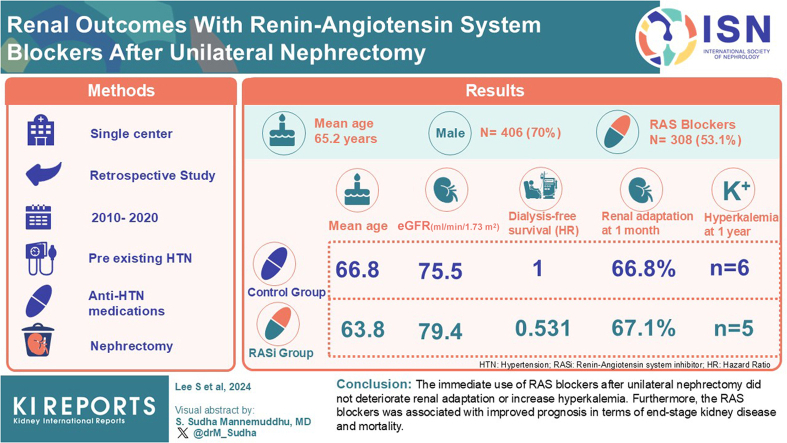
Abstract
Introduction
Despite the benefits of renin-angiotensin system (RAS) blockers, their immediate use after nephrectomy has been limited because of concerns about impaired renal adaptation. We aimed to evaluate the effect of RAS blockers immediately after unilateral nephrectomy on renal adaptation.
Methods
This single-center retrospective cohort study included 580 patients who underwent elective unilateral nephrectomy between 2010 and 2020 and had preexisting hypertension with antihypertensive medications. Patients were divided into groups according to the postnephrectomy RAS blocker use. The primary outcome was renal adaptation defined as (postnephrectomy estimated glomerular filtration rate [eGFR] ÷ prenephrectomy eGFR) × 100 at 1 month after surgery. Secondary outcomes included hyperkalemia during the first year and mortality or end-stage kidney disease within 3 years.
Results
The mean age was 65.2 years, 406 (70%) were male, and 308 (53.1%) received RAS blockers after nephrectomy. The RAS blocker group was younger (63.8 vs. 66.8 years) and had a higher eGFR (79.4 vs. 75.5 ml/min per 1.73 m2) than the control group. There were no differences between the groups in renal adaptation at 1 month (67.1% vs. 66.8%; P = 0.711) or in the incidence of hyperkalemia until 1 year postoperatively. The RAS blocker use was associated with better dialysis-free survival (Adjusted hazard ratio of multivariable Cox-regression model: 0.531; 95% confidence interval: 0.329–0.857; P = 0.010].
Conclusion
The immediate use of RAS blockers after unilateral nephrectomy did not deteriorate renal adaptation or increase hyperkalemia. Furthermore, the RAS blockers were associated with improved prognosis in terms of end-stage kidney disease and mortality.
Keywords: acquired single kidney, end-stage kidney disease, renal adaptation, renin-angiotensin system blockers, survival, unilateral nephrectomy
Graphical abstract
“Acquired solitary kidney” refers to a condition in which a person with bilateral kidneys has a solitary kidney, primarily due to living kidney donation, kidney or urothelial malignancy, or trauma. The incidence of acquired solitary kidney has steadily increased over time, primarily due to kidney or urothelial malignancy.1, 2, 3, 4, 5 After a unilateral nephrectomy, renal adaptation occurs in the patient's remaining kidney to maintain renal function.6 Unlike living kidney donors, patients with renal trauma or kidney cancer often have a poor renal prognosis after nephrectomy.7, 8, 9 Considering that survival rates for localized kidney cancer reached 93% in a recent report, maintaining adequate renal function is critical to improving the quality of life of these patients.5,10
Glomerular hyperfiltration is considered a major risk factor for chronic kidney disease (CKD) progression.11 Various kidney diseases, including diabetic nephropathy, cause glomerular hypertension and hyperfiltration that results in podocyte damage and proteinuria.12 After nephrectomy, a certain degree of single-nephron glomerular hyperfiltration is unavoidable during renal adaptation to maintain the glomerular filtration rate (GFR).6,13,14 The renin-angiotensin system (RAS) plays an important role in glomerular hyperfiltration in disease states.11 However, the role of the RAS on renal hypertrophy after renal mass reduction, such as during nephrectomy, is still being investigated.15,16 The increase in postdonation single kidney GFR, which is driven by hyperfiltration, may play a favorable role in long-term GFR after kidney donation.17
RAS blockers induce the relaxation of efferent arterioles in the glomerulus, which can alleviate glomerular hypertension and slow CKD progression.11,12 However, the effects of RAS blockers on renal adaptation after nephrectomy remain unclear. Despite the numerous potential benefits of RAS blockers, there is some reluctance regarding their use in postnephrectomy patients owing to these uncertainties and concerns about their adverse effects. To date, there have been no large scale published data on the effects of RAS blockers on renal adaptation after radical nephrectomy. Therefore, this study aimed to investigate the effects of RAS blockers on renal adaptation and patient outcomes after unilateral nephrectomy.
Methods
Study Design and Patient Selection
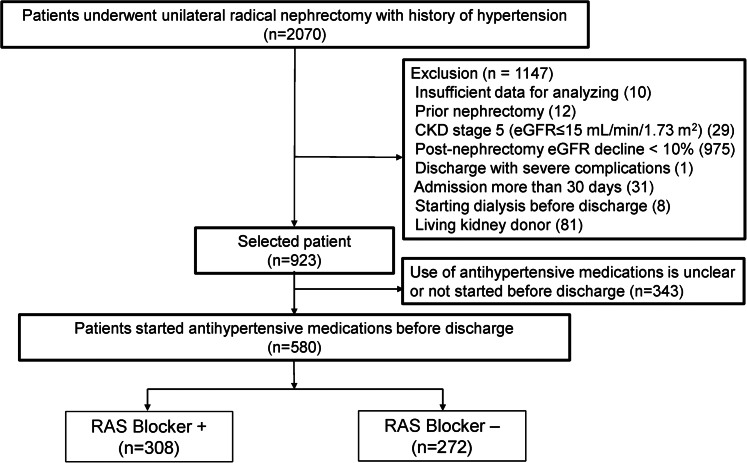
This single-center, retrospective cohort study included 2070 patients with hypertension, who were aged 18 years or older, and who underwent unilateral radical nephrectomy at the Samsung Medical Center between January 2000 and December 2020. The selection process is illustrated in Figure 1. Patients with a postoperative eGFR decline of <10% after surgery were excluded because they were considered to have a virtually nonfunctioning kidney removed.18 In addition, patients with hospital stays longer than 30 days or serious complications during hospitalization were excluded, because factors other than nephrectomy and RAS blockers were likely to affect renal function. Living kidney donors were excluded because of expected differences in their clinical characteristics compared to other patients.18 Furthermore, 343 patients were excluded because of uncertainty about the use of antihypertensive medications, including those who did not start any antihypertensive medications until discharge after surgery. In total, 580 patients were included in the final analysis.
Figure 1.
Flowchart of patient selection. The use of RAS blockers was defined as starting medication within one week after surgery and continuing treatment until discharge.
The study was approved by the Institutional Review Board of the Samsung Medical Center and adhered to the Declaration of Helsinki (IRB number: 2022-12-036). Informed consent was waived because of the anonymized and deidentified data collection.
Clinical Data and Laboratory Findings
Data were extracted from the Clinical Data Warehouse DARWIN-C of the Samsung Medical Center. Preoperative characteristics, including sex (defined as male or female according to their reproductive organs), age, height, and body weight; laboratory findings, including serum creatinine, blood urea nitrogen (BUN), hemoglobin (Hb), potassium (K), uric acid, and urine albumin using the dipstick method; past medical histories, such as diabetes mellitus (DM) and hypertension; cancer types, cancer stages, and adjuvant therapies were extracted from electronic databases. The presence of hypertension was confirmed using anesthesia records.
The cancer types and stages were confirmed from pathological reports. Cancer stage was initially classified according to the 8th edition of the American Joint Committee on Cancer tumor, node, metastasis staging system.19 Subsequently, patients with stage 1 or 2 disease were classified as having limited-stage cancer, whereas those with stage 3 or higher disease were classified as having advanced-stage cancer. We defined chemotherapy as “systemic therapy” for cancer. “Localized therapy” included radiation therapy, radiofrequency ablation, and gamma-knife therapy postoperatively.
RAS blockers include angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers. None of the patients were administered sacubitril/valsartan. Calcium channel blockers (CCB) included dihydropyridine CCB and non-dihydropyridine CCB. Diuretics included thiazide families, loop diuretics, and K-sparing diuretics.
