2021) Effect of neutropenia on survival outcomes of patients with metastatic colorectal cancer receiving trifluridine/tipiracil plus bevacizumab. Oncol Lett 22(5):783. 10.3892/ol.2021.13044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasi PM, Kotani D, Cecchini M, Shitara K, Ohtsu A, Ramanathan RK et al (2016) Chemotherapy induced neutropenia at 1-month mark is a predictor of overall survival in patients receiving TAS-102 for refractory metastatic colorectal cancer: a cohort study. BMC Cancer 16:467. 10.1186/s12885-016-2491-y(Electronic)):467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nose Y, Kagawa YA-O, Hata T, Mori R, Kawai K, Naito A et al (2020) Neutropenia is an indicator of outcomes in metastatic colorectal cancer patients treated with FTD/TPI plus bevacizumab: a retrospective study. Cancer Chemother Pharmacol 86(3):427–433. 10.1007/s00280-020-04129-6 [DOI] [PubMed] [Google Scholar]
- 8.Watanabe D, Fujii H, Ohata K, Iihara H, Makiyama A, Kobayashi R et al (2023) Prognostic impact of severe neutropenia in colorectal cancer patients treated with TAS-102 and bevacizumab, addressing immortal-time bias. BMC Cancer 23(1):1078. 10.1186/s12885-023-11618-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshino T, Cleary JM, Van Cutsem E, Mayer RJ, Ohtsu A, Shinozaki E et al (2020) Neutropenia and survival outcomes in metastatic colorectal cancer patients treated with trifluridine/tipiracil in the RECOURSE and J003 trials. Ann Oncol 31(1):88–95. 10.1016/j.annonc.2019.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 147(8):573–577. 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 11.National Cancer (2017) Institute Common terminology criteria for adverse events (CTCAE), v.5.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50. Accessed 7 Jun 2023
- 12.Fernández Montes A, Carmona-Bayonas A, Jimenez-Fonseca P, Vázquez Rivera F, Martinez Lago N, Covela Rúa M et al (2021) Prediction of survival in patients with advanced, refractory colorectal cancer in treatment with trifluridine/tipiracil: real-world vs clinical trial data. Sci Rep 11(1):14321. 10.1038/s41598-021-93732-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabernero J, Argiles G, Sobrero AF, Borg C, Ohtsu A, Mayer RJ, et al (2020) Effect of trifluridine/tipiracil in patients treated in RECOURSE by prognostic factors at baseline: an exploratory analysis. LID – 10.1136/esmoopen-2020-000752. ESMO Open 4(2059–7029 (Electronic)):e000752. LID - e000752 [DOI] [PMC free article] [PubMed]
- 14.Shiroyama M, Fukuoka S, Masuishi T, Takashima A, Kumekawa Y, Kajiwara T et al (2023) Renal impairment as a risk factor for trifluridine/tipiracil-induced adverse events in metastatic colorectal cancer patients from the REGOTAS study. Sci Rep 13(1):17931. 10.1038/s41598-023-45244-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saif MW, Becerra CR, Fakih MG, Sun W, Popovic L, Krishnamurthi S et al (2021) A phase I, open-label study evaluating the safety and pharmacokinetics of trifluridine/tipiracil in patients with advanced solid tumors and varying degrees of renal impairment. Cancer Chemother Pharmacol 88(3):485–497. 10.1007/s00280-021-04308-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PNG 38.8 KB)
(PNG 57.7 KB)
(PNG 61.1 KB)
Data Availability Statement
No datasets were generated or analysed during the current study.