Abstract
Objective
Mutations in the MTRNR1 gene of mitochondrial DNA are associated with non‐syndromic hearing loss and increased susceptibility to aminoglycoside ototoxicity. The aim of our study was to determine the clinical characteristics of sensorineural hearing loss caused by the m.1555A>G mutation in MTRNR1.
Methods
An observational retrospective study of the m.1555A>G mutation was conducted in patients with suspected hereditary bilateral sensorineural hearing loss in the Department of Otolaryngology of the Marqués de Valdecilla University Hospital (Cantabria, Spain) and in 100 controls with normal hearing.
Results
The m.1555A>G mutation was found in 82 individuals from 20 different families and in none of the controls. Variable degrees of hearing loss were observed, ranging from normal hearing to profound deafness. Patients with a history of streptomycin administration exhibited significantly more pronounced hearing loss. The onset of hearing loss occurred from childhood to adulthood, with progression or stability over the years. No associated vestibular alterations or other clinical manifestations outside the ear were found. Two cochlear implant recipients showed significant improvement in speech comprehension.
Conclusions
Patients with the m.1555A>G mutation in the MTRNR1 gene often develop bilateral, symmetric sensorineural hearing loss, predominantly affecting high frequencies, worsened by streptomycin administration. This mutation does not affect the vestibular function. The variability in the severity of hearing loss, the heterogeneity of phenotypic expression, and the presence of carrier individuals with normal hearing may indicate the existence of modifying factors, both environmental and genetic. Cochlear implantees showed a good response in terms of speech intelligibility. Genetic testing for this mutation is recommended in patients with a family history of hearing loss to prevent the use of aminoglycosides if the mutation is found.
Level of Evidence
4 Laryngoscope, 135:901–907, 2025
Keywords: aminoglycosides, m.1555A>G mutation, mitochondrial DNA, sensorineural hearing loss
INTRODUCTION
Mutations in the mitochondrial genome are responsible for numerous disorders. 1 Most pathologies due to mitochondrial DNA mutations correspond to syndromic conditions, some of which include hearing loss as a clinical sign. However, mutations in mitochondrial genes have been described whose effects appear to be restricted to the inner ear, causing non‐syndromic hearing loss, including the MTRNR1 gene (12S rRNA). 2 , 3
The m.1555A>G mutation is the most prevalent in the MTRNR1 gene, causing non‐syndromic hearing loss. 2 , 4 This mutation is also responsible for hearing loss induced by low doses of aminoglycoside antibiotics. 5 , 6 Other mutations in the MTRNR1 gene, much less frequent, have also been shown to cause sensorineural hearing loss, in some cases induced by aminoglycosides. 7 , 8
The aim of our study was to determine the clinical characteristics of hearing loss caused by the m.1555A>G mutation in the MTRNR1 gene of mitochondrial DNA and the role of aminoglycosides in its development.
METHODS
An observational retrospective study was conducted on patients with non‐syndromic sensorineural hearing loss who underwent genetic testing at the Otorhinolaryngology Department of the Marqués de Valdecilla Hospital (Santander, Spain) from 1996 to 2023.
The criteria of our department for performing a genetic study were:
Patients with bilateral sensorineural hearing loss, defined as average thresholds obtained in the conversational frequency range (500, 1000, and 2000 Hz) above 20 dB.
Suspected genetic origin of hearing loss, based on family history or characteristics of the hearing loss, such as bilateral low‐frequency or mid‐frequency hearing loss, and children with bilateral sensorineural hearing loss.
Patients with a demonstrated cause of hearing loss were excluded from our study, except for cases related to medication.
Both sporadic cases and those with family history of hearing loss were included, and efforts were made to extend the study to as many family members as possible in cases with a genetic origin found. At the same time, a sample of 100 individuals with normal hearing from the general population was obtained, who were interviewed to rule out family history of hearing loss, and an audiogram was performed to confirm the absence of hearing loss. The DNAs obtained from this control group were used to determine the prevalence of the m.1555A>G mutation in the MTRNR1 gene in individuals with normal hearing.
A pedigree of all studied families was created using the CYRILLIC 2 software, version 2.0.2 (Cherwell Scientific Publishing Ltd).
Family and personal history of other diseases that could suggest syndromic hearing loss and possible acquired factors triggering hearing loss were recorded in the medical history.
Regarding hearing loss, the age of onset, mode of onset (sudden or progressive), involvement (uni‐ or bilateral, symmetric or asymmetric), progression (stable or progressive), and the presence of other otologic symptoms accompanying hearing loss (tinnitus, vertigo, otalgia, or discharge) were recorded.