The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) 2009 equation,20 because the race-free CKD-EPI 2021 equation is not yet recommended as a standard method for calculating eGFR outside the United States and showed higher bias in Koreans than the CKD-EPI 2019 in recent studies.21 We performed a sensitivity analysis using eGFR calculated using the creatinine-based CKD-EPI 2021 equation.22
Use of RAS Blockers (Exposure)
The use of RAS blockers was defined as the administration of RAS blockers within 1 week after surgery and the continuance of their use until discharge. All patients who were prescribed RAS blockers after surgery were already taking RAS blockers prior to admission, and none of the patients who were not taking RAS blockers prior to admission initiated taking them after admission. The patients were prescribed a 1-month supply of discharge medications, including a RAS blocker. Patients discharged while receiving RAS blockers during hospitalization were presumed to have continued RAS blocker therapy until their first outpatient follow-up. If patients were prescribed antihypertensive medications at other hospitals after discharge, it was difficult to determine accurately whether they were taking RAS blockers during follow-up. Therefore, the control and RAS blocker groups were defined based on whether patients were taking RAS blockers before discharge, regardless of whether they took RAS blockers after discharge.
Patient Follow-Up After Surgery
At our institution, patients are typically discharged on the 9th postoperative day (interquartile range, 8–10) and are instructed to return for follow-up appointments at 1 and 3 months after surgery. Thereafter, patients with malignant tumors are usually followed-up every 3 to 6 months for 3 years after surgery. Due to the limited availability of medical records beyond 3 years, our analysis focused solely on data within this timeframe.
Outcomes
The primary outcome was renal adaptation 1 month after surgery, defined as (postnephrectomy eGFR/pre-nephrectomy eGFR) × 100. Secondary outcomes were the incidence of hyperkalemia within 1 year postoperatively, acute kidney injury at 1 month, end-stage kidney disease (ESKD), overall survival, and dialysis-free survival within 3 years postoperatively. The incidence of hyperkalemia was defined as a serum K level exceeding 5.5 mmol/l. Acute kidney injury was defined as an increase in serum creatinine level by more than 0.3 mg/dl or by more than 1.5 times compared to serum creatinine levels at discharge. The incidence of ESKD was defined when patients started dialysis.
Subgroups Analysis
We conducted a subgroup analysis of patients prescribed antihypertensive medications at the Samsung Medical Center because we cannot be certain whether patients prescribed antihypertensive medication at other hospitals continued taking it after their first outpatient visit after surgery. The subgroup analysis included patients who continuously adhered to RAS blockers for at least 12 months after surgery, defined as either taking or not taking RAS blockers throughout this period. RAS blockers (+/+): RAS blockers were prescribed immediately after surgery and continued throughout the first year; RAS blockers (+/−): RAS blockers were prescribed immediately after surgery but subsequently discontinued; RAS blockers (−/+): RAS blockers were not prescribed immediately after surgery but started after discharge; RAS blockers (−/−): RAS blockers were not prescribed immediately after surgery and were not started throughout the first year. We also analyzed renal adaptation, overall survival, and dialysis-free survival.
Statistical Analysis
Continuous variables are described as mean ± SD or median (interquartile range) based on the normality test. Categorical variables are presented as counts (percentages). For group comparisons, continuous variables were compared using an independent 2-sample t test or Mann-Whitney U test, depending on normality, and categorical variables were compared using Pearson's chi-square test or Fisher's exact test. Renal adaptation between the groups was compared using an independent 2-sample t test. We further performed analysis of covariance to adjust for covariates when comparing renal adaptation between groups. An analysis of covariance was performed using robust standard errors to account for heteroscedasticity. The log-rank test was used to compare the Kaplan-Meier curves for overall survival, ESKD, and dialysis-free survival based on RAS blocker use. In addition, multivariable analyses were conducted using Cox proportional hazards models for ESKD, overall survival, and dialysis-free survival. We examined the proportional hazards assumption using plots of the log (−log) survival function and Schoenfeld residuals. Reducing the effect of the confounding factors, a multivariable analysis was conducted using 3 models. Model 1 analyzed patient data considering age, sex, body mass index, and medical history including DM, coronary bypass graft or percutaneous coronary intervention history, cerebrovascular accident, and dyslipidemia. Model 2 extended the analysis of model 1 by incorporating preoperative laboratory findings, including eGFR, BUN, Hb, K, and urine albumin. Model 3 further adjusted for cancer type, stages and adjuvant treatment, in addition to the variables in model 2. In addition, sensitivity analysis using propensity score matching was performed to mitigate confounding imbalances. The control and RAS blocker groups were matched at a 1:1 ratio using the nearest neighbor search strategy and a caliper width of 0.1 based on age, sex, body mass index, DM, coronary bypass graft or percutaneous coronary intervention history, cerebrovascular accident, and dyslipidemia; beta blockers, CCB, eGFR, BUN, Hb, K, and urine albumin; cancer types, stages, and adjuvant treatments. The time correlation was not considered. Statistical analyses were performed using IBM SPSS Statistics for Windows (version 27.0; IBM Corp., Armonk, NY) and R (version 4.2.2; R foundation). Statistical significance was defined as a 2-sided P-value of < 0.05.
Results
Patient Characteristics
The mean age was 65.2 years, and 406 (70%) of 580 patients were male. A total of 557 (96%) patients were diagnosed with cancer. Among these patients, 387 (69%) were diagnosed with renal cell carcinoma (RCC), and 128 (23%) were diagnosed with urothelial cell carcinoma. The mean follow-up period was 31.9 ± 7.9 months. Among the patients, 47 were lost to follow-up at 12 months (RAS blocker, 26 vs. control, 21), 95 at 24 months (RAS blocker, 58; control, 37), and 145 at 36 months (RAS blocker, 79; control, 66). The patients were divided into 2 groups based on the use of RAS blockers: the RAS blocker group (n = 308, 53.1%) and the control group (n = 272, 46.9%). There were no individuals who did not take RAS blockers before surgery but started taking them before discharge. The baseline characteristics of the patients included in the analysis are shown in Table 1. The RAS blocker group was younger (RAS blocker, 63.8 ± 10.4 vs. control, 66.8 ± 10.3 years old; P = 0.001) and had a higher body mass index (RAS blocker, 25.6 [23.6–28.2] vs. control, 25.3 [23.3–27.3] kg/m2; P = 0.042), eGFR (RAS blocker, 79.4 ± 17.1 vs. control, 75.5 ± 19.6 ml/min per 1.73 m2; P = 0.013), and uric acid (RAS blocker, 5.7 ± 1.6 vs. control, 5.5 ± 1.6 mg/dl; P = 0.039) levels compared to the control group. In the RAS blocker group, the proportion of patients with preoperative eGFR < 60 ml/min per 1.73 m2 was significantly lower than in the control group (RAS blocker 12.3% vs. control 21.3%; P = 0.004). However, the 2 groups had no significant difference in the proportion of patients with preoperative eGFR < 30 ml/min per 1.73 m2 (RAS blocker, 0.6% vs. control, 1.8%; P = 0.205). There were no differences in other laboratory findings, including BUN, urine albumin, and K levels; or comorbidities, including DM, atrial fibrillation, dyslipidemia, cerebrovascular accident, percutaneous coronary intervention or coronary artery bypass graft events, and cancer types and stages. Hospital stays, interval to first outpatient visit after discharge, social history, such as smoking and drinking habits, and the proportion of adjuvant therapy for cancer did not differ between the groups.
Table 1.
