Introduction
Membranous nephropathy (MN) that occurs posttransplantation can represent either recurrent or de novo disease. Phospholipase A2 receptor (PLA2R) is the predominant antigen in recurrent MN, which is expected given that most cases with MN in native kidneys are PLA2R-positive. De novo MN (DNMN) can occur posttransplantation in 1.5% to 9% of allografts.1 DNMN has been shown to be associated with antibody-mediated rejection (ABMR, primarily C4d positive) in 60%2 and vascular rejection in 80%.3, 4, 5 Several novel antigens were identified in MN (Supplemental References S1–S5), and while PLA2R is the most common antigen in recurrent MN,6,7 the antigen in DNMN remains unidentified, limiting our understanding of this unique entity.
Protocadherin FAT1 was recently identified as an antigen in MN associated with hematopoietic stem cell transplantation.8 Because an alloimmune response may underlie the pathogenesis of the MN that occurs after hematopoietic stem cell transplantation and in DNMN in the presence of rejection, mechanisms and target antigens may be shared among these entities. Therefore, we hypothesized that FAT1 may be the target antigen associated with DNMN.
Results
Clinical Characteristics
We identified 6 patients with FAT1 MN through immunostaining biopsies (Supplementary Figure 1, Supplementary Methods). No patients had MN as the etiology of ESKD. Five patients did not have a native biopsy, and the etiology was based on clinical conjecture regarding the cause, including diabetes (n = 2), obstructive uropathy, congenital renal hypoplasia, or uncontrolled hypertension (Table 1). Of the patients who were FAT1-positive (Table 1), the mean age was 43.3 years with 3 males and 3 females. All demonstrated an elevated creatinine at biopsy (mean = 2.3 ± 1.3 mg/dl). One patient was on dialysis at presentation. None of the study patients had a history of autoimmune disease and 1 had cancer. All patients with available donor-specific antibodies (DSA) results were positive (n = 5), with the most common being human leukocyte antigen (HLA)-DQ (Table 1).
Table 1.
Demographic, clinical, and pathologic characteristics of patients with FAT1-positive de novo membranous nephropathy
| Case | 1A | 1B | 1C | 2 | 3 | 4A | 4B | 5A | 5B | 5C | 6A | 6B |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||||||
| Age | 49 | 43 | 25 | 22 | 47 | 58 | ||||||
| Sex | F | M | M | F | F | M | ||||||
| Race | Black | Hispanic | White | White | White | Asian | ||||||
| Clinical characteristics | ||||||||||||
| HTN | Yes | Yes | Yes | No | Yes | Yes | ||||||
| DM | No | Yes | No | No | No | Yes | ||||||
| Autoimmune | No | No | No | No | No | No | ||||||
| Cancer | Yes | No | No | No | No | No | ||||||
| Cause ESKD | HTN/pre-eclampsia | DM1 | Obstruct | Renal hypoplasia | HTN (focal global sclerosis and hypertensive vascular changes) | DM2 | ||||||
| Time post-tx | 2 yr | 2.5 yr | 2.9 yr | 11 yr | 15 yr | 4 yr | 4.1 yr | 4 yr | 4.5 yr | 5 yr | 3.5 yr | |
| Immunosup. | P, T,MMF | P, T | P, T | None | None | P, T,S | P, T,S | P, T, MMF | P, T,MMF | P, T,MMF | P, T, MMF | |
| Laboratory data | ||||||||||||
| Serum Cr | 1.2 (0.8) | 2.1 | 1.3 | 4.2 | Dialysis | 1.4 | 1.2 | 2.4 | 4.5 | Dialysis | 1.1 | |
| Proteinuria | 0.11 | 0.17 | 0.12 | Sub | Nephrot | 1.5 | 0.5 | 0.7 | 1.5 | Anuria | 1.3 | |
| DSA | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | yes | Yes |
| DSA type/titer | DQ9- DQA1; DQ7-DQA1 × 05 | B72-2,186 DQA1 × 05 3,210 |
DQA1× 05 2,487 | Type unknown, but HLA-DR mismatch | Type unknown | DQA1× 05 21064 |
