Abstract
Alcohol dehydrogenase was partially purified from yeast (Saccharomyces cerevisiae) grown in the presence of 20 muM-MnSO4 without added Zn2+ and from yeast grown in the presence of 1.8 muM-MnSO4. The enzyme from yeast grown with added Zn2+ has the same properties as the crystalline enzyme from commercial supplies of baker's yeast. The enzyme from yeast grown without added An2+ has quite different properties. It has a mol.wt. in the region of 72000 and an S 20 w of 5.8S. The values can be compared with a mol.wt. of 141000 and an S 20 w of 7.6S for the crystalline enzyme. ADP-ribose, a common impurity in commercial samples of NAD+, is a potent competitive inhibitor of the new enzyme (K1 = 0.5 muM), but is not so for the crystalline enzyme. The observed maximum rate of ethanol oxidation at pH 7.05 and 25 degrees C was decreased 12-fold by the presence of 0.06 mol of inhibitor/mol of NAD+ when using the enzyme from Zn2+-deficient yeast, but with crystalline enzyme the maximum rate was essentially unchanged by this concentration of inhibitor. The kinetic characteristics for the two enzymes with ethanol, butan-1-ol, acetaldehyde and butyraldehyde as substrates are markedly different. These kinetic differences are discussed in relation to the mechanism of catalysis for the enzyme from Zn2+-deficient yeast.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREASEN A. A., STIER T. J. B. Anaerobic nutrition of Saccharomyces cerevisiae. I. Ergosterol requirement for growth in a defined medium. J Cell Physiol. 1953 Feb;41(1):23–36. doi: 10.1002/jcp.1030410103. [DOI] [PubMed] [Google Scholar]