Characteristics of patients
| Variables | Total (N = 580) | RAS blocker (+) (n = 308) | RAS blocker (-) (n = 272) | P |
|---|---|---|---|---|
| Male | 406 (70.0) | 220 (71.4) | 186 (68.4) | 0.479 |
| Age, yrs | 65.2 ± 10.5 | 63.8 ± 10.4 | 66.8 ± 10.3 | 0.001 |
| BMI, kg/m2 | 25.5 (23.5–27.8) | 25.6 (23.6–28.3) | 25.3 (23.3–27.4) | 0.058 |
| SBP, mm Hg | 127.5 ± 16.2 | 126.4 ± 16.1 | 128.8 ± 16.3 | 0.062 |
| DBP, mm Hg | 73.0 ± 10.4 | 72.7 ± 10.0 | 73.3 ± 10.9 | 0.523 |
| DM | 164 (28.3) | 92 (29.9) | 72 (26.5) | 0.415 |
| Cancer | 557 (96.0) | 296 (96.1) | 261 (96.0) | 1 |
| Cancer type | 0.584 | |||
| Renal cell carcinoma | 387 (69.7) | 212 (71.9) | 175 (67.3) | |
| Urothelial cell carcinoma | 128 (23.1) | 62 (21.0) | 66 (25.4) | |
| Liposarcoma | 21 (3.8) | 12 (4.1) | 9 (3.5) | |
| Other | 19 (3.4) | 9 (3.1) | 10 (3.8) | |
| Cancer stages | 0.783 | |||
| Stage 1 | 246 (42.4) | 130 (42.2) | 116 (42.6) | |
| Stage 2 | 94 (16.2) | 46 (14.9) | 48 (17.6) | |
| Stage 3 | 171 (29.5) | 97 (31.5) | 74 (27.2) | |
| Stage 4 | 44 (7.6) | 22 (7.1) | 22 (8.1) | |
| Cancer stages | 0.707 | |||
| Limited stages (1∼2) | 340 (58.6) | 176 (57.1) | 164 (60.3) | |
| Advanced stages (3∼4) | 215 (37.1) | 119 (38.6) | 96 (35.3) | |
| Adjuvant Tx., systemic | 87 (15.0) | 49 (15.9) | 38 (14.0) | 0.592 |
| Adjuvant Tx., localized | 34 (5.9) | 16 (5.2) | 18 (6.6) | 0.582 |
| PCI or CABG History | 20 (3.4) | 13 (4.2) | 7 (2.6) | 0.391 |
| CVA | 32 (5.5) | 14 (4.5) | 18 (6.6) | 0.364 |
| Atrial fibrillation | 23 (4.0) | 11 (3.6) | 12 (4.4) | 0.761 |
| Dyslipidemia | 35 (6.0) | 18 (5.8) | 17 (6.2) | 0.976 |
| Smoking History | 0.581 | |||
| None | 303 (60.0) | 161 (60.8) | 142 (59.2) | |
| Ex | 144 (28.5) | 71 (26.8) | 73 (30.4) | |
| Current | 58 (11.5) | 33 (12.5) | 25 (10.4) | |
| Drinking History | 0.896 | |||
| None | 303 (60.4) | 160 (60.6) | 143 (60.1) | |
| Ex | 85 (16.9) | 46 (17.4) | 39 (16.4) | |
| Current | 114 (22.7) | 58 (22.0) | 56 (23.5) | |
| Beta-blockers | 118 (20.3) | 50 (16.2) | 68 (25.0) | 0.012 |
| Calcium channel blockers | 390 (67.2) | 177 (57.5) | 213 (78.3) | < 0.001 |
| Diuretics | 157 (27.1) | 105 (34.1) | 52 (19.1) | < 0.001 |
| Antihypertensive medication count | < 0.001 | |||
| 1 | 282 (48.6) | 67 (21.8) | 215 (79.0) | |
| 2 | 213 (36.7) | 160 (51.9) | 53 (19.5) | |
| 3 | 75 (12.9) | 71 (23.1) | 4 (1.5) | |
| 4 | 10 (1.7) | 10 (3.2) | 0 (0.0) | |
| eGFR, ml/min per 1.73 m2 | 77.6 ± 18.4 | 79.4 ± 17.1 | 75.5 ± 19.6 | 0.013 |
| eGFR < 60 | 96 (16.5) | 38 (12.3) | 58 (21.3) | 0.004 |
| eGFR < 30 | 7 (1.2) | 2 (0.6) | 5 (1.8) | 0.205 |
| BUN, mg/dl | 17.0 ± 5.8 | 16.7 ± 5.2 | 17.2 ± 6.4 | 0.292 |
| Hemoglobin, g/dl | 13.4 ± 1.9 | 13.4 ± 2.0 | 13.4 ± 1.8 | 0.979 |
| Uric acid, mg/dl | 5.6 ± 1.6 | 5.7 ± 1.6 | 5.5 ± 1.6 | 0.039 |
| Potassium, mmol/l | 4.3 ± 0.4 | 4.3 ± 0.4 | 4.3 ± 0.4 | 0.264 |
| Urine albumin | 0.865 | |||
| Negative | 400 (69.3) | 216 (70.8) | 184 (67.6) | |
| Trace | 75 (13.0) | 38 (12.5) | 37 (13.6) | |
| + | 48 (8.3) | 25 (8.2) | 23 (8.5) | |
| ++ | 35 (6.1) | 18 (5.9) | 17 (6.2) | |
| +++ | 19 (3.3) | 8 (2.6) | 11 (4.0) | |
| Hospital stays, d | 8.0 (8.0–10.0) | 8.0 (8.0–9.0) | 8.0 (8.0–10.0) | 0.22 |
| Interval to first visit after surgery, d | 30.0 (26.0–36.0) | 30.0 (26.0–36.0) | 30.0 (27.0–37.5) | 0.307 |
| Follow up periods, mo | 31.9 ± 7.9 | 32.7 ± 6.7 | 30.9 ± 8.9 | 0.005 |
| Overall mortality | 78 (13.4) | 34 (11.0) | 44 (16.2) | 0.070 |
BMI, body mass index; BUN, blood urea nitrogen; CABG, coronary artery bypass graft; CVA, cerebrovascular accident; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; PCI, percutaneous coronary intervention; SBP, systolic blood pressure.
Continuous variables are expressed as mean ± SD or median (interquartile range), and categorical variables are expressed as numbers (%).
The patients in the RAS blocker group received more antihypertensive medications (P < 0.001) than those in the control group. In addition, fewer beta-blockers (RAS blockers, 50 [16.2%] vs. control, 68 [25.0%]; P = 0.012) and CCBs (RAS blockers, 177 [57.5%] vs. control, 213 [78.3%]; P <0.001) were used in the RAS blocker group than in the control group. Diuretics were used more frequently in the RAS blocker group (RAS blocker, 105 [34.1%] vs. control, 52 [19.1%]; P < 0.001).
Renal Adaptation and Renal Outcome
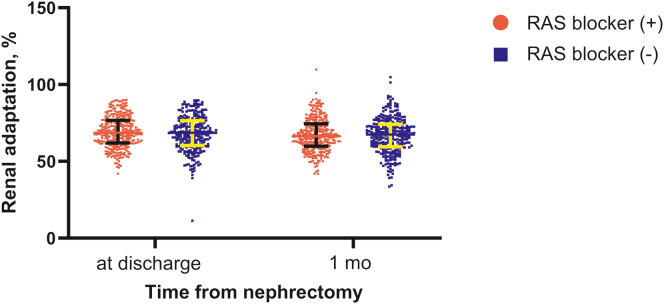
There was no significant difference in renal adaptation between the groups (at discharge, RAS blocker, 69.0 ± 10.3% vs. control, 68.0 ± 11.5%; P = 0.260 from t test and P = 0.341 from analysis of covariance; at 1 month after surgery, RAS blocker, 67.1 ± 10.7% vs. control, 66.8 ± 11.6%; P = 0.711 from t test and P = 0.837 from analysis of covariance), although the RAS blocker group had higher eGFR values than the control group (Table 2 and Figure 2). The incidence of acute kidney injury was comparable between the groups 1 month postoperatively (RAS blocker, 12 [3.9%] vs. control, 14 [5.8%]; P = 0.467) (Table 2).
Table 2.
Renal outcomes and hyperkalemia findings according to RAS blockers usage
| Variables | Time from surgery | RAS blocker (+) n = 308 | RAS blocker (-) n = 272 | P |
|---|---|---|---|---|
| Renal adaptation, % | Discharge | 69.0 ± 10.3 | 68.0 ± 11.5 | 0.260 |
| 1 mo | 67.1 ± 10.7 | 66.8 ± 11.6 | 0.711 | |
| Acute kidney injury, n (%) | 1 mo | 12 (3.9) | 14 (5.8) | 0.467 |
| ESKD (1000 PY) | 3 yrs | 1.4 | 9.8 | 0.037 |
| Hyperkalemia | Discharge | 6/308 (1.9) | 7/272 (2.6) | 0.612 |
| 1 mo | 7/308 (2.3) | 10/272 (3.7) | 0.317 | |
| 6 mo | 8/234 (3.4) | 7/212 (3.3) | 0.945 | |
| 12 mo | 5/216 (2.3) | 6/183 (3.3) | 0.558 |
eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; PY, person-years.
Continuous variables are described as mean ± SD, and categorical variables are presented as counts (percentages). Renal adaptation was defined as (post-nephrectomy eGFR/pre-nephrectomy eGFR) × 100. Acute kidney injury was defined as an increase in serum creatinine level by >0.3 mg/dl or by >1.5 times compared to serum creatinine levels at discharge. The incidence of ESKD was defined when patients started dialysis. Hyperkalemia was defined as a serum potassium level > 5.5 mmol/l.
Figure 2.
Renal adaptation after nephrectomy according to the use of RAS blockers. Renal adaptation (%) was defined as postnephrectomy eGFR ÷ prenephrectomy eGFR × 100. At 1-month follow-up, no significant difference in renal adaptation was observed between the groups.
However, the incidence rate of ESKD was significantly lower in the RAS blocker group than in the control group 3 years postoperatively (RAS blocker, 1.4 per 1000 person-years [PY] vs. control, 9.8 per 1000 PY; P = 0.037) (Table 2). This finding was supported by univariable Cox regression analysis, which revealed a lower risk of ESKD associated with RAS blocker use (HR: 0.143, 95% CI: 0.032–0.633; P = 0.036). However, multivariable Cox regression analysis did not show a statistically significant difference in the risk of ESKD between the 2 groups (full-adjusted HR: 0.005, 95% CI: 0.000–83.522; P = 0.289).