Threshold tonal audiometry was performed on both affected individuals and those reporting normal hearing to rule out subclinical hearing loss. The degree of hearing loss was classified according to the American College of Medical Genetics and Genomics criteria 9 : slight (16–25 decibels [dB]), mild (26–40 dB), moderate (41–55 dB), moderately severe (56–70 dB), severe (71–90 dB), or profound (91 dB or greater). Hearing loss configuration, as seen on audiometric analysis, was classified as sloping, flat, rising (low‐frequency), or mid‐frequency (cookie bite) loss. 9
The data related to auditory brainstem responses (ABRs), otoacoustic emissions (OAEs), and vestibular tests, computed tomography (CT), and/or magnetic resonance imaging (MRI) available in the medical records were also collected, along with additional tests to rule out associated syndromic pathology.
Genetic testing for the m.1555A>G mutation was carried out by PCR amplification of a 339 bp DNA fragment containing the mutation site, followed by digestion with restriction endonuclease HaeIII. In the wild‐type allele, digestion results in two fragments (216 bp and 123 bp). The mutation specifically creates a novel restriction site in the 123‐bp fragment and so digestion results in three fragments (216 bp, 93 bp, and 30 bp). 2 , 5 , 10
Statistical analysis of the data collected in this study was conducted using the SPSS program for Windows, including chi‐square tests, t‐tests, and simple and multiple linear regression.
Informed consent was obtained all individuals participating in the study. The study was approved by the CEIM of Cantabria: Code number: 2022.276. 11/11/2022.
RESULTS
During the study period, 172 proband patients were identified with non‐syndromic sensorineural hearing loss of suspected genetic origin. Among the cases, 135 were familial, and 37 were sporadic. The m.1555A>G mutation in the MTRNR1 gene was identified in 20 cases (11.6% of the total cohort). All of these cases were familial, with more than one affected individual in each, and all exhibited a maternal inheritance pattern of hearing loss (Fig. 1). Furthermore, the mutation was absent in 100 controls without hearing loss.
Fig. 1.
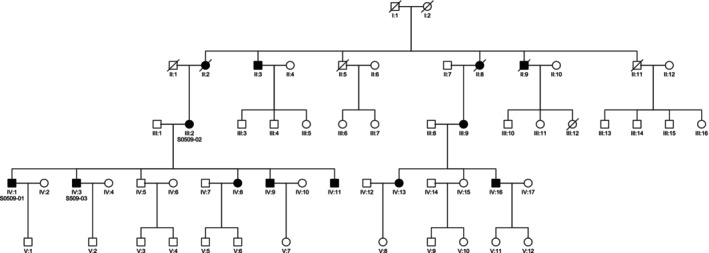
Family tree with code S0509 showing maternal inheritance.
Upon extending the study to relatives, a total of 82 individuals were carriers of the m.1555A>G mutation, including 48 females and 34 males. The age range was between 13 and 92 years (mean 40.51 years, standard deviation 17.03) (Table I).
TABLE I.
Individuals With the m.1555G>G Mutation.
| N | % | |
|---|---|---|
| Sex | ||
| Men | 34 | 41,4 |
| Women | 48 | 58.6 |
| Median age | 40.5 years | |
| Degree of hearing loss | ||
| Normal (<16 dB) | 9 | 10.9 |
| Slight (16–25 dB) | 19 | 23.2 |
| Mild (26–40 dB) | 14 | 17.1 |
| Moderate (41–55 dB) | 11 | 13.4 |
| Moderately severe (56–70 dB) | 13 | 15.9 |
| Severe (71–90 dB) | 12 | 14.6 |
| Profound (>91 dB) | 4 | 4.9 |
| Aminoglycoside exposure | 21 | 25.6 |
| Tinnitus | 19 | 23.2 |
| Vertigo/dizziness/balance disorders | 8 | 9.8 |
% = percentage; dB = decibels; N = number of individuals.
The range of hearing loss severity exhibited considerable diversity (Table S1). Among the individuals, 9 (10.9%) displayed auditory thresholds within the normal range (up to 20 dB) across all frequencies, while others experienced profound hearing impairment. In 28 cases (34.1%), the mean thresholds for frequencies 500, 1000, and 2000 Hz did not surpass 20 dB. Figure 2 illustrates the mean, 25th, and 75th percentiles of auditory thresholds among all individuals carrying the m.1555A>G mutation. Notably, a descending audiometric profile was consistently observed, with a pronounced impact on high frequencies across all cases.
Fig. 2.