- ANDREASEN A. A., STIER T. J. Anaerobic nutrition of Saccharomyces cerevisiae. II. Unsaturated fatty acid requirement for growth in a defined medium. J Cell Physiol. 1954 Jun;43(3):271–281. doi: 10.1002/jcp.1030430303. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brändén C. I., Eklund H., Nordström B., Boiwe T., Söderlund G., Zeppezauer E., Ohlsson I., Akeson A. Structure of liver alcohol dehydrogenase at 2.9-angstrom resolution. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2439–2442. doi: 10.1073/pnas.70.8.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bühner M., Sund H. Yeast alcohol dehydrogenase: SH groups, disulfide groups, quaternary structure, and reactivation by reductive cleavage of disulfide groups. Eur J Biochem. 1969 Nov;11(1):73–79. doi: 10.1111/j.1432-1033.1969.tb00741.x. [DOI] [PubMed] [Google Scholar]
- Ciriacy M. Genetics of alcohol dehydrogenase in Saccharomyces cerevisiae. II. Two loci controlling synthesis of the glucose-repressible ADH II. Mol Gen Genet. 1975;138(2):157–164. doi: 10.1007/BF02428119. [DOI] [PubMed] [Google Scholar]
- Coleman P. L., Weiner H. Growth, isolation, and characterization of a yeast manganese alcohol dehydrogenase. Biochemistry. 1973 Aug 28;12(18):3466–3472. doi: 10.1021/bi00742a017. [DOI] [PubMed] [Google Scholar]
- Creighton D. J., Sigman D. S. A model for alcohol dehydrogenase. The zinc ion catalyzed reduction of 1,10-phenanthroline-2-carboxaldehyde by N-propyl-1,4-dihydronicotinamide. J Am Chem Soc. 1971 Nov;93(23):6314–6316. doi: 10.1021/ja00752a079. [DOI] [PubMed] [Google Scholar]
- Curdel A., Iwatsubo M. Biosynthetic incorporation of cobalt into yeast alcohol dehydrogenase. FEBS Lett. 1968 Aug;1(3):133–136. doi: 10.1016/0014-5793(68)80040-7. [DOI] [PubMed] [Google Scholar]
