Abstract
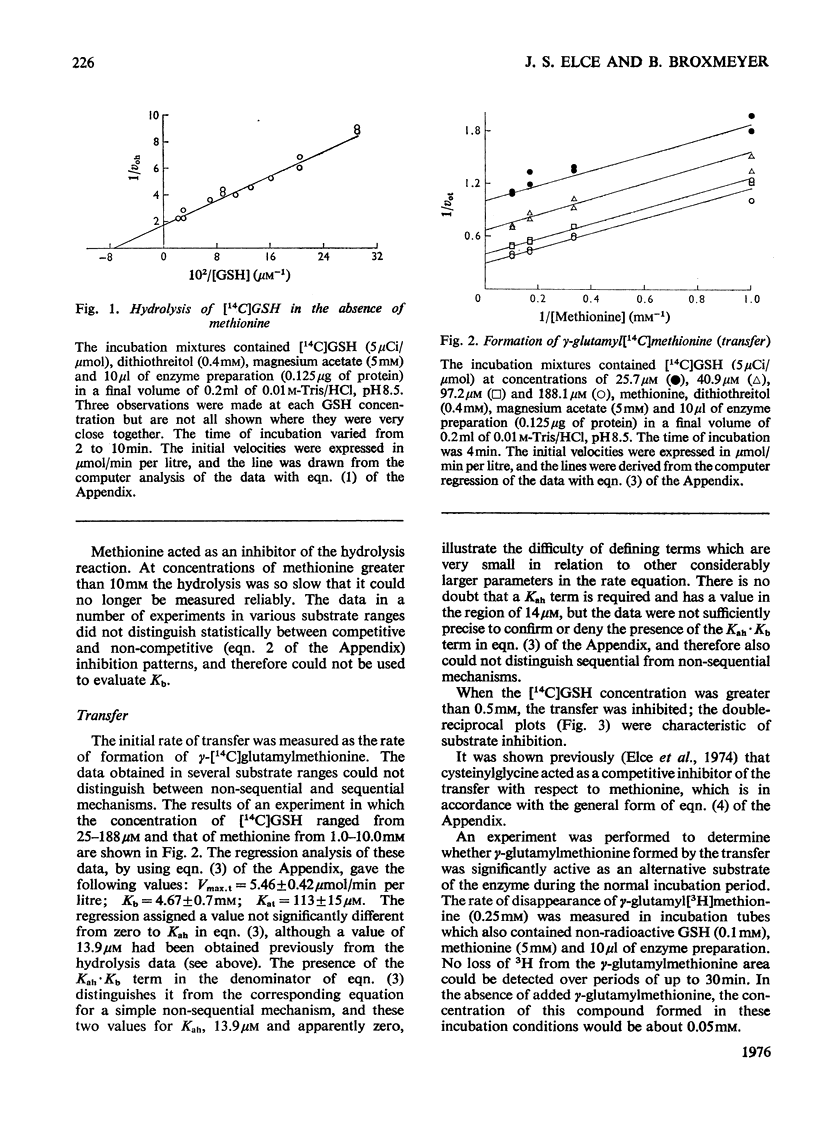
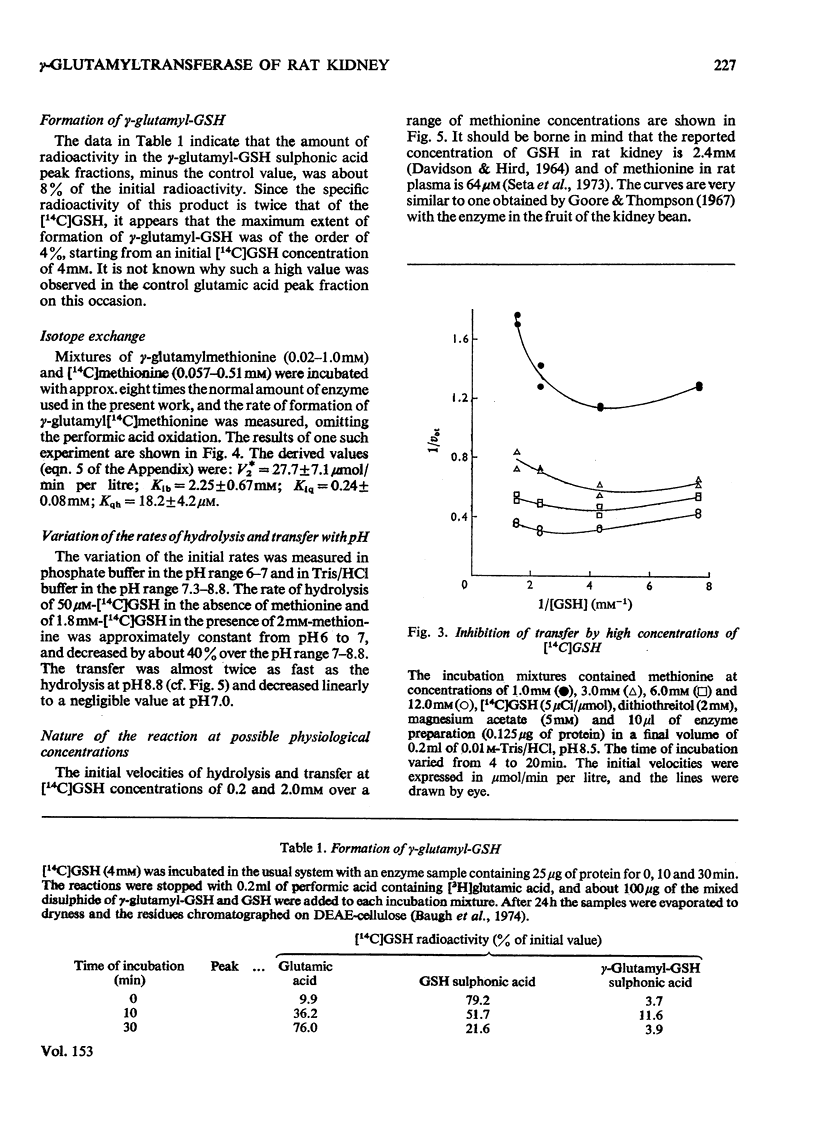
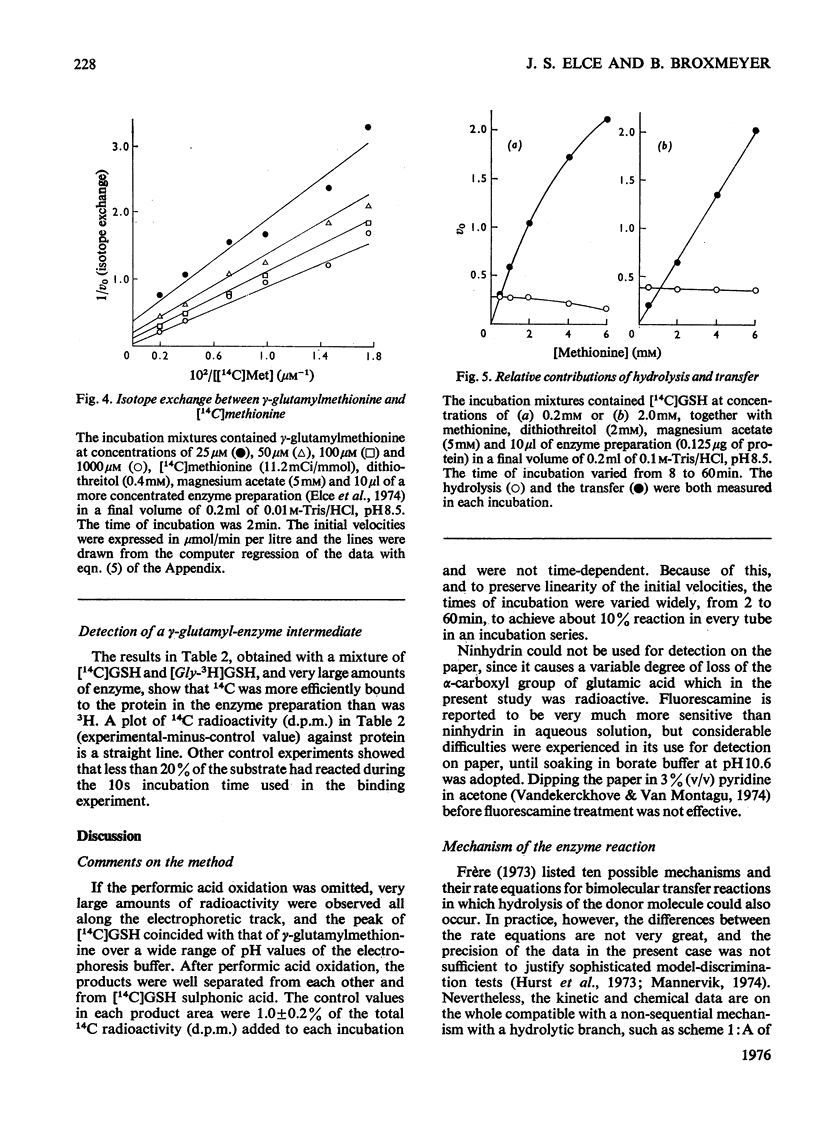
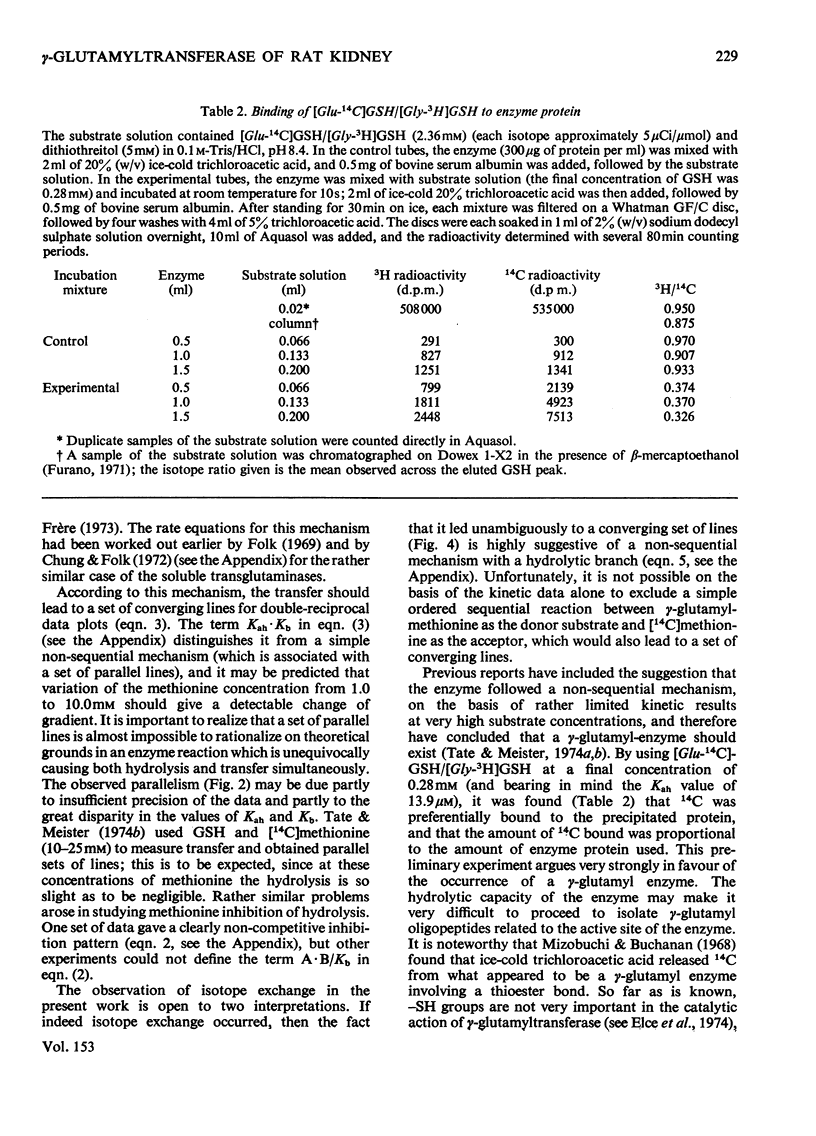
1. The hydrolytic and transfer reactions catalysed by rat kidney-gamma-glutamyltransferase (EC 2.3.2.2) were studied in vitro with substrates [U-14C]glutamic acid-labelled glutathione and methionine. Initial-velocity patterns, isotope-exchange and binding studies were consistent with a branched non-sequential mechanism in which a gamma-glutamyl-enzyme intermediate may react either with water (hydrolysis) or with methionine (gamma-glutamyl transfer). 2. The Michaelis constant for glutathione in hydrolysis was 13.9 +/- 1.4 mum, for glutathione in transfer it was 113 +/- 15 muM and for methionine as substrate it was 4.7 +/- 0.7 mM. At substrate concentrations in the ranges of their respective Michaelis constants, the rate of transfer was about ten times higher than that of hydrolysis, but at concentrations of methionine approximating to the physiological (64 muM in rat plasma) the transfer is negligible. 3. The enzyme is reported to lie on the luminal surface of the proximal straight kidney tubule. In this situation, if the kinetic results obtained with the detergent-solubilized enzyme are relevant to the behavior of the enzyme in vivo, it appears likely that the main function of renal gamma-glutamyltransferase is not in amino acid transport, but rather to hydrolyse glutathione in the renal filtrate.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baugh C. M., Braverman E., Nair M. G. The identification of poly-gamma-glutamyl chain lengths in bacterial folates. Biochemistry. 1974 Nov 19;13(24):4952–4957. doi: 10.1021/bi00721a012. [DOI] [PubMed] [Google Scholar]