We analyzed patients who developed ESKD (Supplementary Table S1). One and 6 patients in the RAS blocker and control groups, respectively, developed ESKD during follow-up. Among the patients who developed ESKD, 2 patients in the control group had DM; however, neither group had underlying medical conditions other than DM and cancer. Six of 7 patients (1 in the RAS blocker group and 5 in the control group) had a preoperative eGFR < 60 ml/min/1.73 m2, of whom 4 in the control group had an eGFR < 30 ml/min per 1.73 m2. Postoperatively, 6 patients (1 in the RAS blocker group and 5 in the control group) had an eGFR < 30 ml/min per 1.73 m2. Two patients in the control group died after developing ESKD.
Hyperkalemia and Laboratory Findings
The occurrence of hyperkalemia events associated with the use of RAS blockers is summarized in Table 2. Hyperkalemia events were analyzed at each visit point up to 1 year, revealing no significant differences between the groups at each time point or in the total number of patients experiencing events during the 1-year follow-up. In addition, no significant differences were observed in Hb, K, and BUN levels between the groups until 1-month after surgery (Supplementary Table S2).
Overall Survival and Dialysis-Free Survival
The incidence rate of mortality tended to be lower in the RAS blocker group than in the control group 3 years postoperatively, although not statistically significant (RAS blocker, 39.6 per 1000 PY vs. control, 61.3 per 1000 PY; P = 0.053). We compared patients who died with those who survived (Supplementary Table S3). Compared with surviving patients, patients who died were older and had lower Hb levels, lower eGFR, a lower proportion of RCC, and a much higher proportion of advanced cancer stage. We also compared patients who died with those who survived according to the use of RAS blockers (Supplementary Table S4). Both the RAS blocker and control groups showed that patients who died had a higher proportion of advanced cancer stages. However, the difference in the proportion of advanced cancer stages between patients who died and those who survived was more pronounced in the RAS blocker group than in the control group (advanced cancer stage in surviving vs. deceased patients: RAS blockers, 33.2% vs. 82.4%; controls, 29.8% vs. 63.6%). In contrast, patients who died had lower preoperative eGFR than those who survived in the control group (survived: 78.5 [66.3–90.6] ml/min per 1.73 m2 vs. died: 67.8 [51.7–85.4] ml/min per 1.73 m2; P = 0.010), whereas eGFR did not differ significantly between patients who died and those who survived in the RAS blocker group. Among patients with an eGFR at discharge <30 ml/min per 1.73 m2, only 1 of 11 (9%) in the RAS blocker group died, compared with 9 of 27 (33%) in the control group.
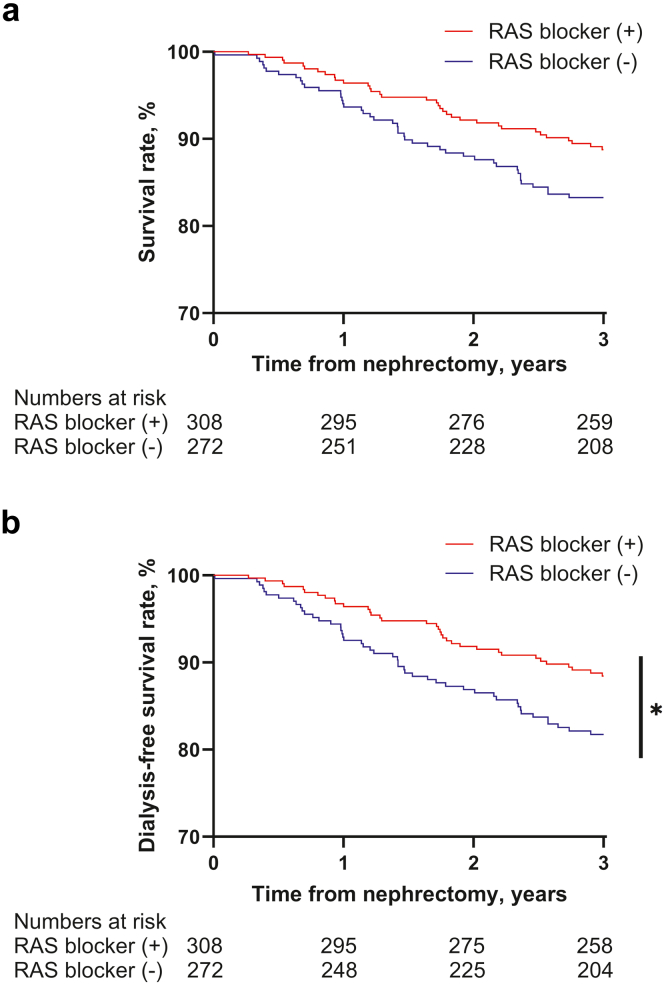
In Cox regression analysis, the use of RAS blockers was associated with a lower risk of mortality (fully adjusted HR for mortality: 0.544; 95% CI: 0.333–0.889; P = 0.015) (Table 3 and Figure 3a). Dialysis-free survival was also significantly better in the RAS blocker group than in the control group (fully adjusted HR for dialysis or mortality: 0.531; 95% CI: 0.329–0.857; P = 0.010) (Table 3 and Figure 3b). Similar results were obtained from the multivariable analysis using CCB instead of beta-blocker as a covariate to account for the association with antihypertensive medication use (Supplementary Table S5).
Table 3.
The risk of mortality and composite outcome of ESKD and mortality according to the use of RAS blockers
| Models | Mortality |
ESKD or mortality |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Univariable | 0.644 | 0.412–1.008 | 0.054 | 0.603 | 0.390–0.932 | 0.023 |
| Model 1 | 0.661 | 0.418–1.044 | 0.076 | 0.632 | 0.404–0.989 | 0.045 |
| Model 2 | 0.608 | 0.381–0.969 | 0.036 | 0.587 | 0.373–0.926 | 0.022 |
| Model 3 | 0.544 | 0.333–0.889 | 0.015 | 0.531 | 0.329–0.857 | 0.010 |
CI, confidence interval; ESKD, end-stage kidney disease; HR, hazard ratio.
Model 1 was adjusted for age, sex, body mass index, medical history (diabetes mellitus, coronary artery bypass graft or percutaneous coronary intervention, cerebrovascular accident, and dyslipidemia), and use of beta blockers.
Model 2: Model 1 + adjusted for initial laboratory tests including estimated glomerular filtration rate, blood urea nitrogen, hemoglobin, potassium, and urine albumin.
Model 3: Model 2 + adjusted for cancer type, stages and adjuvant treatment.
Figure 3.
Overall survival and dialysis free survival according to the use of RAS blockers. (a) Overall survival. In univariate analysis, there was no significant difference between the 2 groups (P-value = 0.054). However, in multivariate analysis, the RAS blocker group showed better overall survival than the control group (full-adjusted hazard ratio: 0.607, 95% confidence interval [CI] 0.373 to 0.987, P-value = 0.044). (b) Dialysis-free survival. The RAS blocker group showed better dialysis-free survival than the control group (full-adjusted hazard ratio 0.576 (95% confidence interval, 0.358 to 0.927; P = 0.023).
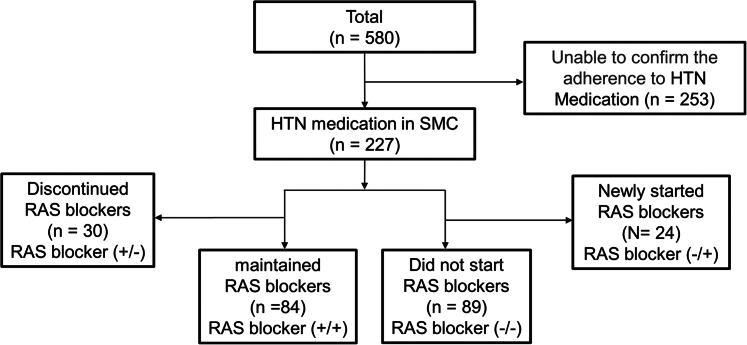
Subgroup Analysis
Among 580 patients, 227 were prescribed antihypertensive medications at our hospital. The medication histories of the patients are shown in Figure 4. Subgroup analyses were conducted on 173 of these patients (84 and 89 in the RAS blocker (+/+) group and RAS blocker (−/−) as the control group, respectively), excluding 30 who discontinued treatment and 24 who were newly started on RAS blockers after discharge. The baseline characteristics are presented in Table 4. The RAS blocker (+/+) group was younger (RAS blocker (+/+), 64 [57;71] years vs. RAS blocker (−/−), 70 [65;77] years; P = 0.001) and had a higher eGFR [RAS blocker (+/+), 79 ± 17 vs. RAS blocker (−/−), 70 ± 22 ml/min per 1.73 m2; P = 0.003] than the RAS blocker (−/−) group.
Figure 4.