- DALZIEL K. Kinetic studies of liver alcohol dehydrogenase. Biochem J. 1962 Aug;84:244–254. doi: 10.1042/bj0840244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALZIEL K. Some observations on the preparation and properties of dihydronicotinamide-adenine dinucleotide. Biochem J. 1962 Aug;84:240–244. doi: 10.1042/bj0840240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALZIEL K. The purification of nicotinamide adenine dinucleotide and kinetic effects of nucleotide impurities. J Biol Chem. 1963 Apr;238:1538–1543. [PubMed] [Google Scholar]
- Dickenson C. J., Dickinson F. M. A study of the oxidation of butan-1-ol and propan-2-ol by nicotinamide-adenine dinucleotide catalysed by yeast alcohol dehydrogenase. Biochem J. 1975 Jun;147(3):541–547. doi: 10.1042/bj1470541. [DOI] [PMC free article] [PubMed] [Google Scholar]
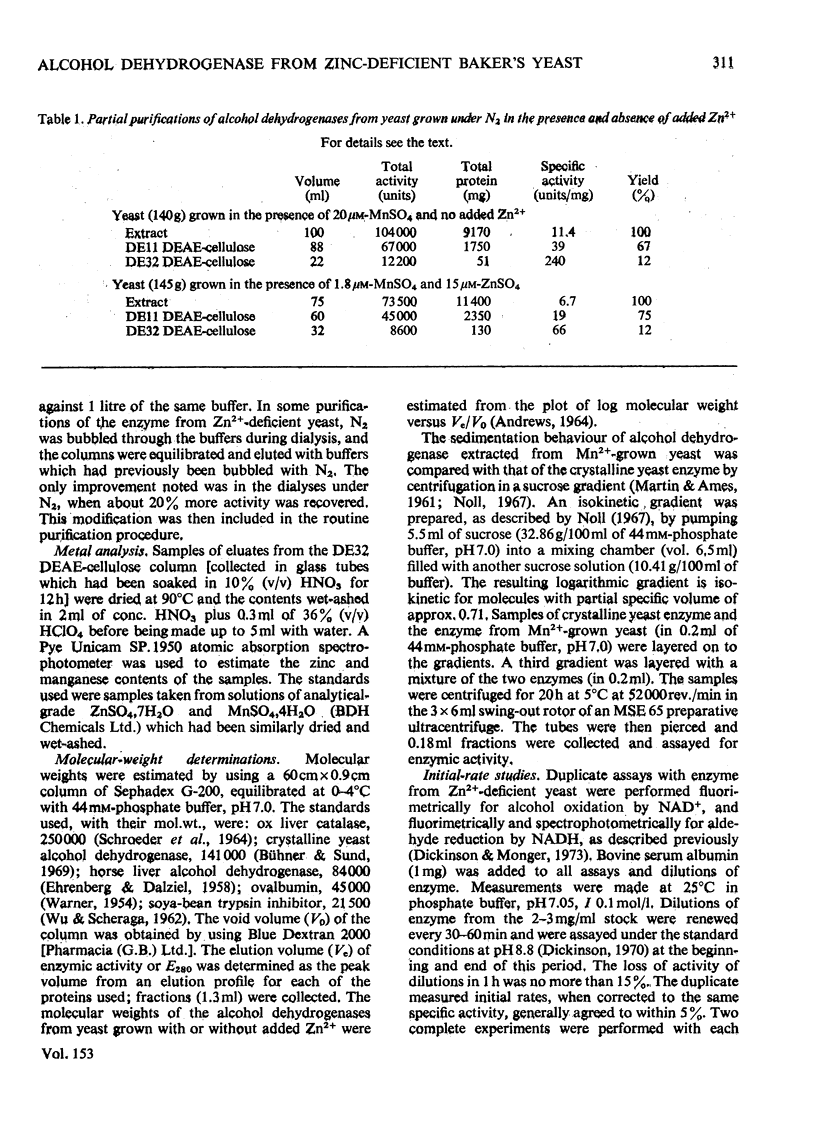
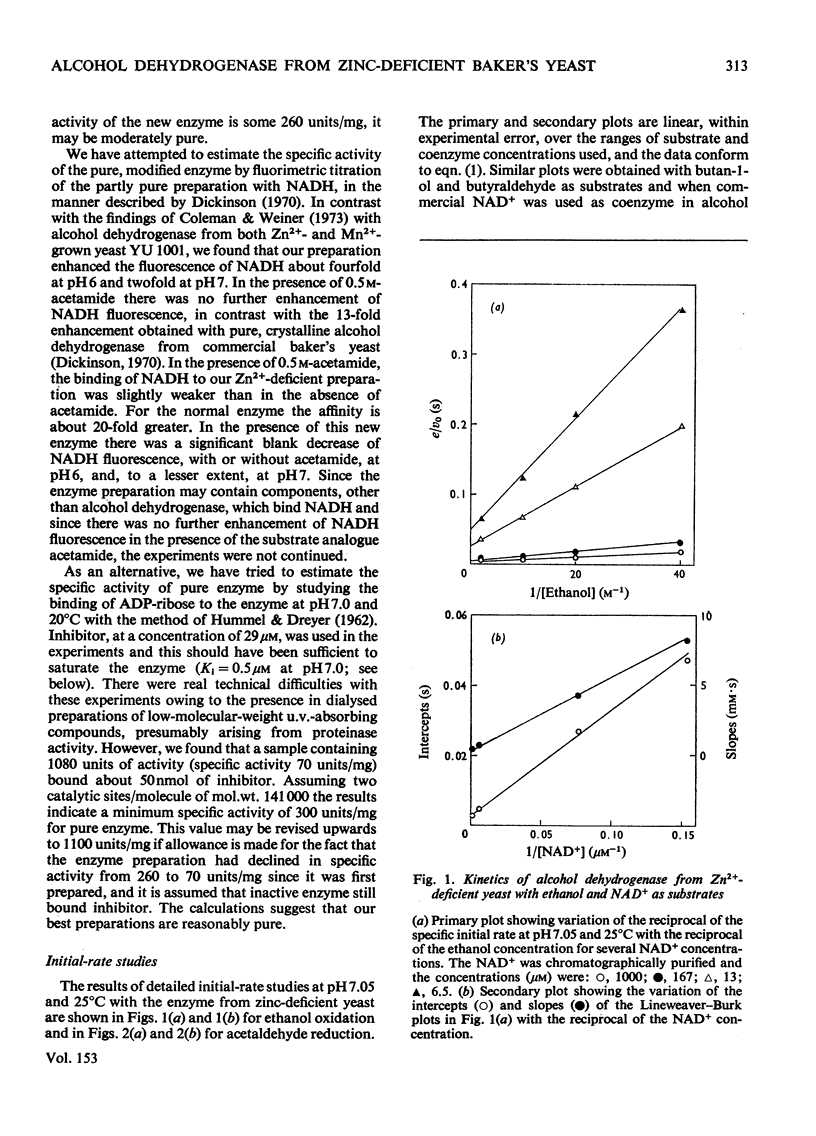
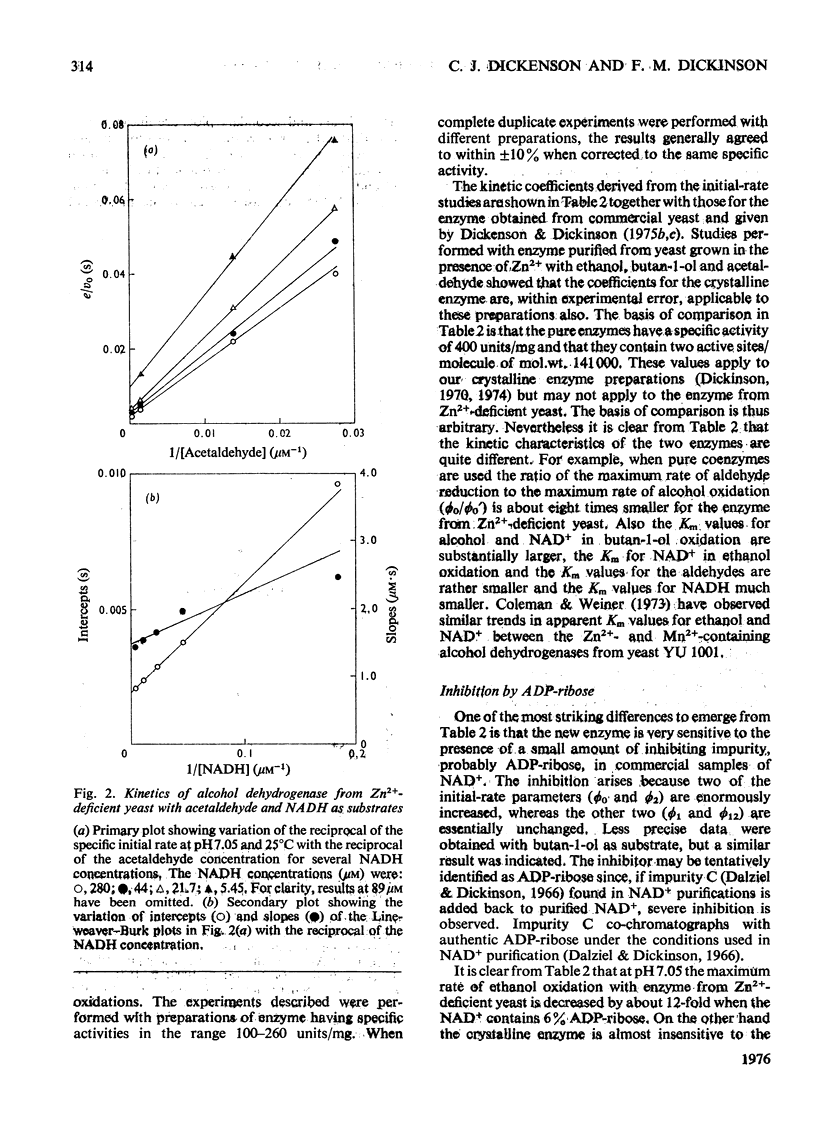
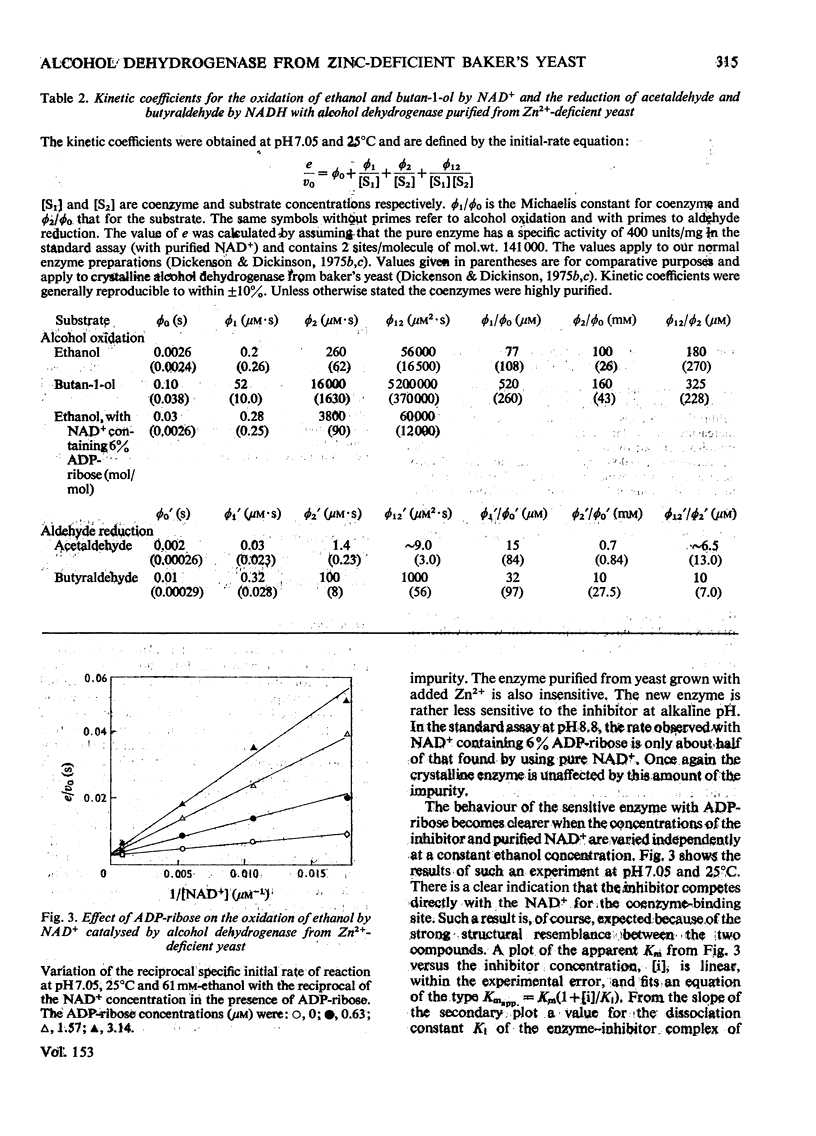
- Dickenson C. J., Dickinson F. M. A study of the pH- and temperature-dependence of the reactions of yeast alcohol dehydrogenase with ethanol, acetaldehyde and butyraldehyde as substrates. Biochem J. 1975 May;147(2):303–311. doi: 10.1042/bj1470303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson C. J., Dickinson F. M. The role of an essential histidine residue of yeast alcohol dehydrogenase. Eur J Biochem. 1975 Apr 1;52(3):595–603. doi: 10.1111/j.1432-1033.1975.tb04031.x. [DOI] [PubMed] [Google Scholar]
- Dickinson F. M., Monger G. P. A study of the kinetics and mechanism of yeast alcohol dehydrogenase with a variety of substrates. Biochem J. 1973 Feb;131(2):261–270. doi: 10.1042/bj1310261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson F. M. Role of the essential thiol groups of yeast alcohol dehydrogenase. Biochem