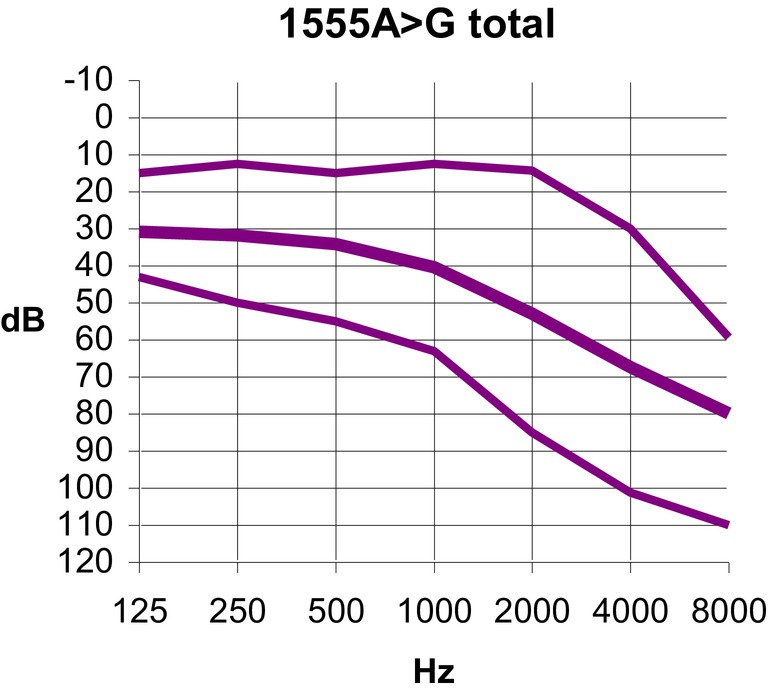
Audiogram depicting the mean audiometric thresholds (thick line) and the 25th and 75th percentiles (thin lines) for all individuals carrying the m.1555A>G mutation. These values are calculated based on the binaural mean for each frequency. [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
Figure 3 presents overlaid audiograms (binaural mean thresholds), distinguishing between individuals with and without a history of aminoglycoside exposure. Figure 4 provides a summary of these findings, depicting the mean, 25th, and 75th percentiles for each frequency. Audiometric thresholds were notably elevated in the group with aminoglycoside exposure across all frequencies (p = 0.0001) (Table II), with no discernible difference between genders concerning the severity of hearing loss (p = 0.2) or the frequency of aminoglycoside exposure (p = 0.8). Patients with hearing loss who were exposed to aminoglycosides were younger (p < 0.01).
Fig. 3.
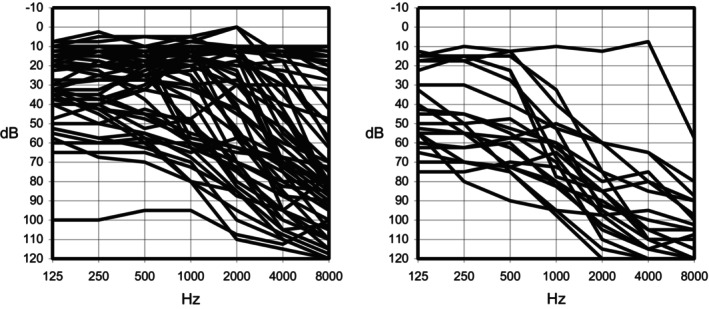
Superposition of audiograms (binaural mean thresholds) for individuals carrying the m.1555A>G mutation. The left panel displays cases not related to medication administration, while the right panel shows cases with a history of medication use (aminoglycosides).
Fig. 4.
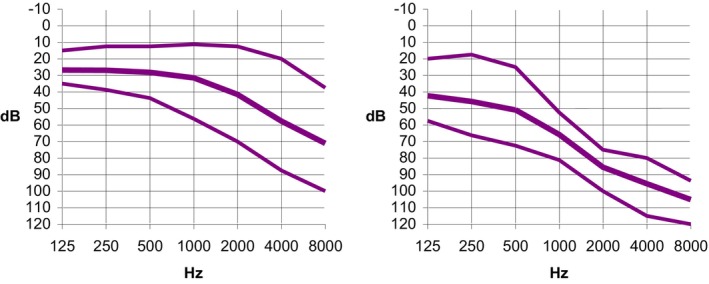
Audiometric thresholds: The thick line represents the mean values, while the thin lines indicate the 25th and 75th percentiles. The left panel shows data not related to medication, and the right panel shows data related to medication. These values are calculated based on the binaural average across each frequency. [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
TABLE II.
Intensity of Hearing Loss in Pure‐Tone Audiometry Based on Previous Exposure to Aminoglycosides.
| Aminoglyside Exposure | Present N (%) | Absent N (%) |
|---|---|---|
| Degree of hearing loss | ||
| Normal (<16 dB) | 1 (4.8) | 8 (13.1) |
| Slight (16–25 dB) | 0 (0.0) | 19 (31.1) |
| Mild (26–40 dB) | 1 (4.8) | 13 (21.3) |
| Moderate (41–55 dB) | 3 (14.3) | 8 (13.1) |
| Moderately severe (56–70 dB) | 7 (33.3) | 6 (9.9) |
| Severe (71–90 dB) | 7 (33.3) | 5 (8.2) |
| Profound (>91 dB) | 2 (9.5) | 2 (3.3) |
| Median age | 46.9 years | 38.4 years |
| Total | 21 (25.6%) | 61 (74.4%) |
% = percentage; dB = decibels; N = number of individuals.