Flowchart of subgroup. Patients tracked for medication use were categorized into 4 groups until 1 year after surgery. RAS blockers (+/+): RAS blockers were prescribed immediately after surgery and continued throughout the first year; RAS blockers (+/−): RAS blockers were prescribed immediately after surgery but were subsequently discontinued; RAS blockers (−/+): RAS blockers were not prescribed immediately after surgery but were started after discharge; RAS blockers (−/−): RAS blockers were not prescribed immediately after surgery and were not started throughout the first year.
Table 4.
Baseline characteristics of patients according to continuation of use of RAS blockers
| Variables | Total |
RAS +/+ |
RAS +/− |
RAS −/+ |
RAS −/− |
P |
|---|---|---|---|---|---|---|
| (N = 227) | (n = 84) | (n = 30) | (n = 24) | (n = 89) | ||
| Sex, male | 161 (70.9) | 60 (71.4) | 20 (66.7) | 16 (66.7) | 65 (73.0) | 0.879 |
| Hospital stays, d | 9 (8–12) | 8 (8–10) | 9 (8–10) | 9 (8–12) | 9 (8–12) | 0.184 |
| Age, yrs | 67 (60–75) | 64 (57–71) | 66 (58–74) | 72 (59–77) | 70 (65–77) | 0.001 |
| BMI, kg/m2, | 25.1 (23.3–27.3) | 25.2 (23.4–27.4) | 24.7 (23.0–26.8) | 25.9 (23.9–27.9) | 24.6 (23.1–26.7) | 0.377 |
| SBP, mm Hg | 129 (118–140) | 126 (114–138) | 126 (118–140) | 132 (122–153) | 131 (119–139) | 0.109 |
| DBP, mm Hg | 73 (67–79) | 73 (65–78) | 74 (67–77) | 76 (71–82) | 72 (65–79) | 0.213 |
| DM | 77 (34) | 31 (37) | 8 (27) | 10 (42) | 28 (32) | 0.589 |
| Cancer | 220 (97) | 80 (95) | 28 (93) | 24 (100) | 88 (99) | 0.114 |
| Cancer type | 0.504 | |||||
| Renal cell carcinoma | 135 (59.5) | 54 (64.3) | 14 (46.7) | 17 (70.8) | 50 (56.2) | |
| Urothelial cell carcinoma | 66 (29.1) | 21 (25) | 12 (40) | 4 (16.7) | 29 (32.6) | |
| Liposarcoma | 9 (4.0) | 2 (2.4) | 1 (3.3) | 1 (4.2) | 5 (5.6) | |
| Other | 10 (4.4) | 3 (3.6) | 1 (3.3) | 2 (8.3) | 4 (4.5) | |
| Noncancer | 7 (3.1) | 4 (4.8) | 2 (6.7) | 0 (0.0) | 7 (3.1) | |
| Cancer stage | 0.059 | |||||
| Limited stage (1∼2) | 137 (60.4) | 46 (54.8) | 23 (76.7) | 18 (75.0) | 50 (56.2) | |
| Advanced stage (3∼4) | 83 (36.6) | 34 (40.5) | 5 (16.7) | 6 (25.0) | 38 (42.7) | |
| Noncancer | 7 (3.1) | 4 (4.8) | 2 (6.7) | 0 (0.0) | 7 (3.1) | |
| PCI or CABG History | 12 (5%) | 5 (6%) | 1 (3%) | 0 (0%) | 6 (7%) | 0.568 |
| CVA | 17 (8%) | 6 (7%) | 1 (3%) | 2 (8%) | 8 (9%) | 0.783 |
| Atrial fibrillation | 12 (5%) | 6 (7%) | 1 (3%) | 1 (4%) | 4 (5%) | 0.806 |
| Dyslipidemia | 18 (8%) | 4 (5%) | 3 (10%) | 4 (17%) | 7 (8%) | 0.279 |
| eGFR, ml/min per 1.73 m2 | 74 ± 21 | 79 ± 17 | 71 ± 22 | 69 ± 21 | 70 ± 22 | 0.003 |
| eGFR < 30, at discharge | 6 (2.6) | 1 (1.2) | 1 (3.3) | 2 (8.3) | 2 (2.2) | 0.282 |
| eGFR < 30, at 1 mo | 27 (11.9) | 4 (4.8) | 3 (10.0) | 4 (16.7) | 16 (18.0) | 0.049 |
| BUN, mg/dl | 16.9 (14.0–20.7) | 16.2 (13.8–20.0) | 16.3 (14.0–20.3) | 17.1 (13.3–24.4) | 17.5 (14.2–21.6) | 0.391 |
| Hb, mg/dl | 13.4 (12.1–14.6) | 13.6 (12.1–14.8) | 13.6 (11.7–13.9) | 13.8 (12.6–14.9) | 12.9 (11.8–14.2) | 0.171 |
| Uric acid, mg/dl | 5.7 ± 1.7 | 5.8 ± 1.7 | 6.0 ± 1.8 | 5.5 ± 1.6 | 5.6 ± 1.8 | 0.356 |
| Potassium, mmol/l | 4.3 ± 0.4 | 4.3 ± 0.4 | 4.3 ± 0.4 | 4.3 ± 0.4 | 4.4 ± 0.4 | 0.795 |
| Urine albumin | ||||||
| negative | 139 (61%) | 57 (68%) | 16 (53%) | 18 (75%) | 48 (54%) | |
| trace | 36 (16%) | 10 (12%) | 5 (17%) | 3 (13%) | 18 (20%) | |
| + | 23 (10%) | 7 (8%) | 4 (13%) | 1 (4%) | 11 (12%) | 0.634 |
| ++ | 16 (7%) | 7 (8%) | 3 (10%) | 0 (0%) | 6 (7%) | |
| +++ | 13 (6%) | 3 (4%) | 2 (7%) | 2 (8%) | 6 (7%) | |
| Beta blockers | 53 (23%) | 17 (20%) | 6 (20%) | 5 (21%) | 25 (28%) | 0.605 |
| Calcium channel blockers | 165 (73%) | 54 (64%) | 22 (73%) | 20 (83%) | 69 (78%) | 0.144 |
| Diuretics | 70 (31%) | 31 (37%) | 14 (47%) | 5 (21%) | 20 (23%) | 0.029 |
BMI, body mass index; BUN, blood urea nitrogen; CABG, coronary artery bypass graft surgery; CVA, cerebrovascular accident; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; PCI, percutaneous coronary intervention; RAS, renin-angiotensin system; SBP, systolic blood pressure.
Continuous variables are expressed as mean ± SD or median (interquartile range), and categorical variables are expressed as numbers (%).
RAS blockers (+/+): RAS blockers were prescribed immediately after surgery and continued throughout the first year.
RAS blockers (+/−): RAS blockers were prescribed immediately after surgery but were subsequently discontinued.
RAS blockers (−/+): RAS blockers were not prescribed immediately after surgery but were started after discharge.
RAS blockers (−/−): RAS blockers were not prescribed immediately after surgery and were not started throughout the first year.
Limited stage includes stages 1 and 2, whereas advanced stage includes stages 3 and 4.
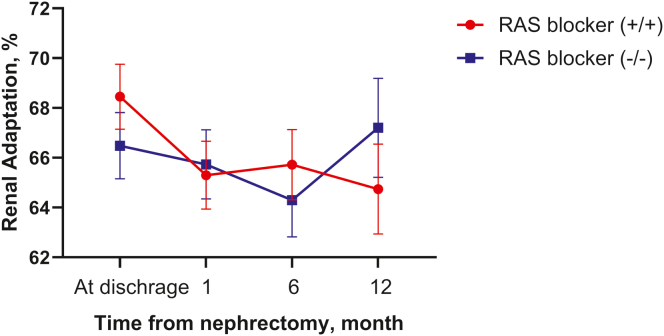
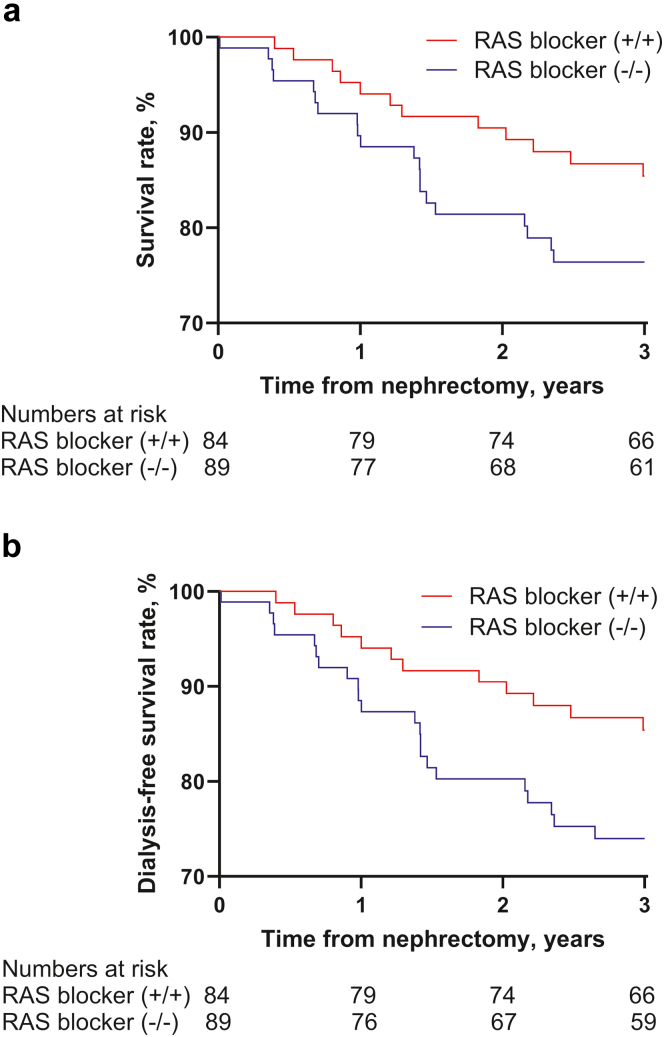
During the 1-year postoperative period, there was no significant difference in renal adaptation between the groups (Figure 5). Overall survival and dialysis-free survival curves of the subgroups are shown in Figure 6. Although the Kaplan-Meier curve indicated a trend toward improved survival with RAS blocker use compared to the control group, the differences were not statistically significant (overall survival: HR adjusted for age and eGFR, 0.674; 95% Cl: 0.319–1.425; P = 0.302 and dialysis-free survival: HR adjusted for age and eGFR, 0.625. 95% CI, 0.298–1.309; P = 0.213).