J. 1972 Jan;126(1):133–138. doi: 10.1042/bj1260133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson F. M. The binding of dihydronicotinamide--adenine dinucleotide and pyridine-3-aldehyde--adenine dinucleotide by yeast alcohol dehydrogenase. Biochem J. 1970 Dec;120(4):821–830. doi: 10.1042/bj1200821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson M. Measurements of the concentration of active sites in preparations of yeast alcohol dehydrogenase. Eur J Biochem. 1974 Jan 3;41(1):31–36. doi: 10.1111/j.1432-1033.1974.tb03240.x. [DOI] [PubMed] [Google Scholar]
- HAYES J. E., Jr, VELICK S. F. Yeast alcohol dehydrogenase: molecular weight, coenzyme binding, and reaction equilibria. J Biol Chem. 1954 Mar;207(1):225–244. [PubMed] [Google Scholar]
- HOCH F. L., WILLIAMS R. J., VALLEE B. L. The role of zinc in alcohol dehydrogenases. II. The kinetics of the instantaneous reversible inhibition of yeast alcohol dehydrogenase by 1,10-phenanthroline. J Biol Chem. 1958 May;232(1):453–464. [PubMed] [Google Scholar]
- HUMMEL J. P., DREYER W. J. Measurement of protein-binding phenomena by gel filtration. Biochim Biophys Acta. 1962 Oct 8;63:530–532. doi: 10.1016/0006-3002(62)90124-5. [DOI] [PubMed] [Google Scholar]
- Jörnvall H. Partial similarities between yeast and liver alcohol dehydrogenases. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2295–2298. doi: 10.1073/pnas.70.8.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAGI J. H., VALLEE B. L. The role of zinc in alcohol dehydrogenase. V. The effect of metal-binding agents on thestructure of the yeast alcohol dehydrogenase molecule. J Biol Chem. 1960 Nov;235:3188–3192. [PubMed] [Google Scholar]