- Chung S. I., Folk J. E. Kinetic studies with transglutaminases. The human blood enzymes (activated coagulation factor 13 and the guinea pig hair follicle enzyme. J Biol Chem. 1972 May 10;247(9):2798–2807. [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- Curthoys N. P., Kuhlenschmidt T. Phosphate-independent glutaminase from rat kidney. Partial purification and identity with gamma-glutamyltranspeptidase. J Biol Chem. 1975 Mar 25;250(6):2099–2105. [PubMed] [Google Scholar]
- Davidson B. E., Hird F. J. The estimation of glutathione in rat tissues. A comparison of a new spectrophotometric method with the glyoxalase method. Biochem J. 1964 Nov;93(2):232–236. doi: 10.1042/bj0930232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elce J. S., Bryson J., McGirr L. G. Gamma-glutamyl transpeptidase of rat kidney. Some properties and kinetic constants. Can J Biochem. 1974 Jan;52(1):33–41. doi: 10.1139/o74-007. [DOI] [PubMed] [Google Scholar]
- Elwyn D. H. Distribution of amino acids between plasma and red blood cells in the dog. Fed Proc. 1966 May-Jun;25(3):854–861. [PubMed] [Google Scholar]
- Elwyn D. H., Parikh H. C., Shoemaker W. C. Amino acid movements between gut, liver, and periphery in unanesthetized dogs. Am J Physiol. 1968 Nov;215(5):1260–1275. doi: 10.1152/ajplegacy.1968.215.5.1260. [DOI] [PubMed] [Google Scholar]
- Folk J. E. Mechanism of action of guinea pig liver transglutaminase. VI. Order of substrate addition. J Biol Chem. 1969 Jul 10;244(13):3707–3713. [PubMed] [Google Scholar]
- Folk J. E. Mechanism of action of guinea pig liver transglutaminase. VI. Order of substrate addition. J Biol Chem. 1969 Jul 10;244(13):3707–3713. [PubMed] [Google Scholar]
- Goore M. Y., Thompson J. F. Gamma-glutamyl transpeptidase from kidney bean fruit. I. Purification and mechanism of action. Biochim Biophys Acta. 1967 Jan 11;132(1):15–26. doi: 10.1016/0005-2744(67)90187-8. [DOI] [PubMed] [Google Scholar]