Generally, individuals reporting ototoxic drug exposure mentioned receiving injections during childhood or adolescence for respiratory tract infections or tuberculosis, associating this with hearing loss onset. However, in most cases, these treatments occurred years ago as outpatient procedures, thus not documented in medical records. Among the 21 individuals more clearly linking hearing loss to drug exposure, one case attributed it to Terramycin (oxytetracycline) injections at age 10, while others implicated streptomycin. Furthermore, a patient treated with intravenous gentamicin, unaware of carrying the m.1555A>G mutation, exhibited no audiometric changes, as previously reported. 11
The age of onset of hearing loss in individuals carrying the m.1555A>G mutation varied widely. Although it is challenging to determine these data precisely, three cases reported the onset of deafness during the first 2 years of age. Of these, the first two were related to drug administration, although the third case did not seem to be. In one case, audiological confirmation was present in the medical history at the age of 5. At the other extreme, two individuals carrying the m.1555A>G mutation, at the ages of 32 and 34, respectively, had audiometric thresholds below 20 dB for all frequencies, including 4000 and 8000 Hz. Another case, at the age of 57, showed a mild–moderate drop limited to frequencies 4000 and 8000 Hz. One patient was deaf‐mute; the rest had developed normal speech.
Regarding the progression of hearing loss, some patients reported stable hearing loss, while in other cases, subjective progression was mentioned. Comparing recent audiograms with old ones revealed that in some cases, hearing loss did not evolve, while in others, progression was evident. We represented the degree of hearing loss for all individuals carrying the m.1555A>G mutation, taken as the mean of thresholds for frequencies 500, 1000, and 2000 Hz, based on their age (Fig. 5). Despite the data dispersion, a simple linear regression model was used to calculate the function predicting the degree of hearing loss from age, minimizing differences between observed and expected values:
Fig. 5.
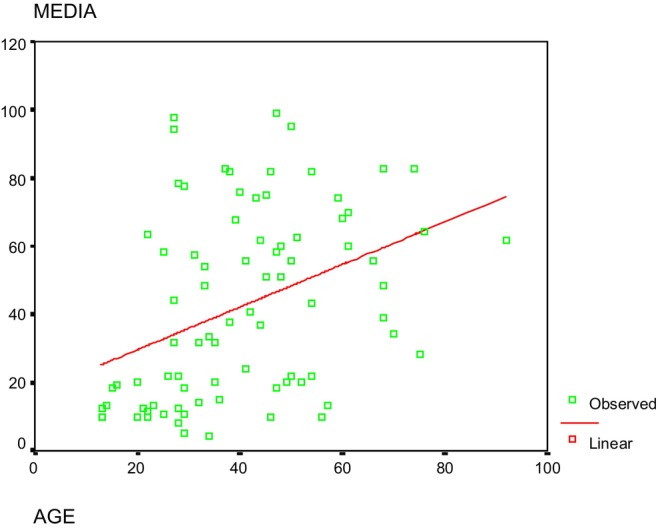
The graph displays the degree of hearing loss (measured by mean thresholds at 500, 1000, and 2000 Hz) plotted against age. A red linear regression line is included to show the trend. R‐value was 0.387 and R square 0.150, p < 0.05. [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
Performing the same analysis independently for individuals with or without a history of aminoglycoside treatment, the following functions were obtained:
In the absence of aminoglycoside exposure:
In the presence of a history of aminoglycoside exposure:
Similarly, through a multiple linear regression model, the function predicting the degree of hearing loss based on age and aminoglycoside exposure history was calculated:
where the aminoglycoside exposure variable (AG) was recoded, assigning a value of 0 in the absence of exposure and a value of 1 if there was exposure.
In 19 cases, patients reported occasional tinnitus associated with hearing loss. The presence of tinnitus was more frequent in individuals with a history of aminoglycoside treatment (p = 0.060). No differences were observed in the distribution of tinnitus between men and women (p = 0.641). Only four individuals from the same family reported regular exposure to loud noise due to working in a canning factory. However, one of them had a normal audiogram, and in the other three, hearing loss was mild.
Eight patients experienced recurrent vertigo, dizziness, or instability. No abnormalities were demonstrated in studies conducted through V‐HIT and/or VNG.
In the 15 cases where exploration was performed through CT and/or MRI, no abnormalities were observed in the inner ear. In 18 patients, ABR tests were conducted, with all findings being consistent with cochlear involvement.
In none of the families were associated diseases found in more than one family member, nor was there a repeating pattern of association with interfamily systemic diseases. Moreover, none of the 82 patients had associated craniofacial malformations.
In all individuals, the m.1555A>G mutation was present in homoplasmy (100% mutant copies), except in one family where the mutation was found in heteroplasmy. The characteristics of this family were previously published. 5
In no family did we detect the mutations m.961delT, m.1095 T > C, and m.1494C > T in MTRNR1, nor the mutations m.7510 T > C or m.7511 T > C in the MTTS1 gene (tRNASer(UCN)).
Of the patients studied, 11 (9.2%) were regular users of hearing aids. Two patients underwent a cochlear implant with good results in speech intelligibility and were still using the implant at the time of the study. The rest of the individuals did not require treatment for hearing loss.