Resolve | DQA1 × 03 16350 | DQA1 × 03 15098 | DQA1 × 03 20398 |
DQ6 | DQ 6 |
| Follow-up time | 26 mo | NA | 14 mo | 1.5 yr | 5 yr | NA | ||||||
| Follow-up Cr | 1.0 | NA | Dialysis | 1.4 | Dialysis | NA | ||||||
| Follow-up Prot | Undetected | NA | Dialysis | Undetected | Dialysis | NA | ||||||
| Remission | CR + DSAs undetected | Unk | NR | CR | NR - Listed for transplantation | Unk | ||||||
| Allograft rejection | ||||||||||||
| Antibody | No | Yes | Yes | Yes | Yes - CA | Yes | No | Yes | Yes | Yes | No | No |
| g | 0 | 1 | 1 | 2 | 3 | 0 | 0 | 1 | 1 | 1 | 0 | 0 |
| ptc | 0 | 2 | 1 | 2 | 2 | 2 | 0 | 3 | 2 | 2 | 0 | 0 |
| C4d | 0 | 2 | 2 | 2 | 2 | 0 | 0 | 2 | 2 | 2 | 0 | 0 |
| T-cell | No | Yes, 1B | No | Yes | No | IB | Yes | 1A | 2B | 2B | No | No |
| i | 0 | 3 | 0 | 2 | 1 | 3 | 2 | 3 | 2 | 1 | 0 | 0 |
| t | 0 | 3 | 0 | 3 | 1 | 3 | 2 | 3 | 2 | 1 | 0 | 0 |
| Vascular | Yes, 2A | Yes, 2A | Yes, 2A | Yes, 2B | Yes, 2A | No | No | No | Yes | Yes | No | No |
| v | 1 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Membranous nephropathy | ||||||||||||
| Seg/global | Seg | Seg | Seg | Seg | Global | Global | Res | Seg | Seg | Seg | Seg | Seg |
| IgG | 3 | 2 to 3 | 1 | 2 to 3 | 3 | 1 | 0 | 3 | 2 | 2 | 2 | 1 |
| C3 | 1 to 2 | 1 | 0 | 0 | 1 | Trace | 0 | 1 | 0 | 1 | 1 | 0 |
| IgA | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| IgM | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C1q | 1 to 2 | 2 to 3 | 1 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Extraglomerular | No | No | No | No | No | No | No | No | No | No | No | No |
| Subepithelial | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Mesangial | Yes | Yes | Yes | No | No | No | No | No | No | No | Yes | Yes |
| Subendo | No | No | No | No | No | No | No | No | No | No | No | No |
| EM stage | 1 to 2 | 2 | 2 | 1 | 3 to 4 | 1 | 0 | 3 to 4 | 3 to 4 | 3 to 4 | 3 to 4 | 3 to 4 |
CA, chronic active; CR, Complete remission; DM, diabetes mellitus; EM, electron microscopy, ESKD, end-stage kidney disease; F, female; g, glomerulitis; Hisp, Hispanic; immunosup, immunosuppression; i, interstitial inflammation; M, male; MMF, mycophenolate mofetil; nephrot, nephrotic range proteinuria; NR, no remission; obstruct, obstructive uropathy; post-tx, posttransplant; P, prednisone; PR, partial remission; ptc, peritubular capillaritis; res, resolved; S, sirolimus; seg, segmental; sub, subnephrotic (range proteinuria); subendo, subendothelial deposits; T, tacrolimus; t, tubulitis; unk, unknown; v, vasculitis; yr, years.
We examined a larger cohort of transplant biopsies to determine the frequency of MN within allografts. Among 24,380 transplant biopsies, 347 (1.4%) had MN. Of biopsies with rejection (n = 10,026), 173 had MN (1.7%). Of these, 53 had ABMR (30.6%), 12 had T cell-mediated rejection (TCMR) (6.9%), 84 had mixed rejection (48.6%), 3 had vascular rejection (1.7%), and 21 had borderline changes for TCMR (6.9%). For patients without rejection (n = 14,354), 174 had recurrent MN (1.2%). Patients with MN with rejection had lower PLA2R positivity than without rejection (30.3% vs. 50.4%), and the lowest was noted for those with mixed rejection (21.7%). The frequency of PLA2R-positivity in MN without rejection was similar to native biopsies.9 The majority of MN with rejection was DNMN (84.4%), rather than recurrence (n = 27, 15.6%). Although DNMN is an important cause of proteinuria, these data demonstrate a low overall frequency within allografts. Further data are included in Supplementary Table 1.