- Hochberg A., Dimant E. The labeling in vitro of glutathione of human blood cells. Biochim Biophys Acta. 1965 Jun 15;104(1):53–62. doi: 10.1016/0304-4165(65)90219-9. [DOI] [PubMed] [Google Scholar]
- Hurst R., Pincock A., Broekhoven L. H. Model discrimination and nonlinear parameter estimation in the analysis of the mechanism of action of beta-hydroxybutyrate dehydrogenase from Rhodopseudomonas spheroides. Biochim Biophys Acta. 1973 Sep 15;321(1):1–26. doi: 10.1016/0005-2744(73)90055-7. [DOI] [PubMed] [Google Scholar]
- Koivusalo M., Uotila L. Enzymic method for the quantitative determination of reduced glutathione. Anal Biochem. 1974 May;59(1):34–45. doi: 10.1016/0003-2697(74)90006-2. [DOI] [PubMed] [Google Scholar]
- Kuhlenschmidt T., Curthoys N. P. Subcellular localization of rat kidney phosphate independent glutaminase. Arch Biochem Biophys. 1975 Apr;167(2):519–524. doi: 10.1016/0003-9861(75)90494-4. [DOI] [PubMed] [Google Scholar]
- Meister A. Glutathione, metabolism and function via the gamma-glutamyl cycle. Life Sci. 1974 Jul 15;15(2):177–190. doi: 10.1016/0024-3205(74)90206-9. [DOI] [PubMed] [Google Scholar]
- Meister A. On the enzymology of amino acid transport. Science. 1973 Apr 6;180(4081):33–39. doi: 10.1126/science.180.4081.33. [DOI] [PubMed] [Google Scholar]
- Milbauer R., Grossowicz N. Gamma-glutamyl transfer reactions in bacteria. J Gen Microbiol. 1965 Nov;41(2):185–194. doi: 10.1099/00221287-41-2-185. [DOI] [PubMed] [Google Scholar]
- Mizobuchi K., Buchanan J. M. Biosynthesis of the purines. XXX. Isolation and characterization of formylglycinamide ribonucleotide amidotransferase-glutamyl complex. J Biol Chem. 1968 Sep 25;243(18):4853–4862. [PubMed] [Google Scholar]