DISCUSSION
The m.1555A>G mutation is widespread across diverse populations worldwide, occurring at a prevalence of approximately 1:500 in the general populace. 12 , 13 Notably, a higher incidence of associated hearing loss is observed in Spain and China. 2 , 14 In Spain, research indicates that it contributes to 15%–20% of all non‐syndromic familial hearing loss cases, independent of inheritance patterns or onset ages. 2 , 15 This elevated occurrence is attributed to the historical liberal usage of streptomycin for minor upper respiratory tract infections in Spain and China following its commercialization. 6 , 16 Although the prevalence of this mutation likely remains consistent across various populations, countries with more restricted usage of aminoglycosides, particularly streptomycin, may experience lower rates of familial hearing loss. For instance, in other European nations, the frequency among hearing‐impaired individuals ranges notably lower, from 0.4% to 3.6%. 15
While the m.1555A>G mutation is predominantly observed in familial cases of hearing loss, it has also been sporadically identified. 17 In our investigation, we detected the m.1555A>G mutation in 19 out of 135 familial cases (14.0%) and in 1 out of 37 sporadic cases (2.7%), although upon further examination, the latter case was found to be familial as well. Additionally, a recent report highlighted a de novo mutation within a Chinese family. 18
The penetrance of the m.1555A>G mutation exhibits variability, with some families showing few affected individuals while others display nearly universal hearing loss among members. Among seven Chinese families studied, the penetrance ranged from 0% to 17% in the absence of aminoglycoside exposure. 19 In the Spanish population, the estimated penetrance of the mutation is 63%. 20
Clinical assessment of individuals carrying the m.1555A>G mutation underscores the wide‐ranging onset of hearing loss, varying both between families and within a family, from childhood onset to adult onset. 21 Moreover, there exists considerable diversity in the degree of hearing loss, ranging from mild losses affecting primarily high frequencies to profound deafness. Early onset of hearing loss before the age of 10 is often associated with severe to profound hearing impairment. 22 The resultant hearing loss typically manifests as sensorineural, bilateral, symmetrical, with a pronounced impact on high frequencies, indicative of a descending audiometric profile.
The progression of hearing loss in individuals with the m.1555A>G mutation exhibits significant variability. While some patients in our study experienced stable hearing over extended periods, others demonstrated varying degrees of progression, albeit none exhibited rapid deterioration without a history of aminoglycoside exposure. Determining whether this progression is primarily attributable to physiological cochlear aging or the mutation itself poses a challenge. Notably, individuals with a history of aminoglycoside exposure did not show accelerated hearing loss progression.
In contrast to patients with other mitochondrial mutations, such as m.3243A > G in the mitochondrial tRNAleu (UUR) gene, which often present significant vestibular system alterations, those carrying the m.1555A>G mutation predominantly manifest cochlear impairment. 23 Our vestibular studies, including V‐HIT and VNG, revealed no associated vestibular abnormalities, with no reports of persistent instability. Even in cases where hearing loss followed streptomycin treatment, vestibular function tests remained normal. Although our findings align with other studies, 22 , 24 Kawashima et al. reported alterations in vestibular‐evoked myogenic potentials in four patients with the m.1555A>G mutation. 25
The variability in the phenotypic expression of the m.1555A>G mutation and the presence of carriers with normal hearing suggest that the mutation may be considered a predisposing factor for the development of hearing loss, requiring other factors for hearing loss to occur. Modulators of the expression of the m.1555A>G mutation may include environmental and genetic factors. Among environmental factors, the most important is a history of exposure to aminoglycoside antibiotics, although other factors, both drug‐related and non‐drug‐related, may exist. Among genetic factors, aspects related to mitochondrial DNA include the presence of the m.1555A>G mutation in homoplasmy or heteroplasmy and the presence of polymorphisms in mitochondrial DNA. Additionally, nuclear DNA‐related aspects involve nuclear modifier genes. 20 , 26 , 27
Aminoglycosides exert their bactericidal effect by binding to the 16S rRNA within the 30S subunit of the bacterial ribosome, thereby impeding protein synthesis. 28 In humans, mitochondrial ribosomes exhibit greater similarity to bacterial ribosomes than cytoplasmic ribosomes. The m.1555A>G mutation appears to heighten the resemblance between human 12S rRNA and bacterial 16S rRNA, resulting in an enhanced affinity for aminoglycosides, thereby disrupting protein synthesis within the mitochondria. 29 , 30 Among aminoglycosides, streptomycin stands out as the primary culprit responsible for hearing loss in individuals with the m.1555A>G mutation. 6 Notably, there are limited reported instances associated with other commonly used aminoglycosides like gentamicin or amikacin. Furthermore, cases have emerged where patients exposed to gentamicin, unaware of their carrier status for the m.1555A>G mutation, did not subsequently develop hearing loss. 11 In specific cases, associations have been made with dihydrostreptomycin 31 or isepamicin, 32 while in many studies, the specific aminoglycoside implicated remains unspecified. 2 , 15 Mutations occurring at various positions in the 12S rRNA gene may heighten susceptibility to the ototoxic effects of certain aminoglycosides.