Figure 5.
Renal adaptation up to 1 year postoperatively in the subgroups. There were no significant differences between the RAS blocker and control groups. RAS blockers (+/+): RAS blockers were prescribed immediately after surgery and were continued throughout the first year. RAS blockers (−/−): RAS blockers were not prescribed immediately after surgery and were not started throughout the first year.
Figure 6.
Kaplan-Meier curves according to the use of RAS blockers in the subgroup analysis. (a) Overall survival of the subgroup. (b) Dialysis-free survival of the subgroup. RAS blockers (+/+): RAS blockers were prescribed immediately after surgery and were maintained throughout the first year. RAS blockers (−/−): RAS blockers were not prescribed immediately after surgery and were not started throughout the first year.
Sensitivity Analysis Using Propensity Score Matching
In total, 186 patient pairs were matched. The characteristics of the matched cohort and the distribution of propensity scores before and after matching are presented in Supplementary Table S6 and Supplementary Figure S1, respectively. All variables were well-matched between the groups, except for diuretics and antihypertensive medication counts. Renal adaptation did not differ between the groups at 1 month after surgery (RAS blocker 67.4 ± 10.1% vs. control 66.8 ± 11.9%; P = 0.606). There was no difference in the incidence of acute kidney injury at 1 month or hyperkalemia during the first year. The incidence of ESKD was lower in the RAS blocker group than in the control group (RAS blocker 0 vs. control 8.3 per 1000 PY; P = 0.038) (Supplementary Table S7).
We also analyzed survival and dialysis-free survival in the matched cohort. The Kaplan–Meier curves for survival and dialysis-free survival according to the use of RAS blockers are shown in Supplementary Figure S2. In univariable analysis, the use of RAS blockers tended to be associated with better survival (HR: 0.620; 95% CI: 0.361–1.063; P = 0.082) and dialysis-free survival (HR: 0.609; 95% CI: 0.360–1.031; P = 0.065), although not statistically significant. Multivariable analysis showed that the use of RAS blockers was significantly associated with better survival (fully adjusted HR: 0.495; 95% CI: 0.278–0.879; P = 0.016) and dialysis-free survival (fully adjusted HR: 0.504; 95% CI: 0.289–0.881; P = 0.016) (Supplementary Table S8).
Sensitivity Analysis With eGFR Using Creatinine-Based CKD-EPI 2021 Equation
We conducted an additional analysis using eGFR calculated using the creatinine-based CKD-EPI 2021 equation. Compared with the eGFR using CKD-EPI 2009 equation, the difference in preoperative eGFR between the RAS blocker and control groups became more pronounced (RAS blocker 83.6 ± 17.2 vs control 79.7 ± 19.9 ml/min per 1.73 m2; P = 0.01). In addition, better renal adaptations were observed at discharge and at 1 month follow-up in the RAS blocker group than in the control group (at discharge, RAS blocker 64.0 ± 15.7% vs. control 60.3 ± 18.3%; P = 0.01; at 1 month, RAS blocker 62.1 ± 15.6% vs. control 59.2 ± 18.4%; P = 0.044). The adjusted results for survival and dialysis-free survival did not differ significantly from the original results (mortality, in model 2: HR, 0.644; 95% CI: 0.382–0.970; P = 0.037; in model 3: HR, 0.544; 95% CI: 0.333–0.889; P = 0.015; mortality or ESKD, in model 2: HR, 0.588; 95% CI: 0.373–0.927; P = 0.022; in model 3: HR, 0.531; 95% CI: 0.329–0.857; P = 0.010).
Discussion
This study demonstrated that the use of RAS blockers immediately after unilateral nephrectomy had no adverse effects on renal adaptation. ESKD is less likely to occur in patients receiving RAS blockers. Furthermore, the use of RAS blockers was associated with improved survival in patients with acquired solitary kidney, particularly in those with kidney or urothelial cancer. To our knowledge, this is the first study to evaluate the immediate and postoperative effects of RAS blockers on kidney function after unilateral radical nephrectomy. This study's findings strongly support the use of RAS blockers after unilateral nephrectomy, particularly in patients with kidney or urothelial cancers.
Our bodies possess a homeostatic mechanism to adapt to changes.15 After nephrectomy, the remaining kidney undergoes compensatory hypertrophy to accommodate the increased functional load. This adaptation involves increased blood flow and nitrogen excretion,14,23 accompanied by many biochemical changes, including the secretion of growth factors.24, 25, 26 These factors activate the mammalian target of the rapamycin complex signaling pathway, ultimately leading to renal hypertrophy.26, 27, 28 The RAS primarily contributes to the early changes in renal blood flow after nephrectomy.15 Increased adrenocorticotrophic hormone and K levels, along with inhibition of angiotensin II feedback promote renin secretion postnephrectomy.29 This, in turn, stimulates RAS activation, leading to increased renal blood flow and the secretion of growth factors and growth hormones.15,30 Contrarily, these changes may contribute to glomerular hypertension, potentially leading to glomerulosclerosis in the remaining kidney.31,32 RAS blockers can alleviate glomerular hypertension by relaxing the efferent renal arterioles. However, concern exists that RAS blockers may hinder early adaptation by reducing RAS activation, thereby potentially increasing the risk of acute kidney injury.25,33
Despite their long-term benefits, the impact of RAS inhibition on compensatory hypertrophy immediately after nephrectomy remains controversial.15,16,25,34, 35, 36 In animal models, RAS inhibition inhibited regeneration of the residual mesangial cells.34 Attenuated kidney proliferation owing to RAS inhibition after nephrectomy appears to occur independently of blood pressure.35 However, a rat model study showed no decline in kidney function with RAS blocker use, suggesting potential renoprotective effects due to the suppression of TGF-β expression.36 In addition, another study in rats revealed that RAS blockers did not interfere with long-term hypertrophy in the remaining kidney, despite suppressing renal blood flow during the acute phase.15 In humans, the evidence is conflicting. Two randomized controlled trials did not demonstrate superior renal outcomes with RAS blocker use in kidney transplant recipients.37,38 Furthermore, concerns about hyperkalemia and creatinine elevation, which are the potential side effects of RAS blockers, have made many clinicians hesitant to use RAS blockers.
We believe that this study can alleviate concerns regarding impaired renal adaptation and hyperkalemia, which have previously deterred the use of RAS blockers after nephrectomy. In our study, there was no significant difference in hyperkalemia events between the RAS blocker and control groups up to one year after surgery. A study involving 136 kidney donors reported that renal adaptation is most prominent during the first month after nephrectomy.23 Similar to previously published reports on partial nephrectomy and the use of RAS blockers,39,40 our study observed no significant difference in renal adaptation between the groups at this time point. Moreover, when the CKD-EPI 2021 equation was applied, the RAS blocker group showed significantly better early renal adaptation than the control group. However, given that the accuracy of the CKD-EPI 2021 equation has not been adequately validated in Koreans, we cannot conclude that RAS blockers improve renal adaptation. A cohort study that followed 1024 kidney donors for up to 10 years identified that the group with successful early renal adaptation exhibited better long-term kidney function.17 Subgroup analysis of patients who remained on RAS blockers for 1 year postnephrectomy also showed no significant difference in renal adaptation according to RAS blocker use. Moreover, patients treated with RAS blockers had a lower risk of developing ESKD. However, the limited number of ESKD cases makes it difficult to assign a definitive statistical significance to this finding.