- ORLOWSKI M., SZEWCZUK A. Determination of gamma-glutamyl transpeptidase activity in human serum and urine. Clin Chim Acta. 1962 Nov;7:755–760. doi: 10.1016/0009-8981(62)90055-4. [DOI] [PubMed] [Google Scholar]
- Okonkwo P. O., Orlowski M., Green J. P. Enzymes of the gamma-glutamyl cycle in the choroid plexus and brain. J Neurochem. 1974 Jun;22(6):1053–1058. doi: 10.1111/j.1471-4159.1974.tb04336.x. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Wilk S. Intermediates of the gamma-glutamyl cycle in mouse tissues. Influence of administration of amino acids on pyrrolidone carboxylate and gamma-glutamyl amino acids. Eur J Biochem. 1975 May 6;53(2):581–590. doi: 10.1111/j.1432-1033.1975.tb04101.x. [DOI] [PubMed] [Google Scholar]
- Palekar A. G., Tate S. S., Meister A. Formation of 5-oxoproline from glutathione in erythrocytes by the gamma-glutamyltranspeptidase-cyclotransferase pathway. Proc Natl Acad Sci U S A. 1974 Feb;71(2):293–297. doi: 10.1073/pnas.71.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross L. L., Barber L., Tate S. S., Meister A. Enzymes of the gamma-glutamyl cycle in the ciliary body and lens. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2211–2214. doi: 10.1073/pnas.70.8.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman J. D., Goodman S. I., Mace J. W., Patrick A. D., Tietze F., Butler E. J. Glutathionuria: inborn error of metabolism due to tissue deficiency of gamma-glutamyl transpeptidase. Biochem Biophys Res Commun. 1975 Jul 8;65(1):68–74. doi: 10.1016/s0006-291x(75)80062-3. [DOI] [PubMed] [Google Scholar]
- Seta K., Sansur M., Lajtha A. The rate of incorporation of amino acids into brain proteins during infusion in the rat. Biochim Biophys Acta. 1973 Feb 4;294(1):472–480. doi: 10.1016/0005-2787(73)90103-2. [DOI] [PubMed] [Google Scholar]