The probability of developing hearing loss by the age of 30 in individuals carrying the m.1555A>G mutation is reported to be 40% in the absence of exposure to aminoglycosides, increasing to 96% if they have received this treatment. 2 In our study, the multiple linear regression model calculates a mean hearing loss 30 dB higher in individuals with a history of aminoglycoside administration. A single dose of aminoglycosides can trigger hearing loss. Families in which the m.1555A>G mutation is detected in heteroplasmy are very rare. 5 It has been observed that most individuals with less than 20% mutant copies have normal hearing, while subjects with more than 52% mutant copies have hearing loss. 5 This could be interpreted as there being a threshold in the proportion of mutant molecules necessary for hearing loss to occur. However, it is not uncommon for individuals carrying the mutation in homoplasmy to have normal hearing. It should be noted that heteroplasmy levels for a mutation can vary in different tissues of a person, so the percentage of mutant copies present in the blood may differ from that in the inner ear. 33
Imaging tests, encompassing CT and MRI scans, revealed no abnormalities in both our study and those conducted by other researchers. 34 Neurophysiological investigations employing ABR demonstrate involvement of the cochlea. Within our cohort, no concurrent pathologies were detected among multiple family members or across families, corroborating the notion that hearing loss represents the primary manifestation of this mutation.
In our study, patients with moderate hearing loss demonstrated noteworthy improvement upon adapting to hearing aids. Additionally, the two individuals who underwent cochlear implantation exhibited substantial enhancements in speech intelligibility, maintaining long‐term usage of the implants. Similar positive outcomes have been documented in patients with mitochondrial genetic hearing loss, including carriers of the m.1555A>G mutation in the MTRNR1 gene, irrespective of aminoglycoside exposure. 34 , 35
Chen et al., in their analysis of 865 newborns, identified six carriers of the m.1555A>G mutation, all of whom displayed normal OAEs and ABRs. 36 Consequently, routine genetic screening for this mutation in neonatal hearing assessments may not be warranted, except in cases with a maternal history of hearing loss or ototoxicity, aiming to prevent the inadvertent use of aminoglycosides that could precipitate hearing loss in susceptible individuals. 37
The limitations of our study are primarily related to its retrospective nature, which hinders the achievement of a more homogeneous patient cohort. Additionally, being a single‐center study, the generalizability of the results to other populations may be limited. Another constraint is that aminoglycoside administration was self‐reported by patients, introducing a risk of recall bias. Nevertheless, this study represents the largest series published to date on this mutation.
CONCLUSIONS
The prevalence of the m.1555A>G mutation in the 12S rRNA gene of mitochondrial DNA is 14.0% among familial hearing loss cases in Cantabria, showing great variability in the degree of hearing loss and the age of onset. In these patients, hearing deficit is less in the absence of exposure to aminoglycoside antibiotics (especially streptomycin) and when the percentage of mutant copies is lower in cases with the mutation in heteroplasmy. Patients carrying this mutation who have severe or profound hearing loss respond well to rehabilitation with hearing aids or cochlear implants.
It is advisable to conduct genetic studies for this mutation in patients with a family history of hearing loss, to avoid the use of aminoglycosides if the mutation is present.
Supporting information
Supplementary Table S1. List of individuals carrying the 1555A>G mutation, indicating their age in years at the time of audiometry (E), relationship of hearing loss with drug administration (F), and audiometric thresholds (in dB) in both ears within the range of 0.125–8 kHz. S: yes. N: no.
Editor's Note: This Manuscript was accepted for publication on September 13, 2024.
The authors declare that there are no conflicts of interest.