Our findings indicate that RAS blocker use is associated with significantly improved overall survival and dialysis-free survival rates. These findings align with those of Nyame et al.,39 who observed that immediate RAS blocker use after partial nephrectomy reduces heart failure and severe renal dysfunction. In a rat model, preoperative RAS blocker administration resulted in less hypertrophy and glomerulosclerosis in the remnant kidney compared to postoperative initiation of the medication. In addition, the cardiovascular benefit of RAS blocker use in patients with CKD is well-recognized.41 Our patients in the RAS blocker group were already administered RAS blockers before surgery, which may have contributed to the observed favorable outcomes. Patients in the control group who died had relatively higher proportion of limited-stage cancer but lower eGFR than those in the RAS blocker group. Therefore, the control group may have had additional deaths of patients with limited-stage cancer for reasons unrelated to cancer, such as complications associated with lower eGFR or cardiovascular diseases. These results suggest that the renal and cardiovascular protective effects of RAS blockers may have contributed to the improved survival.
Potential confounding variables in the relationship between RAS blockers and cancer warrant consideration when interpreting these results. Notably, over 95% of patients undergo nephrectomy for cancer and more than two-thirds are diagnosed with RCC. Compared with healthy individuals, patients with RCC exhibit significantly reduced angiotensin-converting enzyme activity.42 RAS blocker use increases angiotensin I level, leading to the upregulation of angiotensin I-7. Angiotensin I-7 reduces RCC development and progression.43 Previous studies also found an association between RAS blocker use and improved survival in patients with RCC.44,45
Our study has several limitations. First, it inherits the inherent weaknesses of a retrospective study, including the potential for unmeasured confounders, owing to the reliance on data extracted from past medical records. Second, information on the use of RAS blockers throughout the observation period was not available for patients who were prescribed antihypertensive medications at other institutions. Nevertheless, similar trends were observed when analyzing patients who had been taking RAS blockers for up to 1 year compared to those who did not, among those for whom the type of antihypertensive medication could be identified. Finally, our study primarily included patients with cancer, raising uncertainty as to whether the observed positive effect of RAS blockers on survival reflects their direct effect on cancer or other factors.
Conclusion
In patients with hypertension, immediate use of RAS blockers after unilateral nephrectomy did not negatively affect renal adaptation or increase hyperkalemia. Moreover, RAS blocker use was associated with a significantly improved prognosis in terms of ESKD and mortality. Further research is necessary to determine the generalizability of these findings to patients without cancer or hypertension.
Disclosure
All the authors declared no conflicting interests.
Acknowledgments
JJ was supported by the National Research Foundation of Korea grant funded by the Korean Government (MSIT) (2022R1F1A1068198), the [Bio&Medical Technology Development Program] of the National Research Foundation (NRF) funded by the Korean government (MSIT) (No. RS-2023-00222838), and a Young Investigator Research Grant from the Korean Nephrology Research Foundation (2021). KL was supported by grants from the Korea Health Industry Development Institute (HI19C1337), the National Research Foundation of Korea (2021R1A6A3A03039863), and a Young Investigator Research Grant from the Korean Nephrology Research Foundation (2023). HRJ was supported by the National Research Foundation of Korea (2022R1A2B5B01001298, 2019R1A5A2027340), a Korean Fund for Regenerative Medicine (KFRM) grant (22A0302L1-01) funded by the Korean government (Ministry of Science and ICT, Ministry of Health & Welfare), and the Korean Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (HR22C1363). WCC was supported by the National Research Foundation of Korea grant, funded by the Korean government (MSIT) (2022R1A2C3004595). We would like to thank Editage (www.editage.co.kr) for English language editing.
Funding
This study was supported by the National Research Foundation of Korea (South Korea) grant funded by the Korean Government (MSIT) (2022R1F1A1068198).
Data Availability Statement
Data are available on reasonable request due to privacy/ethical restrictions.
Author Contributions
JJ conceptualized this study. SL and JJ designed this study. SL, JJ, and SY analyzed the data. SL, JJ, SK, HJ, KL, HRJ, JL, and WH interpreted the data. SL drafted the manuscript. SL and JJ revised the manuscript for intellectual content. HRJ, JL, and WH supervised the study. All the authors have read and approved the final version of the manuscript.
Footnotes
Figure S1. Distribution of propensity scores before and after matching.
Figure S2. Kaplan-Meier curves according to RAS blocker use after propensity score matching.
Table S1. Characteristics of patients who developed end-stage kidney disease.
Table S2. Differences in laboratory findings according to RAS blocker use after nephrectomy.
Table S3. Comparison between survived and deceased patients.
Table S4. Comparison between survived and deceased patients, according to RAS blockers use.
Table S5. The risk of mortality and composite outcome of ESKD and mortality according to RAS blocker use with calcium channel blocker instead of beta-blocker.
Table S6. Characteristics of patients after propensity score matching.
Table S7. Renal outcomes and hyperkalemia findings according to RAS blocker use after propensity score matching.
Table S8. The risk of mortality and composite outcome of ESKD and mortality after propensity score matching.
STROBE Statement.
Supplementary Material
Figure S1. Distribution of propensity scores before and after matching. Figure S2. Kaplan-Meier curves according to RAS blocker use after propensity score matching. Table S1. Characteristics of patients who developed end-stage kidney disease. Table S2. Differences in laboratory findings according to RAS blocker use after nephrectomy. Table S3. Comparison between survived and deceased patients. Table S4. Comparison between survived and deceased patients, according to RAS blockers use. Table S5. The risk of mortality and composite outcome of ESKD and mortality according to RAS blocker use with calcium channel blocker instead of beta-blocker. Table S6. Characteristics of patients after propensity score matching. Table S7. Renal outcomes and hyperkalemia findings according to RAS blocker use after propensity score matching. Table S8. The risk of mortality and composite outcome of ESKD and mortality after propensity score matching. STROBE Statement.
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J., Soerjomataram I., Dikshit R., et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Fenig Y., Suresh S., Rochon C. Long-term survival after kidney transplantation. N Engl J Med. 2022;386:499. doi: 10.1056/NEJMc2115207. [DOI] [PubMed] [Google Scholar]
- 4.May A.M., Guduru A., Fernelius J., et al. Current trends in partial nephrectomy after guideline release: health disparity for small renal mass. Kidney Cancer. 2019;3:183–188. doi: 10.3233/KCA-190066. [DOI] [Google Scholar]
- 5.Tantisattamo E., Dafoe D.C., Reddy U.G., et al. Current management of patients with acquired solitary kidney. Kidney Int Rep. 2019;4:1205–1218. doi: 10.1016/j.ekir.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen K.W., Wu M.W., Chen Z., et al. Compensatory hypertrophy after living donor nephrectomy. Transplant Proc. 2016;48:716–719. doi: 10.1016/j.transproceed.2015.12.082. [DOI] [PubMed] [Google Scholar]
- 7.Sun M., Bianchi M., Hansen J., et al. Chronic kidney disease after nephrectomy in patients with small renal masses: a retrospective observational analysis. Eur Urol. 2012;62:696–703. doi: 10.1016/j.eururo.2012.03.051. [DOI] [PubMed] [Google Scholar]
- 8.Streja E., Kalantar-Zadeh K., Molnar M.Z., Landman J., Arah O.A., Kovesdy C.P. Radical versus partial nephrectomy, chronic kidney disease progression and mortality in US veterans. Nephrol Dial Transplant. 2018;33:95–101. doi: 10.1093/ndt/gfw358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Digiacomo J.C. The role of nephrectomy in the acutely injured. Arch Surg. 2001;136:1045. doi: 10.1001/archsurg.136.9.1045. [DOI] [PubMed] [Google Scholar]
- 10.Tarver T. Cancer facts & figures 2022. American Cancer Society. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2022/2022-cancer-facts-and-figures.pdf Accessed August 31, 2024.