Histopathologic Characteristics
Twelve biopsies were available from these 6 patients with FAT1, 4 with serial biopsies. Eleven were positive for FAT1 within immune deposits and 1 was a follow-up case with remission. All cases were negative for PLA2R, NELL1, and THSD7A with histologic features of rejection (Supplementary Figure 2). Global IgG staining along glomerular basement membranes was seen in 2 biopsies, 9 with segmental staining. C3 staining had weaker intensity than IgG in all cases, as previously reported in FAT1 MN.8 No cases demonstrated tubular basement membrane staining. One had mesangial deposits (Figure 1, Table 1). This varied from recurrent MN, which demonstrates global IgG staining, C3 codominance, and PLA2R positivity (Supplementary Figure 3). We assessed the presence of HLA-DQ by immunostaining and did not identify HLA-DQ within glomeruli of FAT1 cases (Supplementary Figure 4) or PLA2R controls.
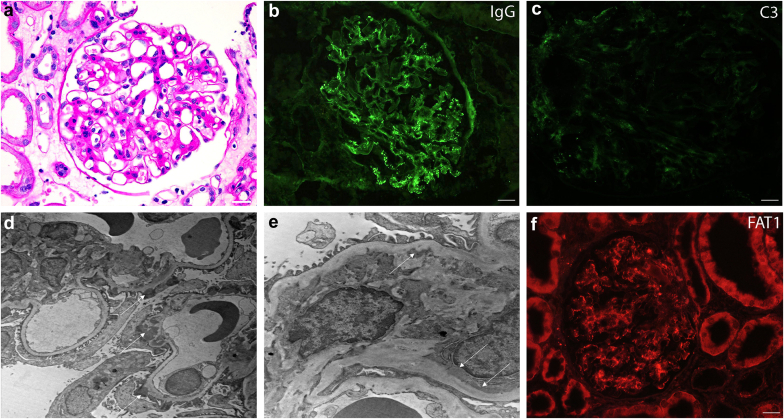
Figure 1.
Histopathology of the membranous nephropathy component of a typical case of FAT1-positive DNMN. (a) PAS stain demonstrating mesangial matrix expansion and prominent capillary loops, scale bar = 20 μm; (b) immunofluorescence for IgG showing an incomplete global granular capillary loop pattern of staining, scale bar = 20 μm; (c) immunofluorescence for C3 is of lower intensity, scale bar = 20 μm; (d) ultrastructural photomicrograph showing stage I to II subepithelial electron dense immune type deposits, magnification 5000x; (e) ultrastructural photomicrograph showing mesangial electron dense deposits, magnification 12000×; and (f) positive immunofluorescence for FAT1, scale bar = 20 μm. DNMN, de novo membranous nephropathy; PAS, periodic acid-Schiff.
FAT1 staining was specific to DNMN, with immunostaining showing positivity for FAT1 in DNMN (Supplementary Figure 1), but not in other types of MN from known antigens (PLA2R, THSD7A, EXT1/2, and NELL1-associated cases, n = 5 each, Supplementary Figure 5). FAT1 was also negative within other proteinuric diseases, including fibrillary glomerulopathy, focal segmental glomerulosclerosis, minimal change disease, and diabetic glomerulopathy (n = 5 each, data not shown). FAT1 staining was present in podocytes (Supplementary Figure 6).
In FAT1-positive cases, the majority demonstrated mixed antibody and TCMR, 1 had ABMR alone, and 1 had a positive DSA without histologic features of rejection. Four had concurrent vascular rejection (Table 1). Among patients with ABMR, 4 were C4d-positive. Five demonstrated active ABMR and 1 showed chronic active ABMR. Of TCMR cases, 2 were Banff 1A and 2 were Banff 1B. Of the vascular rejection cases, 3 were Banff class 2A and 1 was 2B (Table 1).
Outcomes
We examined serial biopsies to investigate whether FAT1 DNMN correlates with DSAs. FAT1 was present with acute rejection and correlated with presence of DSAs. In 2 patients with serial biopsies, ABMR was persistently present with MN. In 1 case with resolution of rejection, FAT1 was negative and correlated with disappearance of DSAs. Interestingly, FAT1 MN persisted after TCMR resolution, indicating a potential association with ABMR, but not TCMR. Moreover, DNMN preceded ABMR in 1 case, and DNMN occurred in the setting of elevated DSA with no evidence of rejection in 1 patient.
FAT1 MN was not an early cause of graft dysfunction (mean 7.2 years posttransplant, Table 1). Four patients received multitargeted therapy, including a calcineurin inhibitor, prednisone, and mycophenolate mofetil (or sirolimus). All patients with ABMR were treated with plasmapheresis, steroids, and i.v. Ig. At follow-up (range of 14 months–5 years), 2 achieved remission, 2 required dialysis, and follow-up data were not available for 2 patients.