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
BIBLIOGRAPHY
- 1. DiMauro S, Davidzon G. Mitochondrial DNA and disease. Ann Med. 2005;37:222‐232. [DOI] [PubMed] [Google Scholar]
- 2. Estivill X, Govea N, Barceló E, et al. Familial progressive sensorineural deafness is mainly due to the mtDNA A1555G mutation and is enhanced by treatment of aminoglycosides. Am J Hum Genet. 1998;62:27‐35. 10.1086/301676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fischel‐Ghodsian N. Mitochondrial deafness. Ear Hear. 2003;24:303‐313. [DOI] [PubMed] [Google Scholar]
- 4. Mutai H, Watabe T, Kosaki K, Ogawa K, Matsunaga T. Mitochondrial mutations in maternally inherited hearing loss. BMC Med Genet. 2017;18:32. 10.1186/s12881-017-0389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. del Castillo FJ, Rodríguez‐Ballesteros M, Martín Y, et al. Heteroplasmy for the 1555A>G mutation in the mitochondrial 12S rRNA gene in six Spanish families with non‐syndromic hearing loss. J Med Genet. 2003;40:632‐636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gallo‐Terán J, Arellano B, Morales‐Angulo C, et al. Prevalence of the A1555G mutation in the mitochondrial DNA in patients with cochlear or vestibular damage due to aminoglycoside‐induced ototoxicity. Acta Otorrinolaringol Esp. 2004;55:212‐217. 10.1016/s0001-6519(04)78511-8. [DOI] [PubMed] [Google Scholar]
- 7. Bacino C, Prezant TR, Bu X, Fournier P, Fischel‐Ghodsian N. Susceptibility mutations in the mitochondrial small ribosomal RNA gene in aminoglycoside induced deafness. Pharmacogenetics. 1995;5:165‐172. [DOI] [PubMed] [Google Scholar]
- 8. Tessa A, Giannotti A, Tieri L, Vilarinho L, Marotta G, Santorelli FM. Maternally inherited deafness associated with a T1095C mutation in the mDNA. Europ J Hum Genet. 2001;9:147‐149. [DOI] [PubMed] [Google Scholar]
- 9. Li MM, Tayoun AA, DiStefano M, et al. ACMG professional practice and guidelines committee. Clinical evaluation and etiologic diagnosis of hearing loss: a clinical practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2022;24:1392‐1406. 10.1016/j.gim.2022.03.018. [DOI] [PubMed] [Google Scholar]
- 10. Morales Angulo C, Gallo Terán J, del Castillo I, Moreno Pelayo MA, García‐Mantilla J, Moreno HF. Audiometric features of familial hearing impairment transmitted by mitochondrial inheritance (A1555G). Acta Otorrinolaringol Esp. 2002;53:641‐648. 10.1016/s0001-6519(02)78358-1. [DOI] [PubMed] [Google Scholar]
- 11. Salomón‐Felechosa C, Gallo‐Terán J, Morales‐Angulo C. Absence of hypersensitivity to the ototoxic effect of gentamicin in a patient carrying the 1555A>G mutation in the MT‐RNR1 gene. Acta Otorrinolaring Esp. 2023;74:403‐405. 10.1016/j.otoeng.2023.03.009. [DOI] [PubMed] [Google Scholar]
- 12. Bitner‐Glindzicz M, Pembrey M, Duncan A, et al. Prevalence of mitochondrial 1555A>G mutation in European children. N Engl J Med. 2009;360:640‐642. 10.1056/NEJMc0806396. [DOI] [PubMed] [Google Scholar]
- 13. Vandebona H, Mitchell P, Manwaring N, et al. Prevalence of mitochondrial 1555A>G mutation in adults of European descent. N Engl J Med. 2009;360:642‐644. 10.1056/NEJMc0806397. [DOI] [PubMed] [Google Scholar]
- 14. Li Z, Li R, Chen J, et al. Mutational analysis of the mitochondrial 12S rRNA gene in Chinese pediatric subjects with aminoglycoside‐induced and non‐syndromic hearing loss. Hum Genet. 2005;117:9‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Del Castillo I, Morín M, Domínguez‐Ruiz M, Moreno‐Pelayo MA. Genetic etiology of non‐syndromic hearing loss in Europe. Hum Genet. 2022;141:683‐696. 10.1007/s00439-021-02425-6. [DOI] [PubMed] [Google Scholar]
- 16. Gallo‐Terán J, Morales‐Angulo C, del Castillo I, et al. Incidence of A1555G mutations in the mitocondrial DNA and 35delG in the GJB2 gene (connexion‐26) in families with late onset non‐syndromic sensorineural hearing loss from Cantabria. Acta Otorrinolaringol Esp. 2002;53:563‐571. 10.1016/s0001-6519(02)78349-0. [DOI] [PubMed] [Google Scholar]
- 17. Morales Angulo C, Gallo‐Terán J, Señaris B, Fontalva A, González‐Aguado R, Fernández‐Luna JL. Prevalence of the A1555G MTDNA mutation in sporadic hearing‐impaired patients without known history of aminoglycoside treatment. Acta Otorrinolaringol Esp. 2011;62:83‐86. 10.1016/j.otorri.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 18. Gu P, Wang G, Gao X, Kang D, Dai P, Huang S. Clinical and molecular findings in a Chinese family with a de novo mitochondrial A1555G mutation. BMC Med Genomics. 2022;15:121. 10.1186/s12920-022-01276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tang X, Yang L, Zhu Y, et al. Very low penetrance of hearing loss in seven Han Chinese pedigrees carrying the deafness‐associated 12S rRNA A1555G mutation. Gene. 2007;393:11‐19. [DOI] [PubMed] [Google Scholar]