- Stein S., Böhlen P., Udenfriend S. Studies on the kinetics of reaction and hydrolysis of fluorescamine. Arch Biochem Biophys. 1974 Jul;163(1):400–403. doi: 10.1016/0003-9861(74)90491-3. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Meister A. Interaction of gamma-glutamyl transpeptidase with amino acids, dipeptides, and derivatives and analogs of glutathione. J Biol Chem. 1974 Dec 10;249(23):7593–7602. [PubMed] [Google Scholar]
- Tate S. S., Meister A. Stimulation of the hydrolytic activity and decrease of the transpeptidase activity of gamma-glutamyl transpeptidase by maleate; identity of a rat kidney maleate-stimulated glutaminase and gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3329–3333. doi: 10.1073/pnas.71.9.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Van Der Werf P., Stephani R. A., Meister A. Accumulation of 5-oxoproline in mouse tissues after inhibition of 5-oxoprolinase and administration of amino acids: evidence for function of the gamma-glutamyl cycle. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1026–1029. doi: 10.1073/pnas.71.4.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerckhove J., Van Montagu M. Sequence analysis of fluorescamine-stained peptides and proteins purified on a nanomole scale. Application to proteins of bacteriophage MS2. Eur J Biochem. 1974 May 2;44(1):279–288. doi: 10.1111/j.1432-1033.1974.tb03483.x. [DOI] [PubMed] [Google Scholar]
- WAELSCH H. Certain aspects of intermediary metabolism of glutamine, asparagine, and glutathione. Adv Enzymol Relat Subj Biochem. 1952;13:237–319. doi: 10.1002/9780470122587.ch7. [DOI] [PubMed] [Google Scholar]
- Young V. R., Vilaire G., Newberne P. M., Wilson R. B. Plasma insulin and amino acid concentrations in rats given an adequate or low protein diet. J Nutr. 1973 May;103(5):720–729. doi: 10.1093/jn/103.5.720. [DOI] [PubMed] [Google Scholar]