Discussion
In our cohort, DNMN comprised 50% of cases of MN within allografts. There was a lower proportion of PLA2R-positive cases, as previously reportedS6 and the target antigen was unknown in the majority of cases. Previous studies examined whether the target antigens were HLA with the autoantibodies being DSAs. In this study, we found FAT1 to be an antigen in DNMN. While we did not observe HLA antigens within glomeruli, there was an association with DSAs. FAT1 was previously discovered as an antigen by through tissue-based proteomics.8 We similarly demonstrated FAT1 as an antigen (Supplementary Figure 7) through retrieval of immune complexes from biopsy tissue.S2
MN is seen in the setting of graft-versus-host disease, representing an alloimmune response inducing renal autoimmunity, which can occur in DNMN. Our study provides further evidence of correlation with circulating DSA and ABMR. An interesting hypothesis about why FAT1 may represent a target antigen in MN associated with 2 separate alloimmune states may be related to the high mutational rate of this large gene (19,752 variants listed in UniProt) such that there may be private epitopes tolerated by the donor but recognized as foreign by the recipient (or vice versa). This may complicate detection of a “universal” circulating anti-FAT1 antibodies able to react with the “consensus” sequence of human FAT1 in an assay similar to detection of anti-PLA2R autoantibodies.
Conclusion
This study highlights FAT1 as an antigen implicated in DNMN. Therefore, in patients with PLA2R-negative MN associated with rejection, evaluation of FAT1 may be helpful to determine the antigen type. Our study provides further evidence of correlation with circulating DSA in ABMR. Understanding the pathogenesis of these fascinating alloimmune phenomena and DSAs can offer new insights in MN. Further work is required to understand the pathobiology of disease and the clinical implications of FAT1 in DNMN.
Disclosure
LHB is a consultant for Novartis, Travere Therapeutics, Canbridge Pharmaceuticals, and Cerium Pharmaceuticals, and is an author for UpToDate, Inc. All the other authors declared no conflicting interests.
Acknowledgments
This work was in part supported by a grant from the NIH #R44DK130702-02 awarded to Arkana Laboratories (authors AS, CPL, TNC) and #R01-DK126978 awarded to Boston Medical College (LHB). An abstract containing a subset of these data was presented at the 2023 American Society of Nephrology Annual Scientific Meeting as a poster titled "FAT1 as an antigen in antibody-mediated rejection associated membranous nephropathy."
Data Availability Statement
Essentially all data is included in this manuscript. However, questions should be directed to the corresponding authors (LA-R or TC).
Footnotes
Supplementary Data.
Supplementary Methods.
Supplementary References.
Figure S1. FAT1 immunofluorescence in the 5 additional cases of FAT1-associated DNMN demonstrating granular mesangial and capillary loop staining.
Figure S2. Representative case of FAT1-associated membranous nephropathy in a patient with both antibody-mediated and cellular rejection.
Figure S3. Histopathology of a typical case of recurrent PLA2R-positive membranous nephropathy.
Figure S4. Immunofluorescence for HLA-DQ was negative within podocytes or along the capillary loops in all FAT1 positive cases.
Figure S5. FAT1 staining is negative along the glomerular capillary loops within cases of (A) PLA2R, (B) THSD7A, (C) NELL1, and (D) EXT1-associated MN, demonstrating specificity of staining.
Figure S6. FAT1 immunohistochemistry.
Figure S7. Mass spectrometry confirms protocadherin FAT1 is an antigen in MN through an independent technique than previously reported.
Table S1. Histopathologic characteristics of MN cases in renal allografts in the presence or absence of allograft rejection.
Contributor Information
Laith F. Al-Rabadi, Email: Laith.Al-Rabadi@hsc.utah.edu.
Tiffany N. Caza, Email: Tiffany.caza@arkanalabs.com.