- 20. Ballana E, Morales E, Rabionet R, et al. Mitochondrial 12S rRNA gene mutations affect RNA secondary structure and lead to variable penetrance in hearing impairment. Biochem Biophys Res Commun. 2006;341:950‐957. [DOI] [PubMed] [Google Scholar]
- 21. Noguchi Y, Yashima T, Ito T, Sumi T, Tsuzuku T, Kitamura K. Audiovestibular findings in patients with mitochondrial A1555G mutation. Laryngoscope. 2004;114:344‐348. 10.1097/00005537-200402000-00031. [DOI] [PubMed] [Google Scholar]
- 22. Matsunaga T, Kumanomido H, Shiroma M, Ohtsuka A, Asamura K, Usami S. Deafness due to A1555G mitochondrial mutation without use of aminoglycoside. Laryngoscope. 2004;114:1085‐1091. [DOI] [PubMed] [Google Scholar]
- 23. Inoue A, Iwasaki S, Fujimoto C, Kinoshita M, Yamasoba T. Progresion of peripheral vestibular dysfuntions in patients with a mitochondrial A3243G mutation. Otol Neurotol. 2019;40:359‐364. 10.1097/MAO.0000000000002091. [DOI] [PubMed] [Google Scholar]
- 24. Tono T, Kiyomizu K, Matsuda K, et al. Different clinical characteristics of aminoglycoside‐induced deafness with and without the 1555 a>G mitochondrial mutation. ORL J Otorhinolaryngol Relat Spec. 2001;63:25‐30. [DOI] [PubMed] [Google Scholar]
- 25. Kawashima Y, Noguchi Y, Ito T, Kitamura K. Vestibular evoked myogenic potentials in patients with the mitochondrial A1555G mutation. Laryngoscope. 2009;119:1874‐1879. 10.1002/lary.20584. [DOI] [PubMed] [Google Scholar]
- 26. Bykhovskaya Y, Mengesha E, Wang D, et al. Phenotype of non‐syndromic deafness associated with the mitochondrial A1555G mutation is modulated by mitochondrial RNA modifying enzymes MTO1 and GTPBP3. Mol Genet Metab. 2004;83:199‐206. [DOI] [PubMed] [Google Scholar]
- 27. Bykhovskaya Y, Mengesha E, Wang D, et al. Human mitochondrial transcription factor B1 as a modifier gene for hearing loss associated with the mitochondrial A1555G mutation. Mol Genet Metab. 2004;82:27‐32. [DOI] [PubMed] [Google Scholar]
- 28. Prezant TR, Agapian JV, Bohlman MC, et al. Mitochondrial ribosomal RNA mutation associated with both antibiotic‐induced and non‐syndromic deafness. Nat Genet. 1993;4:289‐294. [DOI] [PubMed] [Google Scholar]
- 29. Hamasaki K, Rando RR. Specific binding of aminoglycosides to a human rRNA construct based on a DNA polymorfism which causes aminoglycoside‐induced deafness. Biochemistry. 1997;36:12323‐12328. [DOI] [PubMed] [Google Scholar]
- 30. Hutchin T, Haworth I, Higashi K, et al. A molecular basis for human hypersensitivity to aminoglycoside antibiotics. Nucleic Acid Res. 1993;21:4174‐4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsuiki T, Murai K, Murai S, Kitamura K, Tamagawa Y. Audiologic features of hearing loss due to the 1555 mutation of mitochondrial DNA. Ann Otol Rhinol Laryngol. 1997;106:643‐648. [DOI] [PubMed] [Google Scholar]
- 32. Usami S, Abe S, Tono T, Komune S, Kimberling WJ, Shinkawa H. Isepamicin sulfate‐induced sensorineural hearing loss in patients with the 1555 a>G mitochondrial mutation. ORL J Otorhinolaryngol Relat Spec. 1998;60:164‐169. [DOI] [PubMed] [Google Scholar]
- 33. Matthews PM, Hopkin J, Brown RM, Stephenson JBP, Hilton‐Jones D, Brown GK. Comparison of the relative levels of the 3243 (a>G) mtDNA mutation in heteroplasmic adult and fetal tissues. J Med Genet. 1994;31:41‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Usami S, Kasai AS, Shinkawa H, Moeller B, Kenyon JB, Kimberling WJ. Genetic and clinical features of sensorineural hearing loss associated with the 1555 mitochondrial mutation. Laryngoscope. 1997;107:483‐490. [DOI] [PubMed] [Google Scholar]
- 35. Tono T, Ushisako Y, Kiyomizu K, et al. Cochlear implantation in a patient with profound hearing loss with the A1555G mitochondrial mutation. Am J Otol. 1998;19:754‐757. [PubMed] [Google Scholar]
- 36. Chen G, Wang X, Fu S. Prevalence of A1555G mitochondrial mutation in Chinese newborns and the correlation with neonatal hearing screening. Int J Pediatr Otorhinolaryngol. 2011;75:532‐534. 10.1016/j.ijporl.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 37. Gürtler N, Schmuziger N, Kim Y, Mhatre AN, Jungi M, Lalwani AK. Audiologic testing and molecular analysis of 12S rRNA in patients receiving aminoglycosides. Laryngoscope. 2005;115:640‐644. 10.1097/01.mlg.0000161355.28073.f5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. List of individuals carrying the 1555A>G mutation, indicating their age in years at the time of audiometry (E), relationship of hearing loss with drug administration (F), and audiometric thresholds (in dB) in both ears within the range of 0.125–8 kHz. S: yes. N: no.