- Leskovac V., Pavkov-Pericin D. Evidence for a histidine and a cysteine residue in the substrate-binding site of yeast alcohol dehydrogenase. Biochem J. 1975 Mar;145(3):581–590. doi: 10.1042/bj1450581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutstorf U., Megnet R. Multiple forms of alcohol dehydrogenase in Saccharomyces cerevisiae. I. Physiological control of ADH-2 and properties of ADH-2 and ADH-4. Arch Biochem Biophys. 1968 Sep 10;126(3):933–944. doi: 10.1016/0003-9861(68)90487-6. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Noll H. Characterization of macromolecules by constant velocity sedimentation. Nature. 1967 Jul 22;215(5099):360–363. doi: 10.1038/215360a0. [DOI] [PubMed] [Google Scholar]
- Rabin B. R., Cruz J. R., Watts D. C., Whitehead E. P. The reaction of yeast alcohol dehydrogenase with iodoacetamide as determined with a silver-silver iodide electrode. Biochem J. 1964 Mar;90(3):539–542. doi: 10.1042/bj0900539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHROEDER W. A., SHELTON J. R., SHELTON J. B., OLSON B. M. SOME AMINO ACID SEQUENCES IN BOVINE-LIVER CATALASE. Biochim Biophys Acta. 1964 Jul 8;89:47–65. doi: 10.1016/0926-6569(64)90100-2. [DOI] [PubMed] [Google Scholar]
- SILVERSTEIN E., BOYER P. D. EQUILIBRIUM REACTION RATES AND THE MECHANISMS OF LIVER AND YEAST ALCOHOL DEHYDROGENASE. J Biol Chem. 1964 Nov;239:3908–3914. [PubMed] [Google Scholar]
- WU Y. V., SCHERAGA H. A. Studies of soybean trypsin inhibitor. I. Physicochemical properties. Biochemistry. 1962 Jul;1:698–705. doi: 10.1021/bi00910a025. [DOI] [PubMed] [Google Scholar]
- Wenger J. I., Bernofsky C. Mitochondrial alcohol dehydrogenase from Saccharomyces cerevisiae. Biochim Biophys Acta. 1971 Mar 10;227(3):479–490. doi: 10.1016/0005-2744(71)90001-5. [DOI] [PubMed] [Google Scholar]