- 11.Cortinovis M., Perico N., Ruggenenti P., Remuzzi A., Remuzzi G. Glomerular hyperfiltration. Nat Rev Nephrol. 2022;18:435–451. doi: 10.1038/s41581-022-00559-y. [DOI] [PubMed] [Google Scholar]
- 12.Benzing T., Salant D. Insights into glomerular filtration and albuminuria. N Engl J Med. 2021;384:1437–1446. doi: 10.1056/NEJMra1808786. [DOI] [PubMed] [Google Scholar]
- 13.Takagi T., Mir M.C., Sharma N., et al. Compensatory hypertrophy after partial and radical nephrectomy in adults. J Urol. 2014;192:1612–1618. doi: 10.1016/j.juro.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman J.M., Siegel N.J., Hayslett J.P. Functional and hemodynamic adaptation to progressive renal ablation. Circ Res. 1975;36:286–293. doi: 10.1161/01.res.36.2.286. [DOI] [PubMed] [Google Scholar]
- 15.Shimada S., Yang C., Kurth T., Cowley A.W., Jr. Divergent roles of angiotensin II upon the immediate and sustained increases of renal blood flow following unilateral nephrectomy. Am J Physiol Ren Physiol. 2022;322:F473–F485. doi: 10.1152/ajprenal.00376.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valentin J.P., Sechi L.A., Griffin C.A., Humphreys M.H., Schambelan M. The renin-angiotensin system and compensatory renal hypertrophy in the rat. Am J Hypertens. 1997;10:397–402. [PubMed] [Google Scholar]
- 17.van der Weijden J., Mahesh S.V.K., van Londen M., et al. Early increase in single-kidney glomerular filtration rate after living kidney donation predicts long-term kidney function. Kidney Int. 2022;101:1251–1259. doi: 10.1016/j.kint.2022.01.034. [DOI] [PubMed] [Google Scholar]
- 18.Delanaye P., Weekers L., Dubois B.E., et al. Outcome of the living kidney donor. Nephrol Dial Transplant. 2012;27:41–50. doi: 10.1093/ndt/gfr669. [DOI] [PubMed] [Google Scholar]
- 19.Amin M.B., Greene F.L., Edge S.B., et al. 8th ed. Springer; 2017. AJCC Cancer Staging Manual. [Google Scholar]
- 20.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeong T.D., Hong J., Lee W., Chun S., Min W.K. Accuracy of the new creatinine-based equations for estimating glomerular filtration rate in Koreans. Ann Lab Med. 2023;43:244–252. doi: 10.3343/alm.2023.43.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inker L.A., Eneanya N.D., Coresh J., et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385:1737–1749. doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Z., Fang J., Li G., et al. Compensatory changes in the retained kidney after nephrectomy in a living related donor. Transplant Proc. 2012;44:2901–2905. doi: 10.1016/j.transproceed.2012.06.074. [DOI] [PubMed] [Google Scholar]
- 24.Nam U.C., Lee C.H., Chang S.G. Clinical study of insulin-like growth factor-I and compensatory renal growth in patients with donor nephrectomy. Transplant Proc. 1999;31:2114–2117. doi: 10.1016/s0041-1345(99)00279-1. [DOI] [PubMed] [Google Scholar]
- 25.Mok K.Y., Sandberg K., Sweeny J.M., Zheng W., Lee S., Mulroney S.E. Growth hormone regulation of glomerular AT1 angiotensin receptors in adult uninephrectomized male rats. Am J Physiol Ren Physiol. 2003;285:F1085–F1091. doi: 10.1152/ajprenal.00383.2002. [DOI] [PubMed] [Google Scholar]
- 26.Nagasu H., Satoh M., Kuwabara A., et al. Overexpression of klotho protein modulates uninephrectomy-induced compensatory renal hypertrophy by suppressing IGF-I signals. Biochem Biophys Res Commun. 2011;407:39–43. doi: 10.1016/j.bbrc.2011.02.089. [DOI] [PubMed] [Google Scholar]
- 27.Nagasu H., Satoh M., Kidokoro K., et al. Endothelial dysfunction promotes the transition from compensatory renal hypertrophy to kidney injury after unilateral nephrectomy in mice. Am J Physiol Ren Physiol. 2012;302:F1402–F1408. doi: 10.1152/ajprenal.00459.2011. [DOI] [PubMed] [Google Scholar]
- 28.Rojas-Canales D.M., Li J.Y., Makuei L., Gleadle J.M. Compensatory renal hypertrophy following nephrectomy: when and how? Nephrol (Carlton) 2019;24:1225–1232. doi: 10.1111/nep.13578. [DOI] [PubMed] [Google Scholar]
- 29.Baba K., Doi Y., Franco-Saenz R., Mulrow P.J. Mechanisms by which nephrectomy stimulates adrenal renin. Hypertension. 1986;8:997–1002. doi: 10.1161/01.hyp.8.11.997. [DOI] [PubMed] [Google Scholar]
- 30.Ruster C., Wolf G. Renin-angiotensin-aldosterone system and progression of renal disease. J Am Soc Nephrol. 2006;17:2985–2991. doi: 10.1681/ASN.2006040356. [DOI] [PubMed] [Google Scholar]
- 31.Celsi G., Savin J., Henter J.I., Sohtell M. The contribution of ultrafiltration pressure for glomerular hyperfiltration in young nephrectomized rats. Acta Physiol Scand. 1991;141:483–487. doi: 10.1111/j.1748-1716.1991.tb09109.x. [DOI] [PubMed] [Google Scholar]
- 32.Fogo A.B. Glomerular hypertension, abnormal glomerular growth, and progression of renal diseases. Kidney Int Suppl. 2000;75:S15–S21. doi: 10.1046/j.1523-1755.2000.07505.x. [DOI] [PubMed] [Google Scholar]
- 33.Alabdan N., Gosmanova E.O., Tran N.Q., et al. Acute kidney injury in patients continued on renin-angiotensin system blockers during hospitalization. Am J Med Sci. 2017;353:172–177. doi: 10.1016/j.amjms.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Weissgarten J., Berman S., Efrati S., et al. Apoptosis and proliferation of mesangial cells isolated from kidneys undergoing compensatory growth following contralateral nephrectomy: role of the renin-angiotensin system. Med Sci Monit. 2007;13:BR16–BR23. [PubMed] [Google Scholar]
- 35.Krivosikova Z., Sebekova K., Spustova V., Lajdova I., Dzurik R. Enalapril in subantihypertensive dosage attenuates kidney proliferation and functional recovery in normotensive ablation nephropathy of the rat. Physiol Res. 1999;48:429–435. [PubMed] [Google Scholar]
- 36.Matsuo T., Miyata Y., Sagara Y., et al. Renoprotective effects of telmisartan after unilateral renal ablation in rats. Int J Nephrol Renovasc Dis. 2013;6:207–214. doi: 10.2147/IJNRD.S51216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ibrahim H.N., Jackson S., Connaire J., et al. Angiotensin II blockade in kidney transplant recipients. J Am Soc Nephrol. 2013;24:320–327. doi: 10.1681/ASN.2012080777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knoll G.A., Fergusson D., Chasse M., et al. Ramipril versus placebo in kidney transplant patients with proteinuria: a multicentre, double-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4:318–326. doi: 10.1016/S2213-8587(15)00368-X. [DOI] [PubMed] [Google Scholar]
- 39.Nyame Y.A., Liang H., Arora H.C., et al. Do renin-angiotensin blockers affect renal function and cardiac outcomes in patients undergoing partial nephrectomy? J Urol. 2017;197:566–573. doi: 10.1016/j.juro.2016.10.054. [DOI] [PubMed] [Google Scholar]
- 40.Haehn D.A., Shumate A.M., Bajalia E.M., Thomas Ball C., Irizarry-Alvarado J.M., Thiel D.D. Retrospective evaluation of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on postoperative estimated glomerular filtration rates following robotic-assisted partial nephrectomy. J Endourol. 2021;35:808–813. doi: 10.1089/end.2020.0801. [DOI] [PubMed] [Google Scholar]
- 41.Xie X., Liu Y., Perkovic V., et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis. 2016;67:728–741. doi: 10.1053/j.ajkd.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 42.Takada Y., Hiwada K., Yokoyama M., Ochi K., Takeuchi M., Kokubu T. Angiotensin converting enzyme. A possible histologic indicator for human renal cell carcinoma. Cancer. 1985;56:130–133. doi: 10.1002/1097-0142(19850701)56:1<130::aid-cncr2820560120>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 43.Kohara K., Brosnihan K.B., Ferrario C.M. Angiotensin(1-7) in the spontaneously hypertensive rat. Peptides. 1993;14:883–891. doi: 10.1016/0196-9781(93)90063-m. [DOI] [PubMed] [Google Scholar]
- 44.Eskelinen T., Veitonmaki T., Kotsar A., Tammela T.L.J., Poyhonen A., Murtola T.J. Improved renal cancer prognosis among users of drugs targeting renin-angiotensin system. Cancer Causes Control. 2022;33:313–320. doi: 10.1007/s10552-021-01527-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyajima A., Yazawa S., Kosaka T., et al. Prognostic impact of renin-angiotensin system blockade on renal cell carcinoma after surgery. Ann Surg Oncol. 2015;22:3751–3759. doi: 10.1245/s10434-015-4436-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Distribution of propensity scores before and after matching. Figure S2. Kaplan-Meier curves according to RAS blocker use after propensity score matching. Table S1. Characteristics of patients who developed end-stage kidney disease. Table S2. Differences in laboratory findings according to RAS blocker use after nephrectomy. Table S3. Comparison between survived and deceased patients. Table S4. Comparison between survived and deceased patients, according to RAS blockers use. Table S5. The risk of mortality and composite outcome of ESKD and mortality according to RAS blocker use with calcium channel blocker instead of beta-blocker. Table S6. Characteristics of patients after propensity score matching. Table S7. Renal outcomes and hyperkalemia findings according to RAS blocker use after propensity score matching. Table S8. The risk of mortality and composite outcome of ESKD and mortality after propensity score matching. STROBE Statement.
Data Availability Statement
Data are available on reasonable request due to privacy/ethical restrictions.