Supplementary Material
Supplementary Data. Supplementary Methods. Supplementary References. Figure S1. FAT1 immunofluorescence in the 5 additional cases of FAT1-associated DNMN demonstrating granular mesangial and capillary loop staining. Figure S2. Representative case of FAT1-associated membranous nephropathy in a patient with both antibody-mediated and cellular rejection. Figure S3. Histopathology of a typical case of recurrent PLA2R-positive membranous nephropathy. Figure S4. Immunofluorescence for HLA-DQ was negative within podocytes or along the capillary loops in all FAT1 positive cases. Figure S5. FAT1 staining is negative along the glomerular capillary loops within cases of (A) PLA2R, (B) THSD7A, (C) NELL1, and (D) EXT1-associated MN, demonstrating specificity of staining. Figure S6. FAT1 immunohistochemistry. Figure S7. Mass spectrometry confirms protocadherin FAT1 is an antigen in MN through an independent technique than previously reported. Table S1. Histopathologic characteristics of MN cases in renal allografts in the presence or absence of allograft rejection.
References
- 1.Heidet L., Gagnadoux M.E., Beziau A., Niaudet P., Broyer M., Habib R. Recurrence of de novo membranous glomerulonephritis on renal grafts. Clin Nephrol. 1994;41:314–318. [PubMed] [Google Scholar]
- 2.Truong L., Gelfand J., D’Agati V., et al. De novo membranous glomerulonephropathy in renal allografts: a report of ten cases and review of the literature. Am J Kidney Dis. 1989;14:131–144. doi: 10.1016/s0272-6386(89)80189-1. [DOI] [PubMed] [Google Scholar]
- 3.Schwarz A., Krause P.H., Offermann G., Keller F. Impact of de novo membranous glomerulonephritis on the clinical course after kidney transplantation. Transplantation. 1994;58:650–654. doi: 10.1097/00007890-199409270-00002. [DOI] [PubMed] [Google Scholar]
- 4.Honda K., Horita S., Toki D., et al. De novo membranous nephropathy and antibody-mediated rejection in transplanted kidney. Clin Transpl. 2011;25:191–200. doi: 10.1111/j.1399-0012.2010.01213.x. [DOI] [PubMed] [Google Scholar]
- 5.Lim B.J., Kim M.S., Kim Y.S., Kim S.I., Jeong H.J. C4d deposition and multilayering of peritubular capillary basement membrane in posttransplantation membranous nephropathy indicate its association with antibody-mediated injury. Transplant Proc. 2012;44:619–620. doi: 10.1016/j.transproceed.2011.12.052. [DOI] [PubMed] [Google Scholar]
- 6.Stahl R., Hoxha E., Fechner K. PLA2R autoantibodies and recurrent membranous nephropathy after transplantation. N Engl J Med. 2010;363:496–498. doi: 10.1056/NEJMc1003066. [DOI] [PubMed] [Google Scholar]
- 7.Grupper A., Cornell L.D., Fervenza F.C., Beck L.H., Lorenz E., Cosio F.G. Recurrent membranous nephropathy after kidney transplantation: treatment and long-term implications. Transplantation. 2016;100:2710–2716. doi: 10.1097/TP.0000000000001056. [DOI] [PubMed] [Google Scholar]
- 8.Sethi S., Madden B., Casal Moura M., et al. Hematopoietic stem cell transplant-membranous nephropathy is associated with protocadherin FAT1. J Am Soc Nephrol. 2022;33:1033–1044. doi: 10.1681/ASN.2021111488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sethi S., Beck L.H., Jr., Glassock R.J., et al. Mayo Clinic consensus report on membranous nephropathy: proposal for a novel classification. Kidney Int. 2023;104:1092–1102. doi: 10.1016/j.kint.2023.06.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data. Supplementary Methods. Supplementary References. Figure S1. FAT1 immunofluorescence in the 5 additional cases of FAT1-associated DNMN demonstrating granular mesangial and capillary loop staining. Figure S2. Representative case of FAT1-associated membranous nephropathy in a patient with both antibody-mediated and cellular rejection. Figure S3. Histopathology of a typical case of recurrent PLA2R-positive membranous nephropathy. Figure S4. Immunofluorescence for HLA-DQ was negative within podocytes or along the capillary loops in all FAT1 positive cases. Figure S5. FAT1 staining is negative along the glomerular capillary loops within cases of (A) PLA2R, (B) THSD7A, (C) NELL1, and (D) EXT1-associated MN, demonstrating specificity of staining. Figure S6. FAT1 immunohistochemistry. Figure S7. Mass spectrometry confirms protocadherin FAT1 is an antigen in MN through an independent technique than previously reported. Table S1. Histopathologic characteristics of MN cases in renal allografts in the presence or absence of allograft rejection.
Data Availability Statement
Essentially all data is included in this manuscript. However, questions should be directed to the corresponding authors (LA-R or TC).